Editor—Dexmedetomidine is a highly selective α2-adrenergic receptor agonist widely used in clinical anaesthesia as a sedative and analgesic agent.1 It has also been associated with numerous anti-inflammatory effects in preclinical models, including protection against leucocyte-mediated acute lung injury (ALI) after caecal ligation puncture,2 reducing pulmonary oedema in lipopolysaccharide-induced ALI,3 and attenuating cell injury in experimental severe acute pancreatitis via the cholinergic anti-inflammatory pathway.4 In a recent case report, clinical improvement upon dexmedetomidine treatment was suggested to have spared a patient with COVID-19 with worsening hypoxaemia from mechanical ventilation.5 Indeed, there are ongoing clinical trials registered to examine dexmedetomidine in palliative sedation for severe COVID-19 (NCT04350086) and to evaluate its immunomodulatory profile in patients recovering from COVID-19-related acute respiratory distress syndrome (ARDS) (NCT04413864).
Neutrophil extracellular trap formation (NETosis) is a specialised cell death process in which release of chromatin components such as DNA and histones provides a framework for trapping and killing invading microbes.6 However, when dysregulated, NETosis can also aggravate harmful inflammatory responses, including those driving the pathogenesis and thrombosis of severe COVID-19 in lungs and other major organs.7 , 8
In 2020, Jain and collegaues9 hypothesised that ‘given the anti-inflammatory effects of dexmedetomidine, it too may inhibit NETosis and be beneficial in COVID-19 patients’. They went on to provide a detailed schematic illustration of the many feedforward mechanisms potentiating NETosis during COVID-19 and the molecular pathways through which they predicted dexmedetomidine could act to inhibit NET activation.
Our research group has a longstanding interest in the biology and pathobiology of NETs in animal models of infectious diseases such as necrotising fasciitis10 and bacterial pneumonia,11 and recently we studied NET phenotypes in critically ill patients with COVID-19.12 In parallel, we have examined how NETosis is modulated by common medications including statins,13 tamoxifen,14 desferoxamine,15 and propofol.16 With this background, we tested the hypothesis that dexmedetomidine inhibits human NETosis.
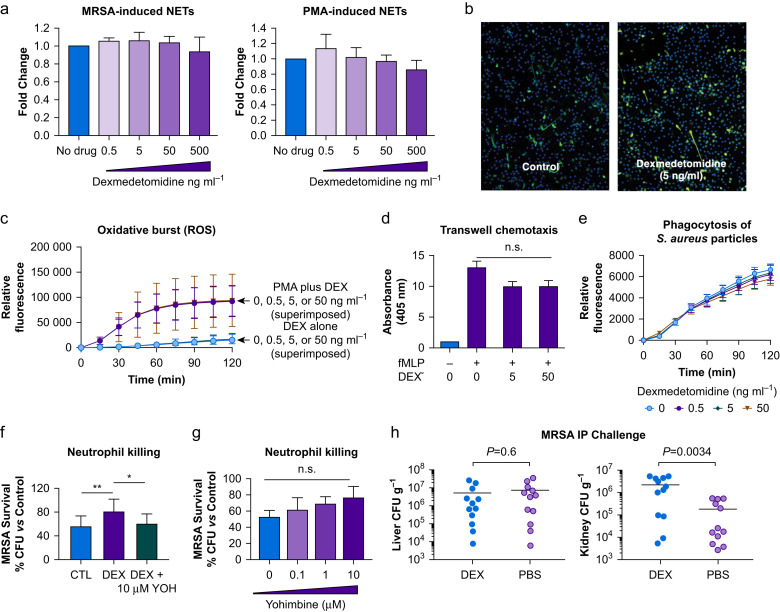
Blood was collected from healthy adults under a protocol approved by the University of California San Diego Institutional Review Board (IRB), and neutrophils were isolated using the PolyMorphPrep™ Kit (Fresenius Kabi, Oslo, Norway) per the manufacturer's instructions. The effective sedative concentration of dexmedetomidine in plasma has been estimated to be 0.2–3.2 ng ml−1 (~1–16 nM).17 We stimulated neutrophils to produce NETs by exposure to live methicillin-resistant Staphylococcus aureus (MRSA) or to the classical NET inducer phorbol myristate acetate (PMA) at 25 nM, in the presence or absence of dexmedetomidine at final concentrations of 0.5, 5, 50, and 500 ng ml−1. For all dexmedetomidine exposures, no inhibition of NET production by MRSA- or PMA-stimulated neutrophils was seen via PICO green quantification of extracellular DNA release (Fig. 1 a) or immunocytochemistry using antibodies against myeloperoxidase (Fig. 1b).
Fig 1.
(a) Fold change in NETosis triggered by either methicillin-resistant Staphylococcus aureus (MRSA; stationary phase, MOI 10, n=4) or phorbol myristate acetate (PMA; 25 nM, n=5) in the presence of increasing concentrations of dexmedetomidine. NET production (extracellular DNA) was quantified using PicoGreen dye. (b) Immunocytochemical analysis of NETosis in response to PMA (25 nM) in the presence or absence of 5 ng/mL (25 nM) dexmedetomidine. Green: myeloperoxidase (staining NETs); blue: DAPI (staining nuclei). (c) Time course of reactive oxygen species (ROS) production by human neutrophils (measured at indicated time points using H2DCFDA, n=3) in the presence or absence of dexmedetomidine either alone or with PMA. (d) Chemotaxis of human neutrophils in response to 100 nM N-formylmethionyl-leucyl phenylalanine (fMLP) in the presence or absence of several concentrations of dexmedetomidine (assessed using Transwell inserts with a 3 μm pore size as described previously, n=5). (e) Phagocytosis time course of S. aureus bioparticles in the presence and absence of several concentrations of dexmedetomidine, n=4. (f) MRSA killing by human neutrophils (MOI 10) in the presence of dexmedetomidine at 5 ng/mL (25 nM) and dexmedetomidine and yohimbine at indicated concentration as compared with the respective control without cells expressed as % colony-forming units (CFU) ml−1, n=8. (g) MRSA killing by human neutrophils (MOI 10) in the presence of several concentrations of yohimbine as compared with the respective control without cells expressed as % CFU ml−1, n=3. (h) amount of MRSA per g of either liver or kidney tissue after an 24 h in vivo intraperitoneal (i.p.) challenge of CD-1 mice that received either dexmedetomidine or phosphate-buffered saline (PBS) i.p. at time of infection and 1 h after infection, n=24, 12 in each group. One-way analysis of variance with post hoc analysis was used to assess significance for data shown here, with the exception of the MRSA i.p. challenge, where unpaired Student's t-test was used. ∗P<0.05; ∗∗P<0.01. CTL, control; DEX, dexmedetomidine; H2DCFDA, 2′,7′-dichlorodihydrofluorescein diacetate; NETOsis, neutrophil extracellular trap formation; NETs, neutrophil extracellular traps; n.s., not significant; YOH, yohimbine.
Neutrophil oxidative burst/generation of reactive oxygen species (ROS) can promote NETosis.18 We found that dexmedetomidine at final concentrations of 0.5, 5.0, and 50 ng ml−1 did not inhibit PMA-induced neutrophil ROS production as measured by a 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Sigma-Aldrich, St Louis, MO, USA) fluorescence assay (Fig. 1c). While examining broader neutrophil functions, we found that similar dexmedetomidine concentrations did not significantly affect neutrophil chemotaxis across a Transwell membrane toward N-formylmethionyl-leucyl-phenylalanine (fMLP) (Fig. 1d), nor did it influence the efficiency of neutrophil phagocytosis of S. aureus-coated particles (pHrodo™ Red S. aureus Bioparticles; Invitrogen Corp., Carlsbad, CA, USA; Fig. 1e). In an ex vivo bactericidal assay, dexmedetomidine (5 ng ml−1) impaired neutrophil killing of MRSA, an effect that was reversed by the α2-adrenergic receptor antagonist yohimbine (Fig. 1f), whereas yohimbine alone did not significantly affect killing (Fig. 1g). Finally, in a murine intraperitoneal MRSA infection model approved by the University of California San Diego Institutional Animal Care and Use Committee (IACUC), treatment with 166 μg kg−1 of dexmedetomidine i.p. at time of infection and again 1 h after bacterial challenge was associated with significantly increased recovery of bacterial colony-forming units (CFU) from kidneys 24 h later (Fig. 1h), although no change was seen in CFUs recovered from the liver.
We conclude that dexmedetomidine at therapeutically relevant concentrations and higher does not directly inhibit production of NETs by human neutrophils in response to commonly used NETosis inducers, nor does it significantly alter neutrophil behaviour in selected other common phenotypic assays including ROS generation, chemotaxis, and phagocytosis. Dexmedetomidine slightly but significantly (1) impaired human neutrophil killing of MRSA in an α2-adrenergic receptor-dependent manner and (2) reduced kidney bacterial burden in a murine systemic infection model, but it is premature to conclude whether these modest phenotypes are related or clinically significant for humans. Of note, dexmedetomidine is mainly hepatically metabolised and can reach liver concentrations much higher than plasma, after which its metabolites are primarily excreted through the kidneys.19
Our study has several limitations. First, we describe in vitro studies with purified human neutrophils and in vivo studies using mice, both relatively distant from the clinical setting. Second, the stimuli used to trigger NETosis and other neutrophil effector functions, although commonly used in the field, are not of viral origin. Follow-up ex vivo studies using COVID-19 patient blood, along with in vitro studies using activators of viral origin, will be important.
Several anaesthetic drugs are known to possess important anti-inflammatory and immunomodulatory properties, including those acting on neutrophils,20 that can influence their pharmacodynamics and clinical effectiveness. Of immediate impact, there is emerging clinical opinion that the immunomodulatory activities of dexmedetomidine might be harnessed to improve patient outcomes in severe COVID-19.9 , 21 Our studies, with the stated limitations, suggest that the proposed benefits do not include direct inhibition of extracellular trap formation by human neutrophils.
Authors' contributions
Project concept: RC, VN, AM
Conduct of experiments: RC, BES, JOl, AM
Data analysis: RC, BES, AM
Data interpretation: VN, AM, RC
Editing of the manuscript: RC, AM, VC
Conduct of initial concept experiments: JOk, AM
Conduct of imaging experiment: AM, JMS
Writing of the manuscript: VN, AM
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
IARS Mentored Research Training Grant (IMRA) to AM, the US National Institutes of Healt (NIH) KL-2 grant1KL2TR001444 to AM,. Pilot Grant 1UL1TR001444-01 to AM, NIH grant R01 AI 145310 NIH/NIAID to VN.
References
- 1.Keating G.M. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs. 2015;75:1119–1130. doi: 10.1007/s40265-015-0419-5. [DOI] [PubMed] [Google Scholar]
- 2.Karabulut G., Bedirli N., Akyurek N., Bagriacik E.U. Dose-related effects of dexmedetomidine on sepsis-initiated lung injury in rats. Braz J Anesthesiol. 2021;71:271–277. doi: 10.1016/j.bjane.2021.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Y., Xia M., Xu JHuang Q., Dai Z., Zhang X. Dexmedetomidine alleviates pulmonary edema through the epithelial sodium channel (ENaC) via the PI3K/Akt/Nedd4-2 pathway in LPS-induced acute lung injury. Immunol Res. 2021;69:162–175. doi: 10.1007/s12026-021-09176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang D.Y., Li Q., Shi C.Y., Hou C.Q., Miao Y., Shen H.B. Dexmedetomidine attenuates inflammation and pancreatic injury in a rat model of experimental severe acute pancreatitis via cholinergic anti-inflammatory pathway. Chin Med J (Engl) 2020;133:1073–1079. doi: 10.1097/CM9.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockton J., Kyle-Sidell C. Dexmedetomidine and worsening hypoxemia in the setting of COVID-19: a case report. Am J Emerg Med. 2020;38:2247 e2241–7, e2242. doi: 10.1016/j.ajem.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkmann V., Reichard U., Goosmann C., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 7.Iliadi V., Konstantinidou I., Aftzoglou K., Iliadis S., Konstantinidis T.G., Tsigalou C. The emerging role of neutrophils in the pathogenesis of thrombosis in COVID-19. Int J Mol Sci. 2021;22:5368. doi: 10.3390/ijms22105368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahilog Z., Zhao H., Wu L. The role of neutrophil NETosis in organ injury: novel inflammatory cell death mechanisms. Inflammation. 2020;43:2021–2032. doi: 10.1007/s10753-020-01294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain A., Lamperti M., Doyle D.J. Dexmedetomidine: another arrow in the quiver to fight COVID-19 in intensive care units. Br J Anaesth. 2021;126:e35–e38. doi: 10.1016/j.bja.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker M.J., Hollands A., Sanderson-Smith M.L., et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007;13:981–985. doi: 10.1038/nm1612. [DOI] [PubMed] [Google Scholar]
- 11.Berends E.T., Horswill A.R., Haste N.M., Nizet M., von Kockritz-BLickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masso-Silva J.A., Moshensky A., Lam M.T.Y., et al. Increased peripheral blood neutrophil activation phenotypes and NETosis in critically ill COVID-19 patients: a case series and review of the literature. Clin Infect Dis. 2021:ciab437. doi: 10.1093/cid/ciab437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow O.A., von Kockritz-BLickwede M., Bright A.T., et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corriden R., Hollands A., Olson J., et al. Tamoxifen augments the innate immune function of neutrophils through modulation of intracellular ceramide. Nat Commun. 2015;6:8369. doi: 10.1038/ncomms9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vollger L., Akong-Moore K., Cox L., et al. Iron-chelating agent desferrioxamine stimulates formation of neutrophil extracellular traps (NETs) in human blood-derived neutrophils. Biosci Rep. 2016;36 doi: 10.1042/BSR20160031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier A., Chien J., Hobohm L., Patras K.A., Nizet V., Corriden R. Inhibition of human neutrophil extracellular trap (NET) production by propofol and lipid emulsion. Front Pharmacol. 2019;10:323. doi: 10.3389/fphar.2019.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebert T.J., Hall J.E., Barney J.A., Uhrich T.D., Colinco M.D. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Kirchner T., Moller S., Klinger M., Solbach W., Laskay T., Behnen M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm. 2012;2012:849136. doi: 10.1155/2012/849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weerink M.A.S., Struys M.M.R.F., Hannivoort L.N., Barends C.R.M., Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56:893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier A., Nizet V. Impact of anesthetics on human neutrophil function. Anesth Analg. 2019;128:569–574. doi: 10.1213/ANE.0000000000003927. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H., Davies R., Ma D. Potential therapeutic value of dexmedetomidine in COVID-19 patients admitted to ICU. Br J Anaesth. 2021;126:e33–e35. doi: 10.1016/j.bja.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]