Abstract
Endomyocardial biopsy (EMB) is used in diagnosing infiltrative and other suspected cardiomyopathies. We present a case in which positron emission tomography– and electroanatomic mapping-guided EMB of the atrial septum confirmed the diagnosis of cardiac sarcoidosis in a patient with negative findings on ventricular and lymph node biopsy. (Level of Difficulty: Advanced.)
Key Words: cardiomyopathy, electroanatomical mapping, imaging, positron emission tomography, sarcoidosis, ultrasound
Abbreviations and Acronyms: 3D, 3-dimensional; EAM, electroanatomical mapping; EMB, endomyocardial biopsy; EP, electrophysiology; FDG, fluorodeoxyglucose; ICD, implantable cardioverter-defibrillator; ICE, intracardiac echocardiography; PET, positron emission tomography
Central Illustration
Introduction
Endomyocardial biopsy (EMB) is useful in patients with suspected infiltrative and other cardiomyopathies to establish a histopathologic diagnosis (1,2). The most common site for biopsy is the right ventricular septum. Unfortunately, sensitivity is low, and false negative rates are high.
Learning Objectives
-
•
To be able to make a specific diagnosis of cardiomyopathy with multimodality imaging and atrial biopsy.
-
•
To understand the role of EP-guided 3D EAM in patients with cardiomyopathy.
-
•
To understand the role of EAM-guided biopsy in patients with cardiomyopathy.
With the advent of advanced 3-dimensional (3D) mapping systems, electroanatomical mapping (EAM), precise guidance of the bioptome catheter to desired regions has allowed operators to obtain biopsy specimens from the right and left ventricles (2).
Atrial biopsies are not routinely performed, given the risk of perforation of the thin atrial wall, but successful and safe sampling of the interatrial septum can be achieved (2). We present a case report of a patient with evidence of inflammation in the right atrium seen on a positron emission tomography (PET) scan. We safely performed an EAM-guided biopsy from the atrial septum and the left ventricle in this patient. Only the atrial biopsy specimens confirmed the diagnosis of sarcoidosis.
History of Present Illness
A 57-year-old woman presented to our facility (Houston Methodist DeBakey Heart and Vascular Center, Houston, Texas) for dyspnea on minimal exertion. She was found to have pneumonia causing an exacerbation of heart failure. After treatment of the pneumonia and stabilization of her heart failure, a cause of her nonischemic cardiomyopathy was pursued.
Past Medical History
She had a history of a transient ischemic attack in 2013, chronic kidney disease, and persistent atrial fibrillation. In 2019, she had a cardiac arrest while visiting her husband in the hospital; she required multiple defibrillations for ventricular tachycardia and ventricular fibrillation. Her underlying rhythm was complete heart block, and she underwent dual-chamber implantable cardioverter-defibrillator (ICD) insertion. Her family history was unremarkable.
Differential Diagnosis
The differential diagnosis of nonischemic cardiomyopathy was broad, including cardiac sarcoidosis, amyloidosis, hemochromatosis, arrhythmogenic right ventricular dysplasia, and myocarditis.
Investigations
Coronary computed tomography angiography performed 1 month earlier showed no significant coronary artery stenosis. On this admission, a transthoracic echocardiogram revealed an ejection fraction of 30%. Because of a non–magnetic resonance imaging–compatible ICD, a cardiac magnetic resonance was unable to be performed. A cardiac PET scan revealed diffuse fluorodeoxyglucose (FDG) uptake in the middle inferior, inferoseptal, and inferolateral walls of the left ventricle. Intense FDG uptake was also seen in the interatrial septum, the right atrium, and the left hilar lymph node (Figure 1). The main differential diagnosis was an inflammatory process such as sarcoidosis or viral myocarditis. The presence of a viral infection was assessed by polymerase chain reaction analysis, and findings were negative. The patient had no extracardiac manifestation of sarcoidosis except for the FDG uptake in the left hilar lymph node. A biopsy of the left hilar lymph node was performed, with nonspecific results.
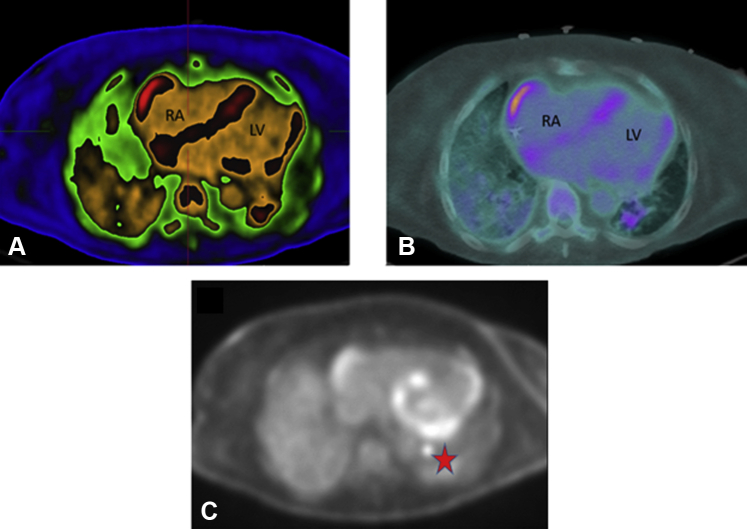
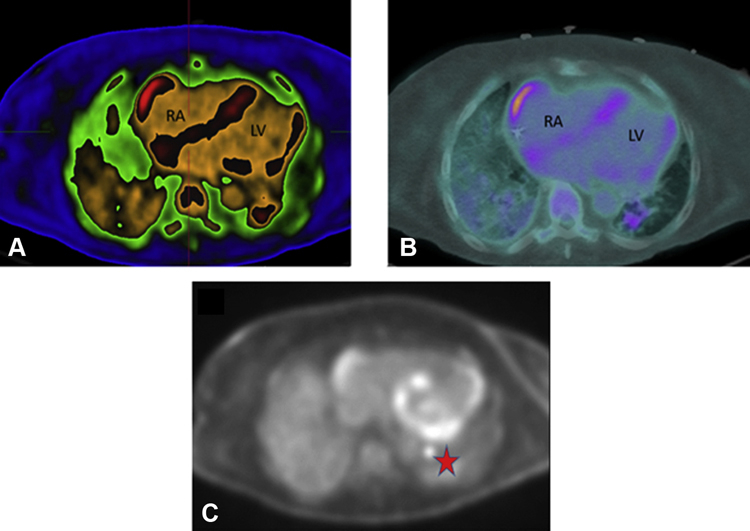
Figure 1.
Positron Emission Tomography Images
Cardiac positron emission tomography scan revealing areas of increased inflammation and fluorodeoxyglucose uptake. (A) ADAC Isocontour, (B) warm metal, and (C) black and white color schemes revealing uptake inside the right lateral atrial wall, the atrial and ventricular septum, left lateral ventricular wall, and left hilar lymph node. The red star indicates fluorodeoxyglucose uptake in left hilar lymph node. LV = left ventricle; RA = right atrium.
Management
Electrophysiology (EP) was consulted for an EAM-guided biopsy. She was brought to the EP laboratory, where an 8.5-F steerable sheath (Agilis, St. Jude Medical) and a 10-F intracardiac echocardiography (ICE) catheter were placed in the right femoral vein. A duodecapolar catheter (Pentaray, Biosense Webster) was placed in the right atrium and used to create a 3D map of the superior vena cava, right atrium, right ventricle, and inferior vena cava. Bipolar EAM revealed patchy areas of low voltage throughout the right atrium, specifically on the interatrial septum just superior to the coronary sinus (Figure 2A). The Pentaray catheter was exchanged for a Bipal 7 bioptome 104 cm (Cordis Corp). Using the Agilis sheath and the ICE catheter, the bioptome was directed to the inferior aspect of the fossa ovalis. Five biopsy specimens were taken from the suspected area and placed in formalin solution for histopathologic analysis (Figures 2B and 2C, Video 1). A transseptal puncture was performed with ICE guidance, and a 3D bipolar EAM was created of the left ventricle. This revealed multiple low=voltage signals along the inferior lateral and septal walls suggestive of unhealthy endocardium and matching the areas of inflammation reported in the PET scan. Six biopsy specimens were obtained from the suspected areas in the left ventricle (total of 8 biopsy specimens) (Figures 3A and 3B).
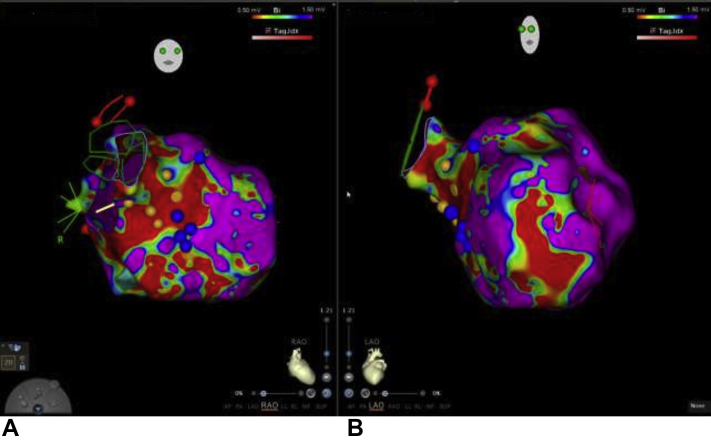
Figure 2.
Electroanatomical Mapping, Fluoroscopy, and ICE Images During IAS Biopsy
(A) Electroanatomical mapping of the intra-atrial septum (IAS). Cranial posteroanterior view revealing atrial septum biopsy sites (white arrow and red dots) just superior to the coronary sinus (CS). (B) Radiograph in the left anterior oblique view illustrating the bioptome biopsy device capturing atrial septal tissue. (C) Intracardiac echocardiography (ICE) image with an open bioptome capturing atrial septal tissue. IVC = inferior vena cava; LA = left atrium; RAA = right atrial appendage; RVL = right ventricular lead; SVC = superior vena cava; TV = tricuspid valve.
Figure 3.
Electroanatomical Mapping of the Left Ventricle
(A) Right anterior oblique view of the left ventricle. Blue dots represent biopsy sites of the left ventricular septum at the border of low and normal voltage; yellow dots indicate the Purkinje fiber network. (B) Left anterior oblique view of the left ventricle.
Interestingly, all atrial and ventricular biopsy specimens revealed marked hypertrophy, except for 1 biopsy in the atrium revealing a noncaseating granuloma, consistent with cardiac sarcoidosis (Figures 4A and 4B). On the basis of the histologic findings and the clinical findings of ventricular tachycardia, complete heart block, and nonischemic cardiomyopathy, the patient received a diagnosis of sarcoidosis.
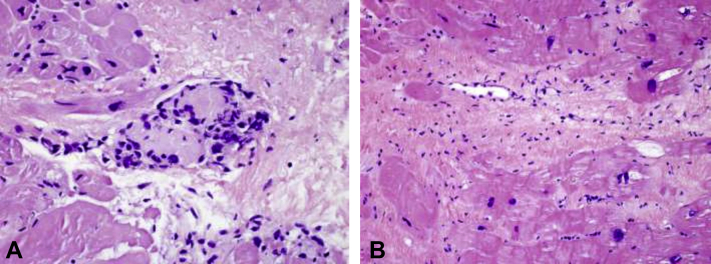
Figure 4.
Histologic Endomyocardial Biopsy Specimen From Both the Atrium and the Ventricle
(A) Atrial endomyocardial biopsy histologic specimen: single noncaseating granuloma (×400 magnification). (B) Left ventricular septum endomyocardial biopsy histologic specimen: focal interstitial fibrosis with focal endocardial lymphocytic infiltration, no granuloma identified.
Discussion
EMB has proven utility in the diagnosis of infiltrative and other cardiomyopathies (1). Although EMB can provide a histopathologic diagnosis of cardiomyopathies such as cardiac sarcoidosis, arrhythmogenic right ventricular dysplasia, myocarditis, and others, it has been plagued by low sensitivity and high false negative rates. The reason is believed to be a combination of patchy distribution of the cardiomyopathy, an inability to assess tissue characteristics for biopsy in the short term, and dependence on ventricular septal biopsy.
Atrial biopsy is uncommon but has been performed. In 1984, Sekiguchi et al (3) described atrial biopsies for the diagnosis of Loffler endocarditis and endomyocardial fibrosis. Frustaci et al (4) reported a case series describing septal atrial biopsies for assessing the histopathologic changes in patients diagnosed with lone atrial fibrillation versus a control group. A total of 12 patients underwent EMB on the atrial septum safely. Results were mixed but did add value to the ventricular septal biopsies (4). More recently, Sepehri Shamloo et al (5) described 4 patients who safely underwent atrial biopsy of the septum for characterization of atrial fibrillation tissue.
Our case contributes to the limited evidence that atrial biopsies can be performed safely and the increased sensitivity it provides in diagnosing the etiology of a nonischemic cardiomyopathy (6,7). FDG uptake from PET, EAM, ICE, and fluoroscopy were used to guide the ultimate location of our biopsy. EAM did reveal areas of patchy endocardial tissue with areas of low voltage and healthy tissue. However, this heterogeneous pattern was also seen in the left ventricle, which yielded a nonspecific result. ICE guidance was critical in our case for guidance of the bioptome because the biopsy specimens were taken superior to the coronary sinus on the intra-atrial septum. A single granuloma was appreciated in the histopathologic analysis, thus providing the diagnosis of cardiac sarcoidosis.
Follow-Up
The patient was started on high-dose corticosteroids and was eventually discharged home in stable condition.
Conclusions
This case provides further insight into the safety, utility, and increased efficacy of atrial biopsy for cardiologists who seek increased sensitivity with EMB.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Atrial Biopsy. Fluoroscopic and intracardiac echocardiography recordings of an atrial biopsy taken from the intra-atrial septum.
References
- 1.Chimenti C., Russo M.A., Frustaci A. Atrial biopsy evidence of Fabry disease causing lone atrial fibrillation. Heart. 2010;96(21):1782–1783. doi: 10.1136/hrt.2010.196162. [DOI] [PubMed] [Google Scholar]
- 2.Casella M., Dello Russo A., Bergonti M., et al. Diagnostic yield of electroanatomic voltage mapping in guiding endomyocardial biopsies. Circulation. 2020;142(13):1249–1260. doi: 10.1161/CIRCULATIONAHA.120.046900. [DOI] [PubMed] [Google Scholar]
- 3.Sekiguchi M., Yu Z.X., Take M., et al. Ultrastructural features of the endomyocardium in patients with eosinophilic heart disease. An endomyocardial biopsy study. Jpn Circ J. 1984;48(12):1375–1382. doi: 10.1253/jcj.48.1375. [DOI] [PubMed] [Google Scholar]
- 4.Frustaci A., Chimenti C., Bellocci F., Morgante E., Russo M.A., Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96(4):1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 5.Sepehri Shamloo A., Husser D., Buettner P., Klingel K., Hindricks G., Bollmann A. Atrial septum biopsy for direct substrate characterization in atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31(1):308–312. doi: 10.1111/jce.14308. [DOI] [PubMed] [Google Scholar]
- 6.Goete A., Kalman J.M., Auguinaga L., et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2016;18(10):1455–1490. doi: 10.1093/europace/euw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaidya V.R., Abudan A.A., Vasudevan K., et al. The efficacy and safety of electroanatomic mapping-guided endomyocardial biopsy: a systematic review. J Interv Card Electrophysiol. 2018;53(1):63–71. doi: 10.1007/s10840-018-0410-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Atrial Biopsy. Fluoroscopic and intracardiac echocardiography recordings of an atrial biopsy taken from the intra-atrial septum.