Abstract
AIM
To compare the frequencies of neurosurgical procedures to treat comorbid conditions of myelomeningocele in patients who underwent fetal surgery versus postnatal surgery for closure of the placode.
METHOD
By utilizing the National Spina Bifida Patient Registry in a comparative effectiveness study, 298 fetal surgery patients were matched by birthdate (±3mo) and spina bifida clinic site with one to three postnatal surgery patients (n=648). Histories were obtained by record review on enrollment and yearly subsequently. Multivariable Poisson regression was used to compare frequencies of procedures between cohorts, with adjustments for sex, ethnicity, insurance status, spinal segmental level of motor function, age at last visit recorded in the Registry, and, for shunt revision in shunted patients, age at cerebrospinal fluid (CSF) diversion.
RESULTS
The median age at last visit was 4 years. In fully adjusted analyses in patients aged at least 12 months old, fetal surgery was associated with decreased frequency of CSF diversion for hydrocephalus by ventriculoperitoneal shunt insertion or endoscopic third ventriculostomy compared with postnatal surgery (46% vs 79%; incidence rate ratio=0.61; 95% confidence interval [CI] 0.53–0.71; p<0.01). Over all ages, fetal surgery was associated with decreased frequency of Chiari decompression for brainstem dysfunction (3% vs 7%; incidence rate ratio=0.41; 95% CI 0.19–0.88; p=0.02). Also over all ages, differences were not significant in frequencies of shunt revision in shunted patients (53% vs 55%; incidence rate ratio=0.87; 95% CI 0.69–1.11; p=0.27), nor tethered cord release for acquired spinal cord dysfunction (18% vs 16%; incidence rate ratio=1.11; 95% CI 0.84–1.47; p=0.46).
INTERPRETATION
Even with the variations inherent in clinical practice, fetal surgery was associated with lower frequencies of CSF diversion and of Chiari decompression, independent of covariates.
Myelomeningocele, perhaps better called spina bifida aperta (see Appendix S1, online supporting information for a full explanation), is caused by failure of the caudal neuropore to close during embryological neurulation. This results in a midline defect in mesoderm-derived tissue (bone, muscle, and dura), through which the atypically formed spinal cord and leptomeninges protrude.1,2
Traditionally myelomeningocele has been treated by postnatal closure of the placode. Its comorbid conditions of hydrocephalus, the Chiari II malformation, and spinal cord tethering often require subsequent neurosurgical procedures.3
Cerebrospinal fluid (CSF) diversion is used to treat hydrocephalus. Insertion of a ventriculoperitoneal shunt (henceforth, shunt) was the only procedure used for this for decades, but it has risks of shunt dysfunction and infection.3 A newer option for CSF diversion, endoscopic third ventriculostomy (ETV), was developed to avoid the complications of shunting.4
The Chiari II malformation is a congenital brain anomaly associated with myelomeningocele. Its principal features are abnormalities of the midbrain, cerebellum, and brainstem, and herniation of the cerebellar vermis, cerebellar tonsils, and medullary elements through the foramen magnum into the cervical spinal canal (henceforth, hindbrain herniation). These abnormalities are thought to result from changes in vectors of fetal brain growth that are caused by continuous CSF venting out the open neural tube defect.5 In some cases, the Chiari II malformation manifests with symptoms of brainstem dysfunction. Surgical decompression of the posterior fossa (Chiari decompression) benefits selected patients with a symptomatic Chiari II malformation.3 Tethering of the spinal placode to the overlying scar after myelomeningocele closure can lead to progressive worsening of spinal cord function and/or intractable pain that requires surgical release of the tethered cord.6 Shunt dysfunction and infection necessitate shunt revision.3 The neurosurgical procedures are only performed on patients with signs, symptoms, or other indications related to each condition. Therefore, in a large sample, their frequencies can be used as surrogates for the frequencies of the conditions themselves.
Fetal surgery for closure of myelomeningocele was first successfully performed in 1997.7,8 The surgical technique used was similar to postnatal surgery, but was done at 23 to 26 weeks gestational age through a hysterotomy.8 It is thought by most that fetal surgery eliminates CSF venting, normalizes CSF pressure dynamics, and thereby normalizes fetal brain growth patterns. This more typical pattern of brain growth in turn reverses fetal hindbrain herniation, relieving obstruction to CSF flow and thus preventing hydrocephalus.9
Early experience with fetal surgery suggested that it decreased frequencies of CSF diversion and imaging evidence of the Chiari II malformation. However, it also increased risks of preterm delivery and uterine dehiscence.10,11 A randomized controlled trial of fetal surgery versus postnatal surgery was conducted to determine if the benefits of fetal surgery outweighed the risks. The Management of Myelomeningocele Study (MOMS) was funded by the National Institutes of Health12 and ran from 2003 to 2010. Enrollment in the MOMS was stopped early, for proof of efficacy of fetal surgery.13
Analysis of the MOMS participants aged at least 12 months old confirmed that fetal surgery was associated with a lower frequencies of CSF diversion13–15 and hindbrain herniation on magnetic resonance imaging, but that the risks of preterm birth and of uterine dehiscence were higher.13
While the MOMS clearly showed the benefit of fetal surgery for some outcomes, the trial was performed in selected participants at the three most experienced centers, raising questions about the generalizability of the MOMS findings to other fetal surgery centers.2 Since the MOMS results were published in 2011,13 fetal surgery for myelomeningocele has become widely utilized in the United States and around the world, but there are still divergent opinions about its value among pediatric neurosurgeons.16 Since 2011, there have been only four single-institution, retrospective, case–control studies comparing frequencies of neurosurgical procedures in fetal surgery and postnatal surgery patients.17–20 Therefore, we sought to determine if relevant findings of the MOMS could be generalized to a larger, broader, and less selected patient population, a study made possible by using the National Spina Bifida Patient Registry (NSBPR).
The NSBPR is maintained and directed by the Centers for Disease Control and Prevention with the goal of improving the care of people with spina bifida through clinical research.21–25 It is the largest clinical database of patients with spina bifida in the world. Through 2017, the NSBPR had 35 participating clinics and had enrolled 8662 patients with all forms of spina bifida. From its inception, its purpose has been to provide nationwide data to study the clinical characteristics of patients with spina bifida, the treatments used for their comorbidities, and the outcomes of their treatments.21 Because of its large sample size and the involvement of so many spina bifida clinics, the NSBPR is well suited to study typical outcomes in patients with myelomeningocele.24–26
A randomized controlled trial, like the MOMS, determines the efficacy of an intervention under ideal conditions. A comparative effectiveness study determines if a new intervention changes outcomes in the ‘real world’, outside of the ideal conditions of a randomized controlled trial. Comparative effectiveness studies are necessary because indications for interventions evolve in clinical practice27 in known and unknown ways, resulting in clinical variations, in contrast to the rigid exclusion and inclusion criteria of a randomized controlled trial. Patient registries can be used for comparative effectiveness studies.27
Variations by fetal surgery center in fetal surgery exclusion criteria have arisen since 2011, becoming less stringent today than they were during the MOMS.28 Two types of fetal surgery are now done for placode closure: via hysterotomy and by fetoscopy. Variations also exist in postnatal management. Frequencies of CSF diversion and Chiari decompression vary among spina bifida clinics that participated in our study25,26 and two types of CSF diversion are now used to treat hydrocephalus: shunt insertion and ETV. These variations are all justifications for our comparative effectiveness study.
The aim of this study was therefore to utilize the NSBPR to evaluate the comparative effectiveness of fetal surgery and postnatal surgery in patients with myelomeningocele for CSF diversion (shunt insertion or ETV), shunt revision in shunted patients, Chiari decompression, and tethered cord release. Outcomes were assessed in time frames consistent with those used by the MOMS (age ≥12mo for assessment of CSF diversion status and at any age for other outcomes).
METHOD
Each of the individual spina bifida clinics that contributed data to this study obtained approval from its own institutional review board. There was no multisite institutional review board approval. The standard methods used by the NSBPR for institutional review board approval, data collection, data management, and data quality control have been described previously21–24,26 and are presented in detail in Appendix S2 (online supporting information). Assessment of category of spinal segmental level of motor function (henceforth, motor level) was done by a standard physical examination at the last visit recorded in the NSBPR (Appendix S2).
Patients could be enrolled at any age. Medical and surgical histories were collected retrospectively by record review on enrollment and then prospectively once per year subsequently. Previously incomplete histories could be supplemented at any visit, making the history at the last visit both the most up to date and most complete. Data analyzed for this study were collected and entered from 2009 through 2017.
The primary outcome was the frequency of CSF diversion. This variable combined shunt insertion alone, ETV alone, and the combination of ETV and shunt insertion. Secondary outcomes were frequencies of shunt revision in shunted patients (which included patients with shunt insertion alone or with the combination of ETV and shunt insertion), Chiari decompression, and tethered cord release.
The study population was drawn from all patients with myelomeningocele born from 1997, the year of the first successful fetal surgery,7,8 through 2017. Each fetal surgery patient was matched with one to three postnatal surgery patients by date of birth (±3mo) and by spina bifida clinic site of care. If more than three postnatal surgery patients could be matched with a fetal surgery patient, three postnatal surgery patients were randomly selected from among those who matched. Fetal surgery patients for whom there were no matching postnatal surgery patients were excluded. The entire study population was used for all analyses, with these exceptions: (1) for comparisons of the frequencies of CSF diversion, the study population was limited to fetal surgery patients and matched postnatal surgery patients who were both at least 12 months old at the last visit, the age used for first their analysis by the MOMS;6,10 and (2) for comparison of frequencies of shunt revision ever (henceforth, shunt revision), the study population was limited to patients who had had a shunt inserted.
Statistical analyses
Fisher’s exact test was used to compare the proportion of fetal surgery in patients born in two eras, based on when the results of the MOMS were published: (1) January 1997 through December 2010, and (2) January 2011 through December 2017. Fisher’s exact test was also used to test associations of the distributions of motor levels with CSF diversion frequency in each cohort.
Univariable Poisson regression with robust variance estimators conditioning on the matched pair was used to evaluate both the differences in frequencies of sociodemographic characteristics and motor levels between cohorts, as well as the associations of each sociodemographic covariate with the frequency of each of the four neurosurgical procedures. A similar analysis was done for categories of motor levels with the frequencies of all four outcomes. Univariable linear regression conditioning on the matched pair was used to compare the age at last visit recorded in the NSBPR and age at CSF diversion in the two cohorts.
Multivariable Poisson regression with robust variance estimators conditioning on the matched pair was used to evaluate the differences in frequencies of outcomes between the cohorts after adjusting for covariates. Covariates included in the models were determined a priori: male sex, non-Hispanic white ethnicity, private insurance, motor level, and age at last visit recorded in the NSBPR. For the outcome of shunt revision among shunted patients, age at initial CSF diversion was also included as a covariate.
The Poisson regression method was used instead of logistic regression because the odds ratio obtained using logistic regression overestimates the risk ratio when the outcome is not rare (>10%), which was the case for multiple outcomes.29–32 Results of regression analyses are presented as incidence rate ratios or coefficients with 95% confidence intervals (CIs); p-values less than 0.05 were considered significant. Analyses were conducted using Stata version 16.0 (Statcorp, College Station, TX, USA).
RESULTS
Of the 8662 patients enrolled in the NSBPR through December 2017, 6410 patients were born in 1997 or later and, of these, 4872 had myelomeningocele. From these, 321 fetal surgery patients were identified. Relative to the number of patients enrolled in the NSBPR at the time, the proportion of fetal surgery patients increased significantly after results of the MOMS were reported in 201113 (Fig. 1). Of the 321 fetal surgery patients, 298 patients were matched for date of birth and spina bifida clinic site to 648 postnatal surgery patients at 25 spina bifida clinic sites of care, and the remaining 23 unmatched fetal surgery patients were excluded from analysis. Figure S1 (online supporting information) presents the distribution of patient in the study by spina bifida clinic site.
Figure 1:
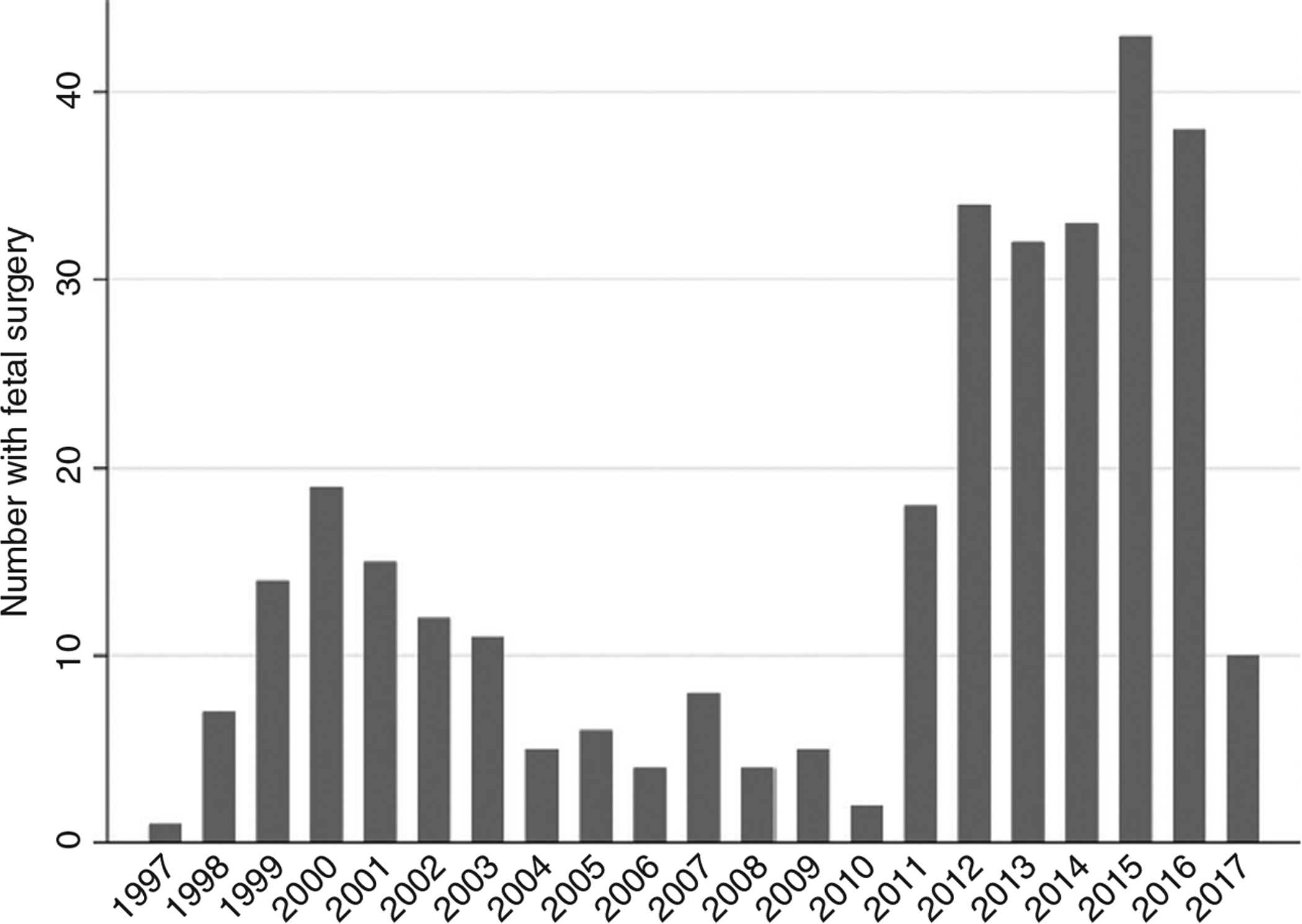
Number of fetal surgery patients, by year of birth (n=321). A higher proportion of patients born in 2011 and after (208 out of 1473, 14%) had fetal surgery compared with before 2011 (113 out of 3399, 3%; p<0.001).
The fetal surgery cohort was more likely to be non-Hispanic white and to have private insurance, and was less likely to be non-Hispanic black or Hispanic. Sex distribution was not different between the two cohorts (Table 1). Demographics of the 23 fetal surgery patients excluded from analysis were similar to those included (48% male, 82% non-Hispanic white, and 65% with private insurance). In addition, we found that every sociodemographic characteristic, except male sex and Hispanic ethnicity, was significantly associated with at least one outcome in the whole study population (Table S1, online supporting information).
Table 1:
Unadjusted comparisons of demographic characteristics, categories of spinal segmental level of motor function, and the age at last visit recorded in the NSBPR between fetal surgery patients (n=298) and postnatal surgery patients matched for date of birth (±3mo) (n=648), assessed when patients in both cohorts were at least 12 months of age
| Covariate | Fetal surgery, n (%) | Postnatal surgery, n (%) | IRR (95% CI); p |
|---|---|---|---|
| Male sex | 147/298 (49) | 321/648 (50) | 1.00 (0.76–1.32); 0.99 |
| Female sex | 151/298 (51) | 327/648 (50) | |
| Non-Hispanic white | 236/285 (83) | 404/631 (64) | 3.06 (2.07–4.54); <0.01 |
| Non-Hispanic black | 10/285 (4) | 75/631 (12) | 0.30 (0.15–0.58); <0.01 |
| Hispanic | 34/287 (12) | 136/641 (21) | 0.41 (0.26–0.66); <0.01 |
| Private insurance | 218/298 (73) | 335/648 (52) | 2.76 (2.00–3.81); <0.01 |
| Sacral level | 102 (34) | 163 (25) | Reference |
| Low lumbar level | 81 (27) | 151 (23) | 0.94 (0.64–1.37); 0.75 |
| Mid-lumbar level | 79 (27) | 176 (27) | 0.75 (0.50–1.11); 0.15 |
| High lumbar level | 16 (5) | 87 (13) | 0.30 (0.16–0.55); <0.01 |
| Thoracic level | 20 (7) | 71 (11) | 0.47 (0.26–0.84); 0.01 |
| Age at last visit in years | 3.67 (1.42–11.09) | 4.08 (2.00–11.50) | 0.02 (−0.13 to 0.17); 0.82 |
All patients had myelomeningocele, were born 1997 through 2017, and were enrolled in the National Spina Bifida Patient Registry (NSBPR) 2009 through 2017. The significance of differences between cohorts in demographic characteristics was assessed by univariable Poisson regression. The significance of differences in frequencies of patients in categories of spinal segmental level of motor function was assessed by Poisson univariable regression, and the significance of differences in age at last visit recorded in the NSBPR was assessed by univariable Poisson linear regression. Percentages may not total 100% because of rounding. IRR, incidence rate ratio; CI, confidence interval.
Relative to the frequency of having a sacral motor level, fetal surgery patients had lower odds ratios of having either a thoracic or a high lumber motor level than did the postnatal cohort (Table 1). In addition, in unadjusted analyses, more rostral motor levels were related to greater frequencies of CSF diversion and of Chiari decompression (Table S2, online supporting information). For both cohorts individually, the distribution of motor level categories was significantly associated with frequency of CSF diversion (Table S3, online supporting information).
The median age at last visit of the cohort was 4 years (25th–75th centile: 1y 8mo–11y 4mo) and mean age was 6 years 4 months. Mean ages were not significantly different between cohorts (Table 1). The median age at first CSF diversion was 91 days (30–153d) for the fetal surgery cohort, compared with 0 days (0–31d) for the postnatal surgery cohort (p=0.03). We therefore adjusted for age at CSF diversion in the analysis of shunt revisions in shunted patients; this was not applicable to other analyses.
We found that the frequencies components of the CSF diversion variable were: shunt only 86 out of 239 (36%); ETV only 14 out of 239 (6%); and both ETV and shunt 10 out of 239 (4%). In adjusted analyses, fetal surgery was associated with a significantly lower risk of CSF diversion and of Chiari decompression. The differences in frequencies between cohorts were not significant for tethered cord release or for shunt revision in shunted patients (Table 2).
Table 2:
Comparison of neurosurgical procedure outcomes between fetal surgery (n=248) and postnatal surgery (n=698) patients assessed when patients in both cohorts were at least 12 months of age
| Outcome | Fetal surgery, n (%) | Postnatal surgery, n (%) | Unadjusted IRR (95% CI); p | Adjusted IRRa (95% CI); p |
|---|---|---|---|---|
| CSF diversion at last visit (shunted or ETV)a | 110/239 (46) | 349/441 (79) | 0.58 (0.50–0.67); <0.01 | 0.61 (0.53–0.71); <0.01 |
| Shunt revision ever/shunted patients at last visitb,c | 51/96 (53) | 185/336 (55) | 0.89 (0.71–1.12); 0.32 | 0.86 (0.67–1.10); 0.23 |
| Chiari decompressionb | 10/298 (3) | 45/648 (7) | 0.50 (0.26–0.96); 0.04 | 0.41 (0.19–0.88); 0.02 |
| Tethered cord releaseb | 54/298 (18) | 102/648 (16) | 1.22 (0.94–1.59); 0.13 | 1.11 (0.84–1.47); 0.46 |
All patients had myelomeningocele, were born 1997 through 2017, and were enrolled in the National Spina Bifida Patient Registry (NSBPR) 2009 through 2017. Outcomes were assessed at last visit recorded in the Registry. Univariable Poisson regression was used for unadjusted analyses. Multivariable Poisson regression was used to adjust for the covariates of non-Hispanic white, insurance status, motor function segmental level, and age at last visit in the NSBPR, in determining adjusted incidence rate ratios (IRRs), 95% confidence intervals (CIs), and significance of differences.
In matched patients ≥12 months old.
In patients of all ages.
For shunt revision in shunted patients, age at shunt insertion was also a covariate.
DISCUSSION
Our comparative effectiveness study found that CSF diversion frequency at age 12 months or older was lower after fetal surgery (46%) than after postnatal surgery (79%), in concordance with the main finding of the MOMS. It thereby directly and independently addressed the generalizability of this MOMS finding. Fetal surgery has been found to reverse fetal hindbrain herniation more frequently than postnatal surgery.13,15,20 Reversal of fetal hindbrain herniation was associated with absence of postnatal hydrocephalus, suggesting a mechanism for the lower frequency in CSF diversion frequency after fetal surgery.20 Neither Tulipan et al.,14 reporting results from the MOMS, nor Flanders et al.20 found an association between anatomic levels of lesion and CSF diversion frequency in either their fetal surgery cohort, or in their postnatal surgery cohort. In contrast, we found significant associations between distributions of patients in categories of motor levels and CSF diversion frequencies in both cohorts (Table S3). We therefore adjusted for category of motor level in this analysis. In common with previous reports,10,20 we found that the age at CSF diversion was significantly older in fetal surgery patients.
We found a significantly lower frequency of Chiari decompression after fetal surgery (10 out of 298, 3%) than after postnatal surgery (45 out of 648, 7%). In contrast, Houtrow et al.,15 studying MOMS outcomes at school age, did not (3 out of 79, 4% vs 9 out of 82, 11%). The most likely explanation for this discrepancy is the greater statistical power of our study from its larger study population. Kim et al.26 found that more rostral motor level categories were associated with more frequent Chiari decompression, a finding that we confirmed and adjusted for in this analysis.
In our study, the frequencies of shunt revisions in shunted patients were not significantly different between cohorts. Flanders et al. also found no significant difference in shunt revision frequency between cohorts.20 Houtrow et al.,15 studying frequencies of shunt insertion ever in MOMS school-age children, found that significantly fewer fetal surgery participants had had shunt revisions compared to postnatal surgery participants.15 The reasons for this discordance are unclear. We matched for clinic site to control partially for the wide variation in indications for shunt revision in myelomeningocele among pediatric neurosurgeons,33 reasoning from experience that neurosurgeons in the same clinic tend to have similar indications for most procedures. We noted that our difference in frequencies of shunt revision in shunted patients between cohorts was small (53% vs 55%), despite the longer time-at-risk for postnatal surgery patients because of their slightly older age at last visit and the shorter time-at-risk for fetal surgery patients because of their older age at CSF diversion. We found no relationship between shunt revision frequency and motor level.
Finally, in adjusted analysis, we found that tethered cord release was not significantly more frequent in fetal surgery patients. In contrast, Houtrow et al. found a higher frequency of tethered cord release in the MOMS fetal surgery cohort.15 The frequency of symptomatic tethered cord increases through childhood,6 but this is unlikely to be the sole explanation for this discrepancy in findings, given the closeness of the mean ages at ascertainment of outcomes in our study and in Houtrow et al.15 (6y 4mo vs 7y 10mo). We found no relationship between motor level and frequency of tethered core release in the study population.
Calling our study a comparative effectiveness study is justified by relevant variations in clinical practice. Variations in fetal exclusion criteria for fetal surgery now occur for abnormal fetal DNA analysis, cerebral gray matter heterotopias, cleft lip, and anatomic levels outside of MOMS inclusion criteria.28 Two procedures for fetal surgery are now used for placode closure, via hysterotomy or by fetoscopy. Variations exist also in indications for neurosurgical procedures to treat the comorbidities of myelomeningocele,33 probably because there are no level 1 or 2 guidelines for any such neurosurgical procedure. It is therefore not surprising that variation also exists among spina bifida clinics participating in the NSBPR in frequencies of CSF diversion, ranging from 50% to 97%,25 and in frequencies of Chiari decompression, ranging from 2% to 23%.26 Finally, two procedures are used for CSF diversion: shunt insertion and ETV.
The NSBPR does not collect any prenatal data except the occurrence of fetal surgery. Therefore, we could not match postnatal surgery patients to fetal surgery patients for fetal surgery inclusion criteria. It is likely that this resulted in our postnatal surgery cohort having a greater frequency of patients with fetal surgery exclusion criteria than our fetal surgery cohort. The relevant fetal surgery exclusion criteria were absence of fetal hindbrain herniation, fetal anatomic level outside of MOMS inclusion criteria, fetal kyphosis, chromosomal abnormality, fetal physical anomalies not associated with myelomeningocele, and twin gestation. Literature searches revealed no reports of an association of any fetal surgery exclusion criterion with any of our four neurosurgical outcome procedures, with the exception of absence of fetal hindbrain herniation. Fetal hindbrain herniation was a fetal surgery exclusion criterion because it was thought to be associated with a lower frequency of postnatal hydrocephalus.5,34 There was no direct evidence for this until recently, when Nagaraj et al. found that only 13% (1 out of 8) of patients with no fetal hindbrain herniation had had shunt insertion.35 Flanders et al. found that 23% of their postnatal surgery cohort had absent fetal hindbrain herniation20 and it is likely that it was more frequent in our postnatal cohort as well. It is likely that the greater frequency of patients without fetal hindbrain herniation in our postnatal surgery cohort decreased the frequency of CSF diversion in this cohort, thereby decreasing the magnitude of the difference in frequencies of CSF diversion between cohorts. In spite of this, we found the difference in frequencies of CSF diversion to be significant. To say this another way, although we were not able to exclude patients without fetal hindbrain herniation from our postnatal surgery cohort, if we had been able to, excluding them would only have increased the magnitude of the significant effect, as the remaining infants in the postnatal surgery group would have had an even greater frequency of CSF diversion.
We then searched the literature for evidence that fetal anatomic levels of lesion were associated with the frequencies of any neurosurgical procedure in patients who had had postnatal surgery. For only one outcome, CSF diversion, had this been studied. No associations were found between fetal anatomic levels and CSF diversion frequencies in postnatal surgery cohorts in two studies.14,20
Because there are no data to support the idea that being unable to match our postnatal surgery patients to fetal surgery patients for fetal surgery exclusion affected the validity of our findings, we concluded that such matching was not mandatory for our study. This issue could be addressed someday by a consortium of fetal surgery centers using a set of common data elements (now in development36) and following patients prospectively.
Finally, we note that the three most recent post-MOMS single-center, observational studies comparing neurosurgical outcomes in fetal surgery and postnatal surgery cohorts also did not use fetal surgery exclusion criteria for selection of their postnatal surgery cohorts, thereby making them effectiveness studies as well.18–20
Our study may be useful in prenatal counseling and decision making by parents because it independently demonstrated that fetal surgery reduced the frequency of CSF diversion. Further, by studying a large national sample, it also addressed the generalizability of this finding. The analyzed experience of individual fetal surgery centers may also be helpful in decision making, since frequencies of CSF diversion by fetal surgery center vary some-what.18,19 Moreover, if an infant is cared for at a hospital where the CSF diversion frequency is low,37 the advantage of fetal surgery to prevent CSF diversion may be relatively less. Our study also found that social determinants of health were differently distributed between our cohorts and, for some neurosurgical procedures, in the whole study population. This issue needs further study. Whether our study applies to regions of the world with fewer resources and/or a different ethical framework for treating patients with spina bifida is unknown.
The process of decision making is extremely complex38 and must include discussions with parents about risks of fetal surgery to mothers and infants, and prognoses for other outcomes of fetal surgery, including cognitive development,15,39 ambulation,15,39 and bladder function,40 all of which are beyond the scope of this study.
Although the MOMS showed no difference in survival between cohorts in school-age children,15 whether fetal surgery improves life expectancy is unknown. However, our study provided evidence that fetal surgery reduced the frequencies of two major causes of mortality, hydrocephalus41 and brainstem dysfunction from the Chiari II malformation.41,42 Long-term follow-up studies will be necessary to address survival and other related issues.43
Some of our study’s limitations are inherent in the NSBPR. First, the reliability of the data collected was not independently validated. However, Centers for Disease Control and Prevention protocols were in place for data quality control, which included queries to spina bifida clinic sites about questionable entries.26 Second, muscle function, and therefore motor level, cannot be as reliably assessed before 5 years old as after.44 Third, results may not be generalizable to patients who are not cared for in a multidisciplinary spina bifida clinic in the United States or elsewhere. Finally, the NSBPR does not record any prenatal data, except for the occurrence of fetal surgery.
The NSBPR also did not record the site of fetal surgery. However, we note that eight of the 25 spina bifida clinics that contributed patients to our study are at institutions that offer fetal surgery. No single spina bifida clinic dominated the study population. The largest number of fetal surgery patients enrolled by a single spina bifida clinic was 60.
It is a strength of our study that we matched postnatal surgery patients with fetal surgery patients for spina bifida clinic, because there is variation by spina bifida clinic for some outcomes.25,26,33 Further, matching for spina bifida clinic also reduced variation in ascertainment of outcomes. Matching for date of birth (±3mo) controlled for the evolution in the neurosurgical management of comorbid conditions of myelomeningocele.25,26 Other strengths were the adjustments in analyses for covariates, the lack of investigator bias in ascertaining outcomes, and the study’s independence from the MOMS investigators. The potential of using the NSBPR for outcomes studies of fetal surgery versus postnatal surgery was recognized by Flanders et al.20
CONCLUSIONS
In this comparative effectiveness study of fetal surgery versus postnatal surgery utilizing the NSBPR, we found a lower frequency of CSF diversion in fetal surgery patients, independent of ethnicity, insurance status, and spinal segmental level of motor function, and time-at-risk, concordant with the main finding of the MOMS.
Supplementary Material
What this paper adds.
Fetal surgery was associated with lower frequencies of cerebrospinal fluid diversion and decompression of Chiari II malformation than postnatal surgery.
Frequencies of ventriculoperitoneal shunt revision and tethered cord release were not significantly different between cohorts.
ACKNOWLEDGEMENTS
Additional investigators were as follows: Judy Thibadeau (Division of Human Development and Disability, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention [CDC], Atlanta, GA); J Richard Adams (Division of Developmental Behavioral Pediatrics, University of Texas Southwestern, Texas Scottish Rite Hospital, Dallas, TX); and Betsy Hopson (Division of Pediatric Neurosurgery, Children’s Hospital of Alabama, Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, AL, USA).
This study was funded by the CDC under the Cooperative Agreement for Research Approaches to Improve the Care and Outcomes of People Living with spina bifida. The authors thank the many individuals with spina bifida and their family members who participated in this research, without whom the NSBPR would not have been possible. The NSBPR has also been successful because of the contributions of the CDC, the Spina Bifida Association, and all members of the NSBPR Coordinating Committee (Appendix S3, online supporting information). The authors thank the reviewers from the NSBPR and from the CDC for their thoughtful critiques. The authors declare no conflict of interests. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. GW and JSW express their appreciation to the Kenneth and Elaine Jones Family, and the Jones-Guerrero Fund, for their support of the Duke Spina Bifida Clinic and its research. We also appreciate the helpful critiques of Dr Read Pukkila-Worley. This paper is dedicated to the memory of Dr Gregory S Liptak, a distinguished developmental pediatrician, whose vision and leadership were instrumental in establishing the NSBPR.
ABBREVIATIONS
- CSF
Cerebrospinal fluid
- ETV
Endoscopic third ventriculostomy
- MOMS
Management of Myelomeningocele Study
- NSBPR
National Spina Bifida Patient Registry
Footnotes
Three additional investigators are listed in the Acknowledgements.
SUPPORTING INFORMATION
The following additional material may be found online:
Appendix S1: Myelomeningocele and spina bifida aperta.
Appendix S2: Methods.
Appendix S3: Members of the NSBPR Coordinating Committee.
Figure S1: Fetal and postnatal surgery patients by spina bifida clinic site of care.
Table S1: Unadjusted associations between demographic and clinical characteristics, and neurosurgery procedure outcomes, in the study population of fetal surgery and matched postnatal surgery patients
Table S2: Unadjusted associations between motor function spinal segmental level categories and neurosurgical procedure outcomes in the study population of fetal surgery and matched postnatal surgery patients
Table S3: Unadjusted associations of distributions of categories of spinal segmental levels of motor function (motor levels) with frequencies of CSF diversion in fetal surgery patients and separately in matched postnatal surgery patients
DATA AVAILABILITY STATEMENT
Data subject to third party restrictions.
REFERENCES
- 1.Mitchell LE, Adzick NS, Melchionne J, et al. Spina bifida. Lancet 2004; 364: 1885–95. [DOI] [PubMed] [Google Scholar]
- 2.Danzer E, Joyeux L, Flake AW, Deprest J. Fetal surgical intervention for myelomeningocele: lessons learned, outcomes, and future implications. Dev Med Child Neurol 2020; 62: 417–25. [DOI] [PubMed] [Google Scholar]
- 3.Dias MS. Neurosurgical management of myelomeningocele (spina bifida). Pediatr Rev 2005; 26: 50–60. [DOI] [PubMed] [Google Scholar]
- 4.Warf BC, Campbell JW. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment of hydrocephalus for infants with myelomeningocele: long-term results of a prospective intent-to-treat study in 115 East African infants. J Neurosurg Pediatr 2008; 2: 310–6. [DOI] [PubMed] [Google Scholar]
- 5.McLone DG, Knepper P. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci 1989; 15: 1–12. [DOI] [PubMed] [Google Scholar]
- 6.Bowman RM, Mohan A, Ito J, Seibly JM, McLone DG. Tethered cord release: a long-term study in 114 patients. J Neurosurg Pediatr 2009; 3: 181–7. [DOI] [PubMed] [Google Scholar]
- 7.Tulipan N, Bruner JP. Myelomeningocele repair in utero: a report of three cases. Pediatr Neurosurg 1998; 28: 177–80. [DOI] [PubMed] [Google Scholar]
- 8.Adzick NS, Sutton LN, Crombleholme TM, Flake AW. Successful fetal surgery for Spina Bifida. Lancet 1998; 352: 1675–6. [DOI] [PubMed] [Google Scholar]
- 9.Sutton LN, Adzick NS, Bilaniuk LT, et al. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA 1999; 282: 1826–31. [DOI] [PubMed] [Google Scholar]
- 10.Bruner JP, Tulipan N, Paschall RL, et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA 1999; 282: 1819–25. [DOI] [PubMed] [Google Scholar]
- 11.Tulipan N, Sutton LN, Bruner JP, et al. The effect of intrauterine myelomeningocele repair on the incidence of shunt-dependent hydrocephalus. Pediatr Neurosurg 2003; 38: 27–33. [DOI] [PubMed] [Google Scholar]
- 12.Gross P, Reed GT, Engelmann R, Kestle JR. Hydrocephalus research funding from the National Institutes of Health: a 10-year perspective. J Neurosurg Pediatr 2014; 13: 145–50. [DOI] [PubMed] [Google Scholar]
- 13.Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011; 364: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tulipan N, Wellons JC 3rd, Thom EA, et al. Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. J Neurosurg Pediatr 2015; 16: 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houtrow AJ, Thom EA, Fletcher JM, et al. Prenatal repair of myelomeningocele and school-age functional outcomes. Pediatrics 2020; 145: e20191544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley JS, Antiel RM, Flake AW, et al. Pediatric neurosurgeons’ views regarding prenatal surgery for myelomeningocele and the management of hydrocephalus: a national survey. Neurosurg Focus 2019; 47: E8. [DOI] [PubMed] [Google Scholar]
- 17.Zamłyński J, Olejek A, Koszutski T, et al. Comparison of prenatal and postnatal treatments of spina bifida in Poland–a non-randomized, single-center study. J Matern Fetal Neonatal Med 2014; 27: 1409–17. [DOI] [PubMed] [Google Scholar]
- 18.Cools M, Northam W, Goodnight W, et al. Thirty-day medical and surgical readmission following prenatal versus postnatal myelomeningocele repair. Neurosurg Focus 2019; 47: E14. [DOI] [PubMed] [Google Scholar]
- 19.Riddle S, Huddle R, Lim FY, et al. Morbidity and cost burden of prenatal myelomeningocele repair. J Matern Fetal Neonatal Med published online 25 July 2019, doi: 10.1080/14767058.2019.1645827 (E-pub ahead of print). [DOI] [PubMed] [Google Scholar]
- 20.Flanders TM, Heuer GG, Madsen PJ, et al. Detailed analysis of hydrocephalus and hindbrain herniation after prenatal and postnatal myelomeningocele closure: report from a single institution. Neurosurgery 2020; 86: 637–45. [DOI] [PubMed] [Google Scholar]
- 21.Thibadeau JK, Ward EA, Soe MM, et al. Testing the feasibility of a National Spina Bifida Patient Registry. Birth Defects Res A Clin Mol Teratol 2013; 97: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawin KJ, Liu T, Ward E, et al. The National Spina Bifida Patient Registry: profile of a large cohort of participants from the first 10 clinics. J Pediatr 2015; 166: 444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schechter MS, Liu T, Soe M, et al. Sociodemographic attributes and spina bifida outcomes. Pediatrics 2015; 135: e957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alabi NB, Thibadeau J, Wiener JS, et al. surgeries and health outcomes among patients with spina bifida. Pediatrics 2018; 142: e20173730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim I, Hopson B, Aban I, et al. Treated hydrocephalus in individuals with myelomeningocele in the National Spina Bifida Patient Registry. J Neurosurg Pediatr 2018; 22: 646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim I, Hopson B, Aban I, et al. Decompression for Chiari malformation type II in individuals with myelomeningocele in the National Spina Bifida Patient Registry. J Neurosurg Pediatr 2018; 22: 652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah BR, Drozda J, Peterson ED. Leveraging observational registries to inform comparative effectiveness research. Am Heart J 2010; 160: 8–15. [DOI] [PubMed] [Google Scholar]
- 28.Moise KJ Jr, Moldenhauer JS, Bennett KA, et al. Current selection criteria and perioperative therapy used for fetal myelomeningocele surgery. Obstet Gynecol 2016; 127: 593–7. [DOI] [PubMed] [Google Scholar]
- 29.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–6. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280: 1690–1. [DOI] [PubMed] [Google Scholar]
- 31.Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr 2015; 169: e153785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ 2012; 184: 895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alford EN, Hopson BD, Safyanov F, et al. Care management and contemporary challenges in spina bifida: a practice preference survey of the American Society of Pediatric Neurosurgeons. J Neurosurg Pediatr 2019; 24 (5): 539–48 (E-pub ahead of print). [DOI] [PubMed] [Google Scholar]
- 34.Walsh DS, Adzick NS, Sutton LN, Johnson MP. The rationale for in utero repair of myelomeningocele. Fetal Diagn Ther 2001; 16: 312–22. [DOI] [PubMed] [Google Scholar]
- 35.Nagaraj UD, Bierbrauer KS, Zhang B, Peiro JL, Kline-Fath BM. Hindbrain herniation in Chiari II malformation on fetal and postnatal MRI. AJNR Am J Neuroradiol 2017; 38: 1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altoukhi S, Whitehead CL, Ryan G, et al. Development of a Core outcome set for fetal Myelomeningocele (COSMiC): study protocol. Trials 2020; 21: 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakraborty A, Crimmins D, Hayward R, Thompson D. Toward reducing shunt placement rates in patients with myelomeningocele. J Neurosurg Pediatr 2008; 1: 361–5. [DOI] [PubMed] [Google Scholar]
- 38.Van Calenbergh F, Joyeux L, Deprest J. Maternal-fetal surgery for myelomeningocele: some thoughts on ethical, legal, and psychological issues in a Western European situation. Childs Nerv Syst 2017; 33: 1247–52. [DOI] [PubMed] [Google Scholar]
- 39.Farmer DL, Thom EA, Brock JW 3rd, et al. The Management of Myelomeningocele Study: full cohort 30-month pediatric outcomes. Am J Obstet Gynecol 2018; 218: 256. e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brock JW 3rd, Thomas JC, Baskin LS, et al. Effect of prenatal repair of myelomeningocele on urological outcomes at school age. J Urol 2019; 202: 812–8. [DOI] [PubMed] [Google Scholar]
- 41.Oakeshott P, Hunt GM, Poulton A, Reid F. Expectation of life and unexpected death in open spina bifida: a 40-year complete, non-selective, longitudinal cohort study. Dev Med Child Neurol 2010; 52: 749–53. [DOI] [PubMed] [Google Scholar]
- 42.Worley G, Schuster JM, Oakes WJ. Survival at 5 years of a cohort of newborn infants with myelomeningocele. Dev Med Child Neurol 1996; 38: 816–22. [DOI] [PubMed] [Google Scholar]
- 43.Oakeshott P, Reid F, Poulton A, et al. Neurological level at birth predicts survival to the mid-40s and urological deaths in open spina bifida: a complete prospective cohort study. Dev Med Child Neurol 2015; 57: 634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald CM, Jaffe KM, Shurtleff DB. Assessment of muscle strength in children with meningomyelocele: accuracy and stability of measurements over time. Arch Phys Med Rehabil 1986; 67: 855–61. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data subject to third party restrictions.


