Abstract
Growing evidence suggests that oxytocin (OT) plays an important factor for the control of food intake, body weight, and energy metabolism in human and non-human animals. It has reported previously, the downregulation in oxytocin receptors (OTRs) expression is linked with the development of obesity, but exogenous OT reverse body weight and food intake in obese animal model. It is important to know that, whether intraperitoneal administration crosses blood brain barrier. Therefore, in the present experiment, we study the impact of intraperitoneal administration of synthetic OT 0.0116 mg/kg and antagonist atosiban (OTA) 1 mg/kg on food intake, and body weight of female mice, Mus musculus for different duration i.e. 30, 60, and 90 days. In this study, it was observed that there was significant decrease (p<0.001, one-way analysis of variance [ANOVA]) in the body weight (BW), food intake, and gonadosmatic indices (GSI) after the intraperitoneal exposure of OT at dose 0.0116 mg/kg up to 90 days and inhibits via antagonist atosiban. These results indicates that intraperitoneal administration of OT can be used for treatment for longer duration without any side effects and maintains homeostasis in physiologic system regulates body weight and gonadal weight in female mice, which represent an important therapeutic tool for the obesity and metabolic disorder in female.
Keywords: OT, Oxytocin; OTRs, Oxytocin Receptors; OTA, Antagonist Atosiban; ANOVA, One-Way Analysis of Variance; BW, Body Weight; GSI, Gonadosomatic Indices; PVN, Paraventricular Nuclei; SON, Supraoptic Nuclei; GPCR, G-Protein Coupled Receptor; AN, Arcuate Nucleus; VTA, Ventral Tegmental Area; NTS, Nucleus Tractus Solitarius; SIM1, Single Minded 1 Gene; BBB, Blood Brain Barrier; CNS, Central Nervous System; ICV, Intracerebroventricular; I.P., Intraperitoneal; SEM, Standard Error of Mean; GI, Gastrointestinal; VP, Vasopressin; HPG, Hypothalamic-Pituitary-Gonadal Axis; PCOS, Polycystic Ovary Syndrome
Keywords: Oxytocin, Body weight, Food intake, Gonadosomatic indices, Energy metabolism
1. Introduction
Oxytocin (OT) is a hypothalamic peptide synthesized by paraventricular (PVN) and supraoptic nuclei (SON) [1] binds with oxytocin receptor (OTRs) the member of G-protein coupled receptor (GPCR) and regulates different physiological function in central and periphery system. The oxytocinergic neurons of PVN and SON release oxytocin through dendritic diffusion to different regions of the brain i.e. the arcuate nucleus (AN) [2], ventral tegmental area (VTA) [3], nucleus accumbens [4], spinal cord [5,6], and nucleus tractus solitarius (NTS) [6,7] and play an important role in energy metabolism. Furthermore, magnocellular neurons projection to posterior pituitary and traditionally known for various endocrine and neuroendocrine function [8,9]. It has also reported previously, OT also released in gastrointestinal tract [10] via the activation of OTRs and shows autocrine and paracrine function [11,12]. Various evidences suggest that the downregulation in OTRs expression is linked with the development of obesity. Along with this, haploinsufficiency of single minded 1 (SIM1) gene necessary for the formation of PVN, results downregulation in the OTRs in hypothalamus and associated with hyperphagic obesity and significant increase in susceptibility to diet induced obesity in mice [[13], [14], [15], [16]] and human [17,18], but exogenous OT reverses weight gain as well as excessive food intake [14]. These studies suggest that the OT signaling plays an important role in energy metabolism regulation, and supported by a study on mice deficient with either OT [19] or OTRs [20] results in the development of obesity in later stage.
It has also reported in previous research exogenous administration of OT by central or peripheral route in mice, monkey, and rat showed decrease in body weight, increasing energy expenditure, and enhance lipolysis [5,[21], [22], [23], [24], [25], [26]], indicates therapeutic targets for obesity and metabolic disorders. But, peripheral administration of OT mimics the effects of centrally administered OT [27] and it is important to know that how peripheral OT transfer information to the brain to inhibit feeding. But, it have also been the matter of debate how OT reaches brain area, whether it crosses blood brain barrier (BBB). BBB is a semipermeable border composed of an endothelial membrane and bound together by tight junctions, astrocytes, pericytes, microglia, and distinct basement membranes which separate circulating blood from brain and extracellular fluid in the central nervous system (CNS) as well as exclude molecules and pathogens from the brain [28,29]. Even though, previous research suggests that BBB may be permeable to certain peptide [30] and exact mechanism is not understood how peptides crosses the BBB. The half life of circulating OT is very small nearly 1∼2min [31] and only 0.002% peripheral OT reaches to the brain [32]. Interestingly, it has been reported intraperitoneal (i.p.) administration of OT causes upregulation in c-fos expression in certain areas of hindbrain including, NTS, and AP binding with OTRs, these areas are known for the control of meal size [22,23].
However, large body of animal research showed intracerebroventricular (ICV) treatment of OT decreased food intake in many species including mice [33], rat [34], birds [35] and reversed by OTRs antagonist [21]. But, ICV treatment method is impractical in human trail. Therefore, in the present experiment, we study the impact of intraperitoneal administration of synthetic OT and antagonist atosiban (OTA) on food intake, and body weight of female mice, Mus musculus and the findings might be helpful for the future therapeutic targets for obesity and metabolic disorder in clinical investigation.
2. Material & methods
Animals: Seven to eight week old mature female mice, Mus musculus weighing 25 ± 5 g was used in present study. All test animals was housed in temperature controlled condition i.e. 25 ± 3⸰C under 12:12h light and dark photoperiod. The animals had ad libitum access to water and standard chow diet. All the experimental work was done with the approval of the university ethical committee i.e. Institutional Animal Ethics Committee (IAEC) of CPCSEA, (Ref No.1885/GO/S/16/CPCSEA/IAEC/B.U./19).
2.1. Drug and dose
Oxytocin (OT) 0.0116 mg/kg and antagonist atosiban (OTA) 1 mg/kg was prepared separately in 0.89% normal saline as we described previously [36].
2.2. Experimental Procedure
Animal were divided into four groups of eighteen each. The group 1st i.e. control group received a standard diet, water ad libitum, and normal saline (0.89%) for 30, 60, and 90 days, while Group 2nd received a standard diet, water ad libitum and was treated daily with OT 0.0116 mg/kg intraperitoneally (i.p.) for 30, 60, and 90 days. However, group 3rd also received a standard diet, water ad libitum, and were treated daily with OT 0.0116 mg/kg along with OTA 1 mg/kg i.p. for 30, 60, and 90 days. Apart from this, group 4th received a standard diet, water ad libitum, and was treated daily only with OTA 1 mg/kg i.p. for 30, 60, and 90 days.
2.3. Body weight and food intake study
The initial body weight of all animals i.e. Mus musculus were taken with the help of laboratory weighing balance on day first i.e. 0 days of experiment and final body weight was observed after the completion of different intervals i.e. 30, 60, and 90 days treatment of all the animals and then sacrificed. Abdominal cavity was opened and ovary were carefully removed and weighed for the assay of gonadosomatic indices. Food intake was determined by placing preweighed standard food pellets in cages and weighing the remained food after 24 h in individual cages.
2.4. Statistical analysis
Data are expressed as the mean and standard error of mean (SEM) of different groups. Statistical analysis was measured using a one way analysis of variance (ANOVA) followed by Tukey’s test [37]. P value of <0.001 and < 0.05 were considered statistically highly significant and significant respectively.
3. Results
Intraperitoneal administration of oxytocin (OT) (0.0116 mg/kg) in 30, days treated group showed significant decrease (p<0.05, one way analysis of variance [ANOVA]) in final BW as compared to initial body weight (BW). But, the animal treated with OT for 60, and 90 days showed highly significant (p<0.001, one way analysis of variance [ANOVA]) decrease in BW as compared to initial BW. Furthermore, when antagonist atosiban (OTA) (1 mg/kg) was administered with OT and OTA alone for 30, 60, and 90 days there were also decrease in body weight as compared to initial BW (Fig. 1).
Fig. 1.
Comparison of Body weight (BW) (g) after different duration i.e. 30, 60, and 90 days treatment of Oxytocin (OT), Oxytocin (OT)+Oxytocin antagonist Atosiban (OTA), Oxytocin Antagonist Atosiban (OTA) alone and Control female animal Mus musculus. Data represent mean ± SEM values (n = 6 per group), *p< 0.05, ***p< 0.001, NS = non significant from the control vs. treated by one way ANOVA.
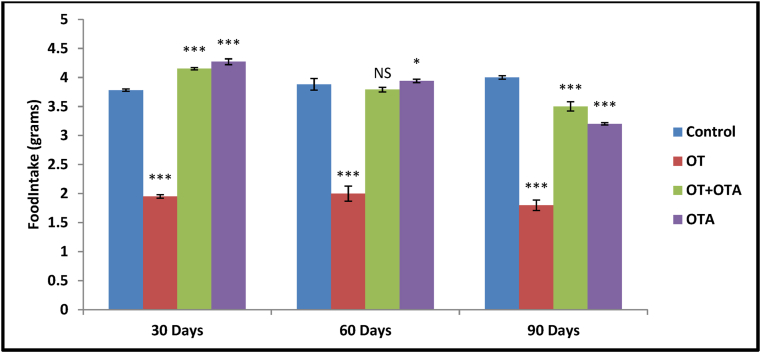
Along with this, OT administration for different duration i.e. 30, 60, and 90 days showed significant decrease (p<0.001, one way analysis of variance [ANOVA]) in food intake in female animal as compared to control group. But significant increase in food intake was observed when OTA administered along with OT for 30, 60 and 90 days group (Fig. 2).
Fig. 2.
Comparison of Food Intake (grams) after different duration i.e. 30, 60, and 90 days treatment of Oxytocin (OT), Oxytocin (OT)+Oxytocin antagonist Atosiban (OTA), Oxytocin Antagonist Atosiban (OTA) alone and Control female animal Mus musculus. Data represent mean ± SEM values (n = 6 per group), *p< 0.05, ***p< 0.001, NS = non significant from the control vs. treated by one way ANOVA.
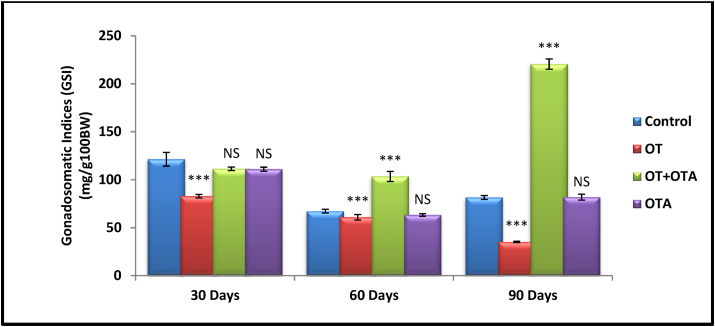
Apart from this, OT administration for different duration i.e. 30, 60, and 90 days showed significant decrease (p<0.001, one way analysis of variance [ANOVA]) in GSI in female animal as compared to control group. But significant recovery was observed when OTA administered along with OT for 60 and 90 days, and no significant in 30 days group. Apart from this, the animals treated with OTA only for 30, 60, and 90 days showed no significant alteration in gonadosomatic indices (GSI) when compared to control group (Fig. 3).
Fig. 3.
Comparison of Gonadosomatic indices (GSI) (mg/g100BW) after different duration i.e. 30, 60, and 90 days treatment of Oxytocin (OT), Oxytocin (OT)+Oxytocin antagonist Atosiban (OTA), Oxytocin Antagonist Atosiban (OTA) alone and Control female animal Mus musculus. Data represent mean ± SEM values (n = 6 per group), *p< 0.05, ***p< 0.001, NS = non significant from the control vs. treated by one way ANOVA.
4. Discussion
Limbic system plays an important role in behavioral organization in mammalian brain which is essential for survival of the organism like, feeding, drinking, defense or attack behavior etc. Hypothalamus is the important part of the limbic system responsible for integrating information to maintain homeostasis in physiological system including, blood pressure, fluid balance, thermal regulation, and body weight (BW) [38,39]. Research indicates oxytocin receptors (OTRs) present in hypothalamic nuclei [40] are responsible for regulation of food intake and energy expenditure in human [[41], [42], [43]] as well as in nonhuman primates [[44], [45], [46]]. In the present study intraperitoneal (i.p.) administration of oxytocin (OT) in female mice showed significantly decrease in final BW as compared to initial BW in 30, 60, and 90 days of treatment and agrees with the finding of Maejima and co-authors [47] who observed reduction in BW after peripheral administration in female mice as compared to initial BW. This reduction has been linked to degradation of visceral and subcutaneous fat mass. It is known that OTRs are present in adipocytes [48], and preclinical study indicates activation of these receptor induce lipolysis and oxidation of fat which reduced BW and body fat [25,49]. Therefore, OT has drawn attention of scientist towards its therapeutic potential for the treatment of obesity, hyperphagia, and autism. Furthermore, [50] reported that i.p. treatment of OT results upregulation in c-fos expression in certain areas of hindbrain including, nucleus tractus solitarius (NTS), and area postrema (AP), these areas are known for the control of meal size. Interestingly, NTS, and AP are the areas of hindbrain where blood-brain-barrier (BBB) is leaky and OTRs are highly expressed [51]. Therefore, it is acceptable long duration exposure of OT at high dose could directly reduce food intake and BW and increasing sensitivity to satiety signals by acting on NTS, and AP [52,53].
Nevertheless, OTRs are also present in gastrointestinal (GI) tract [10], and responsible for autocrine and paracrine effects of OT [11,12,54]. OT action in peripheral system has also been implicated as modulator of appetitive behaviours [49]. Systemic infusion of OT accelerated gastric empting time and increased colonic peristalsis in healthy female [55,56], however OTRs antagonist atosiban (OTA) delays gastric emptying time [57], researcher considered that these effects may be due to the action of vasopressin (VP) receptor with atosiban due to close chemical homology of OTR and VP receptor [58,59] and causes hyperglycemia, nausea, anxiety, and weight loss in female [60].
OTA is a synthetic peptide antagonist, rationally designed to compete with OTRs at myometrial and clinically proved effective tocolytic agent [61]. In addition, OTRs are G-protein coupled receptor (GPCR) and formed by different subunits [31,62] and each subunit can activate different signaling pathway independently [62,63]. Recent data showed that, atosiban a biased agonist activate OTR-Gi signaling and inhibit OTR-Gq [64,65]. Indeed, OTA crosses BBB by intraperitoneal administration [66] and showed central effect on dorsal vagal complex which is involved in regulation of metabolism [67]. In present study, the animal treated with OTA along with OT and OTA alone for 30, 60, and 90 days, showed reduction in final BW as compared to initial BW and could be possible due to biased activity of OTA by activating OTRs signaling in dorsal vagal complex results reduction in BW and meal seize.
In addition to OT involvement in activation of different pathway in central nervous system (CNS), OT plays crucial role in reproduction and acts as a neurotransmitter in the CNS and hormone in peripheral system. Furthermore, reproduction is coordinated by the hypothalamic-pituitary-gonadal (HPG) axis in all vertebrates. Gonads are the main sex steroid producing organs in the body and gonadosomatic indices (GSI) is a tool for analyzing the degree of sexual maturity of animals in co-relation with ovarian development. Number of studies worldwide has reported polycystic ovary syndrome (PCOS) among women and the principle cause of PCOS are endocrine disruption and hormonal imbalance in female due to number of factors such as hyperandrogenemia, insulin resistance, lipid related abnormalities, metabolic syndrome, and obesity showed larger ovarian volume and infertility [[68], [69], [70], [71]]. In our investigation it has been shown that the GSI values of the OT treated female after 30, 60, and 90 days were significantly lower as compared to control group and inhibited via OTA. Which may be due to the reason that OT control metabolism and body weight by increasing energy expenditure, lipolysis and its effects on multiple homeostatic and neurobehavioral pathway [72]. In consistent with this study, [73] have reported that OT level was lower in the PCOS group in comparison to control and systemic administration of OT results ovary volume reduction, BW reduction and balance hormonal profile in women affected by PCOS. Therefore, it is evident that there had been decrease in the gonadal weight in the female treated with OT up to 90 days.
5. Conclusion
Overall, the present investigation reaches its conclusion, that our finding extends previous evidence and suggests that intraperitoneal administration of OT can be used for treatment for longer duration without any side effects and maintains homeostasis in physiologic system regulates body weight and gonadal weight in female mice, which represent an important therapeutic tool for the obesity and metabolic disorder in female.
CRediT authorship contribution statement
Pratibha Thakur: Study design, Writing – original draft, Conceptualization, Resources, Original draft. Renu Shrivastava: Writing – review & editing, Supervision. Vinoy K. Shrivastava: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
NIL.
Acknowledgement
Authors are thankful to the Indian Council of Medical Research (ICMR), New Delhi, India, for financial support in the form of Senior Research Fellowship (SRF) to Dr. Pratibha Thakur, Reference no.45/37/18-PHA/TRANS/BMS.
References
- 1.Vigneaud V du, Ressler C., Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953;205:949–957. doi: 10.1016/S0021-9258(18)49238-1. [DOI] [PubMed] [Google Scholar]
- 2.Maejima Y., Sakuma K., Santoso P., Gantulga D., Katsurada K., Ueta Y., et al. Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS Lett. 2014;588:4404–4412. doi: 10.1016/j.febslet.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Shahrokh D.K., Zhang T.-Y., Diorio J., Gratton A., Meaney M.J. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross H.E., Cole C.D., Smith Y., Neumann I.D., Landgraf R., Murphy A.Z., et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. Epub 2009 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blevins J.E., Baskin D.G. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: insights from rodents, nonhuman primates and humans. Physiol Behav. 2015;52:438–449. doi: 10.1016/j.physbeh.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawchenko P.E., Swanson L.W. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 7.Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Gimpl G., Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 9.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Ohlsson B., Truedsson M., Djerf P., Sundler F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul Pept. 2006;135:7–11. doi: 10.1016/j.regpep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Qin J., Feng M., Wang C., Ye Y., Wang P.S., Liu C. Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats. Neuro Gastroenterol Motil. 2009;21:430–438. doi: 10.1111/j.1365-2982.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- 12.Feng M., Qin J., Wang C., Ye Y., Wang S., Xie D., et al. Estradiol upregulates the expression of oxytocin receptor in colon in rats. Am J Physiol Endocrinol Metab. 2009;296:E1059–E1066. doi: 10.1152/ajpendo.90609.2008. [DOI] [PubMed] [Google Scholar]
- 13.Kublaoui B.M., Jr J.L.H., Gemelli T., Zinn A.R. Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Mol Endocrinol. 2006;20:2483–2492. doi: 10.1210/me.2005-0483. [DOI] [PubMed] [Google Scholar]
- 14.Kublaoui B.M., Gemelli T., Tolson K.P., Wang Y., Zinn A.R. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holder J.L., Zhang L., Kublaoui B.M., DiLeone R.J., Oz O.K., Bair C.H., et al. Sim1 gene dosage modulates the homeostatic feeding response to increased dietary fat in mice. Am J Physiol Endocrinol Metab. 2004;287:E105–E113. doi: 10.1152/ajpendo.00446.2003. [DOI] [PubMed] [Google Scholar]
- 16.Tolson K.P., Gemelli T., Gautron L., Elmquist J.K., Zinn A.R., Kublaoui B.M. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci. 2010;30:3803–3812. doi: 10.1523/JNEUROSCI.5444-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung C.-C.C., Luan J., Sims M., Keogh J.M., Hall C., Wareham N.J., et al. Studies of the SIM1 gene in relation to human obesity and obesity-related traits. Int J Obes. 2007;31:429–434. doi: 10.1038/sj.ijo.0803443. [DOI] [PubMed] [Google Scholar]
- 18.Holder J.L., Jr., Butte N.F., Zinn A.R. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- 19.Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obes (Silver Spring) 2009;17:980–984. doi: 10.1038/oby.2009.12. [DOI] [PubMed] [Google Scholar]
- 20.Takayanagi Y., Kasahara Y., Onaka T., Takahashi N., Kawada T., Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G., Bai H., Zhang H., Dean C., Wu Q., Li J., et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011;69:523–535. doi: 10.1016/j.neuron.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton G.J., Thatcher B.S., Reidelberger R.D., Ogimoto K., Wolden-Hanson T., Baskin D.G., et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab. 2012;302:E134–E144. doi: 10.1152/ajpendo.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maejima Y., Iwasaki Y., Yamahara Y., Kodaira M., Sedbazar U., Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY) 2011;3:1169–1177. doi: 10.18632/aging.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasa T., Matsuzaki T., Mayila Y., Yanagihara R., Yamamoto Y., Kawakita T., et al. Oxytocin treatment reduced food intake and body fat and ameliorated obesity in ovariectomized female rats. Neuropeptides. 2019;75:49–57. doi: 10.1016/j.npep.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Deblon N., Veyrat-Durebex C., Bourgoin L., Caillon A., Bussier A.-L., Petrosino S., et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blevins J.E., Graham J.L., Morton G.J., Bales K.L., Schwartz M.W., Baskin D.G., et al. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2015;308:R431–R438. doi: 10.1152/ajpregu.00441.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasaki Y., Maejima Y., Suyama S., Yoshida M., Arai T., Katsurada K., et al. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. Am J Physiol Regul Integr Comp Physiol. 2015;308:R360–R369. doi: 10.1152/ajpregu.00344.2014. [DOI] [PubMed] [Google Scholar]
- 28.Almutairi M.M., Gong C., Xu Y.G., Chang Y., Shi H. Factors controlling permeability of the blood-brain barrier. Cell Mol Life Sci. 2016;73:57–77. doi: 10.1007/s00018-015-2050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daneman R., Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastin J.A., Pan W. Concepts for biologically active peptides. Curr Pharmaceut Des. 2010;16:3390–3400. doi: 10.2174/138161210793563491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimpl G., Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 32.Mens W.B., Witter A., Greidanus TB. van W. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983 doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 33.Lee J., Moon H., Lee H., Oh Y., Kim C., Lee Y.H., et al. Antagonistic interaction between central glucagon-like Peptide-1 and oxytocin on diet-induced obesity mice. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson B.R., Drutarosky M.D., Chow M.S., Hruby V.J., Stricker E.M., Verbalis J.G. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–118. doi: 10.1016/0196-9781(91)90176-. [DOI] [PubMed] [Google Scholar]
- 35.Jonaidi H., Oloumi M.M., Denbow D.M. Behavioral effects of intracerebroventricular injection of oxytocin in birds. Physiol Behav. 2003;79:725–729. doi: 10.1016/s0031-9384(03)00145-8. [DOI] [PubMed] [Google Scholar]
- 36.Thakur P., Shrivastava R., Shrivastava V.K. Effects of exogenous oxytocin and atosiban antagonist on GABA in different region of brain. IBRO Rep. 2019;6:185–189. doi: 10.1016/j.ibror.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tukey J. Comparing individual mean in the analysis of variance. Biometrics. 1949;5:99–114. [PubMed] [Google Scholar]
- 38.Luiten P.G., Horst GJ ter, Steffens A.B. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol. 1987;28:1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- 39.Henry B.A. Encycl LIFE Sci John Wiley Sons, Ltd WwwElsNet; 2007. Hypothalamic control of food intake and body weight. [DOI] [Google Scholar]
- 40.Williams G., Bing C., Cai X.J., Harrold J.A., King P.J., Liu X.H. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 41.Boccia M.L., Petrusz P., Suzuki K., Marson L., Pedersen C.A. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 42.Loup F., Tribollet E., Dubois-Dauphin M., Dreifuss J.J. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- 43.Loup F., Tribollet E., Dubois-Dauphin M., Pizzolato G., Dreifuss J.J. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res. 1989;500:223–230. doi: 10.1016/0006-8993(89)90317-x. [DOI] [PubMed] [Google Scholar]
- 44.Boccia M.L., Panicker A.K., Pedersen C., Petrusz P. Oxytocin receptors in non-human primate brain visualized with monoclonal antibody. Neuroreport. 2001;12:1723–1726. doi: 10.1097/00001756-200106130-00041. [DOI] [PubMed] [Google Scholar]
- 45.Freeman S.M., Inoue K., Smith A.L., Goodman M.M., Young L.J. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schorscher-Petcu A., Dupré A., Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Maejima Y., Aoyama M., Sakamoto K., Jojima T., Aso Y., Takasu K., et al. Impact of sex, fat distribution and initial body weight on oxytocin's body weight regulation. Sci Rep. 2017;7:8599. doi: 10.1038/s41598-017-09318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gajdosechova L., Krskova K., Olszanecki R., Zorad S. Differential regulation of oxytocin receptor in various adipose tissue depots and skeletal muscle types in obese zucker rats. Horm Metab Res. 2015;47:600–604. doi: 10.1055/s-0034-1395677. [DOI] [PubMed] [Google Scholar]
- 49.Lawson E.A. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol. 2017;13:700–709. doi: 10.1038/nrendo.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maejima Y., Iwasaki Y., Yamahara Y., Kodaira M., Sedbazar U., Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY) 2011;3:1169–1177. doi: 10.18632/aging.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida M., Takayanagi Y., Inoue K., Kimura T., Young L.J., Onaka T., et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blevins J.E., Schwartz M.W., Baskin D.G. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 53.Perello M., Raingo J. Leptin activates oxytocin neurons of the hypothalamic paraventricular nucleus in both control and diet-induced obese rodents. Comp Study PLoS One. 2013;8 doi: 10.1371/journal.pone.0059625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.L G., S N. Oxytocin - the sweet hormone? Trends Endocrinol Metabol. 2017;28:365–376. doi: 10.1016/j.tem.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Ohlsson B., Ringström G., Abrahamsson H., Simrén M., Björnsson E.S. Oxytocin stimulates colonic motor activity in healthy women. Neuro Gastroenterol Motil. 2004;16:233–240. doi: 10.1111/j.1365-2982.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 56.Petring O.U. The effect of oxytocin on basal and pethidine-induced delayed gastric emptying. Br J Clin Pharmacol. 1989;28:329–332. doi: 10.1111/j.1365-2125.1989.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohlsson B., Björgell O., Ekberg O., Darwiche G. The oxytocin/vasopressin receptor antagonist atosiban delays the gastric emptying of a semisolid meal compared to saline in human. BMC Gastroenterol. 2006;6 doi: 10.1186/1471-230X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maggi M., Fantoni G., Baldi E., Cioni A., Rossi S., Vannelli G.B., et al. Antagonists for the human oxytocin receptor: an in vitro study. J Reprod Fertil. 1990;101:345–352. doi: 10.1530/jrf.0.1010345. [DOI] [PubMed] [Google Scholar]
- 59.Rydén G., Andersson R.G., Berg G., Karlsson S.G., Oscarsson Y. Binding of four oxytocin analogues to myometrial oxytocin and arginine-vasopressin binding sites in pregnant women. Gynecol Obstet Invest. 1990;29:6–9. doi: 10.1159/000293289. [DOI] [PubMed] [Google Scholar]
- 60.Worldwide Atosiban versus Beta-agonists Study Group Effectiveness and safety of the oxytocin antagonist atosiban versus beta-adrenergic agonists in the treatment of preterm labour. The worldwide atosiban versus beta-agonists study group. BJOG An Int J Obstet Gynaecol. 2001;108:133–142. [PubMed] [Google Scholar]
- 61.Goodwin T.M., Millar L., North L., Abrams L.S., Weglein R.C., Holland M.L. The pharmacokinetics of the oxytocin antagonist atosiban in pregnant women with preterm uterine contractions. Am J Obstet Gynecol. 1995;173:913–917. doi: 10.1016/0002-9378(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 62.Busnelli M., Saulière A., Manning M., Bouvier M., Galés C., Chini B. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J Biol Chem. 2012;287:3617–3629. doi: 10.1074/jbc.M111.277178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wootten D., Christopoulos A., Marti-Solano M., Babu M.M., Sexton P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat Rev Mol Cell Biol. 2018;19:638–654. doi: 10.1038/s41580-018-0049-3. [DOI] [PubMed] [Google Scholar]
- 64.Williams A.V., Duque-Wilckens N., Ramos-Maciel Stephanie, Campi L.K., et al. Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacology. 2020;1–8 doi: 10.1038/s41386-020-0657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Busnelli M., Bulgheroni E., Manning M., Kleinau G., Chini B. Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. J Pharmacol Exp Therapeut. 2013;346:318–327. doi: 10.1124/jpet.113.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ermisch A., Barth T., Rühle H.J., Skopková J., Hrbas P., Landgraf R. On the blood-brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinol Exp. 1985;19:29–37. [PubMed] [Google Scholar]
- 67.McCann M.J., Rogers R.C. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsilchorozidou T., Overton C., Conway G.S. The pathophysiology of polycystic ovary syndrome. Clin Endocrinol. 2004;60:1–17. doi: 10.1046/j.1365-2265.2003.01842.x. [DOI] [PubMed] [Google Scholar]
- 69.Salehpour S., Esmaeilnia H.S., Entezari A. Evaluation of the prevalence of polycystic ovarian syndrome among adolescent (15-18 Years old) girls in tehran during 2005-2006. Int J Fertil Steril. 2010;4:122–127. [Google Scholar]
- 70.Gharakhani M., Neghab N., Farimani M. Is reducing ovarian volume in polycystic ovarian syndrome patients after administration of metformin associated with improving cardiovascular risk factors? Int J Fertil Steril. 2011;5:90–95. [PMC free article] [PubMed] [Google Scholar]
- 71.Aali B.S., Mahdi S.A., Makhaee N., Soboutipour S., Mehdizadeh A. Are lean and normal weight patients with polycystic ovarian syndrome at risk of preeclampsia? Int J Fertil Steril. 2010;4:5–8. [Google Scholar]
- 72.McCormack S.E., Blevins J.E., Lawson E.A. Metabolic effects of oxytocin. Endocr Rev. 2020;41:121–145. doi: 10.1210/endrev/bnz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.NamavarJahromi B., Dabbaghmanesh M.H., Bakhshaie P., Parsanezhad M.E., Anvar Z., Alborzi M., et al. Assessment of oxytocin level, glucose metabolism components and cutoff values for oxytocin and anti-mullerian hormone in infertile PCOS women. Taiwan J Obstet Gynecol. 2018;57:555–559. doi: 10.1016/j.tjog.2018.06.015. [DOI] [PubMed] [Google Scholar]