Abstract
Purpose:
Interleukin-18 (IL-18) is an immunostimulatory cytokine with antitumor activity in preclinical models. A phase I study of recombinant human IL-18 (rhIL-18) was done to determine the toxicity, pharmacokinetics, and biological activities of rhIL-18 administered at different doses in two different schedules to patients with advanced cancer.
Experimental Design:
Cohorts of three to four patients were given escalating doses of rhIL-18 as a 2-h i.v. infusion either on 5 consecutive days repeated every 28 days (group A) or once a week (group B) forup to 6 months. Toxicities were graded using standard criteria. Blood samples were obtained for safety, pharmacokinetic, and pharmacodynamic measurements.
Results:
Nineteen patients (10 melanoma and 9 renal cell cancer) were given rhIL-18 in doses of 100AE, 500, or 1,000 μg/kg (group A) or 100, 1,000, or 2,000 μg/kg (group B). Common side effects included chills, fever, headache, fatigue, and nausea. Common laboratory abnormalities included transient, asymptomatic grade 1 to 3 lymphopenia, grade 1 to 4 hyperglycemia, grade 1 to 2 anemia, neutropenia, hypoalbuminemia, liver enzyme elevations, and serum creatinine elevations. No dose-limiting toxicities were observed. Biological effects of rhIL-18 included transient lymphopenia and increased expression of activation antigens on lymphocytes. Increases in serum concentrations of IFN-γ, granulocyte macrophage colony-stimulating factor, and IL-18–binding protein were observed following dosing.
Conclusions:
rhIL-18 can be given in biologically active doses by either weekly infusions or daily infusions for 5 days repeated every 28 days to patients with advanced cancer. Toxicity was generally mild to moderate, and a maximum tolerated dose of rhIL-18 by either schedule was not determined.
Interleukin-18 (IL-18) is an immunostimulatory cytokine that regulates both innate and adaptive immune responses (1, 2). IL-18, a member of the IL-1 superfamily of cytokines, is produced by several cell types, including macrophages, dendritic cells, microglial cells, and keratinocytes. The mRNA transcript of the human IL-18 gene encodes a 193–amino acid biologically inactive precursor protein, pro-IL-18. Enzymatic cleavage of the 24-kDa pro-IL-18 polypeptide by caspase-1 yields the biologically active 18-kDa cytokine. The effects of IL-18 are mediated through a specific cell surface receptor complex composed of at least two subunits: an α chain (IL-1Rrp1) and β chain (AcPL; ref. 3). IL-18–binding protein (IL-18 BP), like other circulating binding protein family members such as cytokine-like factor 1 and osteoprotegerin, does not resemble its corresponding cell-bound receptor; however, it can bind IL-18 with high affinity and act as an inhibitor of IL-18 biological activity in vitro and in vivo.
IL-18 enhances the production of IFN-γ by T cells and natural killer cells, augments the cytolytic activity of natural killer cells and CTLs, and promotes the differentiation of activated CD4 T cells into helper effector cells (1, 2). IL-18 acts synergistically with IL-12 to induce IFN-γ production and stimulate Th1 immune responses (4–6). These biological activities indicate that IL-18 may be a particularly useful cytokine for immunotherapy of cancer. Indeed, IL-18 has been found to have significant antitumor activity in preclinical animal models (7–11). Moreover, a previous phase I study has shown that recombinant human IL-18 (rhIL-18) can be given in biologically active doses to patients with cancer (12). In that first-time-in-man study, rhIL-18 was given as a single 5-day course of treatment, and no maximum tolerated dose (MTD) was identified. Thus, additional studies are required to define the optimal dose and schedule of rhIL-18. This report describes the effects of more prolonged therapy with rhIL-18 given i.v. at several escalating doses by two different schedules of administration.
Materials and Methods
Patient selection.
Eligible patients included adults (ages ≥18 y) with histologically confirmed, locally advanced, or metastatic solid tumor or follicular lymphoma that was measurable and refractory to standard therapy or for which no effective therapy was available. Patients were required to have a Karnofsky performance status of at least 70%, an estimated life expectancy of at least 12 wk, hemoglobin ≥9 g/dL, absolute neutrophil count ≥1,500 per μL, platelet count ≥100,000 per μL, serum bilirubin ≤1.5 mg/dL, serum aspartate aminotransferase and alanine aminotransferase ≤3× upper limit of normal, estimated creatinine clearance ≥50 mL/min, and prothrombin and partial thromboplastin ≤1.1× upper limit of normal. Patients with a history of coronary artery disease were required to have a stress test with no clinically significant abnormality. Patients with a history of congestive heart failure, myocardial infarction, or prior anthracycline therapy were required to have a left ventricular ejection fraction of at least 40%. Patients were excluded if they were pregnant or breast-feeding or had severe or uncontrolled infection, known symptomatic or untreated leptomeningeal or brain metastases, significant autoimmune disease, or a history of ventricular arrhythmia requiring drug or device therapy. In addition, patients were required to have no antineoplastic therapy or major surgical procedures within 4 wk before beginning study drug administration and were to have recovered from serious toxicities from prior treatments. Concurrent administration of corticosteroids, chemotherapy, immunotherapy, radiotherapy, or other investigational therapy was not allowed.
Study design.
This open-label, nonrandomized, dose-escalation phase I clinical study was conducted at two centers. The protocol was approved by the Institutional Review Boards at Indiana University Medical Center (Indianapolis, IN) and the Pittsburgh Cancer Institute (Pittsburgh, PA); written informed consent was obtained from each patient before enrollment on study. Study drug SB-485232, a rhIL-18 protein produced in Escherichia coli, was supplied by GlaxoSmithKline. Each dose of rhIL-18 was given by i.v. infusion over 2 h. The dose of rhIL-18 was calculated using actual body weight, except for patients whose actual body weight exceeded their ideal body weight by at least 30%; the dose for the latter patients was calculated using the 130% ideal body weight. Premedication with antipyretics was not given before the first dose of rhIL-18, although after the first dose oral acetaminophen was given for drug-related fever and i.v. meperidine as needed for rigors.
The schedule of rhIL-18 administration was changed during the course of the study. Initially, rhIL-18 was given in 28-d cycles, with each cycle consisting of five consecutive daily infusions of rhIL-18 followed by a 22-d observation period. Such cycles were repeated every 28 d for a maximum of six cycles (maximum planned 30 doses of rhIL-18 per patient). Based on the results of pharmacokinetic and pharmacodynamic studies done on samples obtained from the first 9 patients (group A), the study was amended and the remaining 10 patients (group B) received rhIL-18 infusions once per week for up to 24 consecutive weeks (maximum planned 24 doses of rhIL-18 per patient). If the investigator and sponsor agreed that a patient might be deriving benefit from study drug administration, continuation of treatment beyond cycle 6 or week 24 was allowed for patients without progressive disease or unacceptable toxicity.
Successive cohorts of three to four patients received rhIL-18 in doses of 100, 500, and 1,000 μg/kg (group A) and 100, 1,000, and 2,000 μg/kg (group B). At least three patients were enrolled in each dose cohort and all patients within a dose cohort were followed for at least 14 d (group A) or 4 wk (group B) on study before initiating treatment at the next dose level. If any patient experienced dose-limiting toxicity (DLT) at a particular dose level, then an additional three patients were to be enrolled at that dose level and an additional cohort of three patients was added at a dose level that was 50% of the next planned dose level. Dose escalation was to be halted if more than two patients within a dose cohort experienced DLT. Toxicity was graded using the National Cancer Institute Common Toxicity Criteria version 3.0. DLT was defined as any grade 3 or 4 toxicity assessed to be related to study drug, with the following exceptions: grade 4 neutropenia persisting for <5 d, grade 3 febrile neutropenia, grade 4 lymphopenia, and grade 3 fever managed successfully with antipyretics (unless fever complicated by infection, hypotension, or delirium) and grade 3 nausea or vomiting controlled with supportive care.
Escalation of the rhIL-18 dose within an individual patient was not permitted. Patients who experienced DLT could continue on study as long as the toxicity resolved to grade ≤1 in severity after holding rhIL-18 treatment for no longer than 2 wk. rhIL-18 treatment was resumed with a reduction in dose to the level of the prior dose cohort. Patients who experienced DLT despite dose reduction were to be taken off study.
Pharmacokinetic measurements.
Blood samples were collected and processed for plasma to measure the total (bound + unbound) concentration of rhIL-18 using a specific fluoroimmunoassay method described previously (12). In group A, samples were taken before starting the infusion of rhIL-18 on days 1 to 5 and at 0.67, 1.33, 2, 2.5, 3, and 6 h after starting the infusion on day 1 of cycles 1 and 2. In group B, samples were taken before starting the infusion of rhIL-18 and at 1, 2, 2.5, 3, 4, 6, 8, 24, 48, and 168 h after starting the infusion on weeks 1 and 4.
Pharmacodynamic measurements.
In group A, blood samples were collected prior to and 3 and 6 h after starting the rhIL-18 infusion on days 1 and 5 of cycles 1 and 2 to determine serum concentrations of granulocyte macrophage colony-stimulating factor, IFN-γ, tumor necrosis factor-α, IL-4, IL-6, IL-8, IL-10, IP-10, MCP-1, and MIG using specific ELISA methods and xMAP (Luminex) bead technology. Blood samples were also collected before starting the rhIL-18 infusion on days 1 and 2 of cycles 1 and 2 for measurement of plasma IL-18 BP concentration by a specific ELISA method. Whole blood samples collected prior to and 6, 24, 48, and 168 h after starting the rhIL-18 infusion on day 1 of cycles 1 and 2 were analyzed for leukocyte markers by flow cytometry. Aliquots of blood were incubated with FITC-conjugated, phycoerythrin-conjugated, and peridinin chlorophyll protein–conjugated monoclonal antibodies in erythrocyte lysis buffer, washed, fixed, and analyzed by flow cytometry. The lymphocyte gate was defined by light scatter variables; thresholds for discriminating specific staining above background were established by analysis of samples stained with FITC-conjugated, phycoerythrin-conjugated, and peridinin chlorophyll protein–conjugated isotype control antibodies.
In group B, blood samples were collected prior to and 3, 6, 24, and 48 h after starting the rhIL-18 infusion in weeks 1 and 4 and before and 3 h after the start of rhIL-18 infusion every 4 wk thereafter for determination of serum cytokines. Blood samples were also collected prior to and 24 and 48 h after starting the rhIL-18 infusion in weeks 1 and 4 for measurement of plasma IL-18 BP. Whole blood samples were collected prior to and 6, 24, 48, and 168 h after starting the rhIL-18 infusion in weeks 1 and 4 for analysis of leukocyte markers.
Detection of anti-rhIL-18 antibodies.
For group A, blood samples were collected for detection of antibodies to study drug before enrollment on study, before rhIL-18 infusions on day 1 of each cycle (other than cycle 1), on days 8 and 15 of each cycle, and on the final study visit. For group B, blood samples were collected for detection of antibodies to study drug before enrollment on study, before the rhIL-18 infusion in weeks 1 to 7, 9, 11, 13, 15, 17, 19, 21, and 23, and on the final study visit. Antibodies to rhIL-18 were assessed using an electrochemiluminescence immunoassay that used rhIL-18–coated paramagnetic beads to capture anti-rhIL-18 antibodies. Bound antibodies were detected with ruthenium-conjugated protein A/G (for quantitation of total anti-rhIL-18 antibodies) or ruthenium-conjugated anti-human IgE (for detection of anti-rhIL-18 IgE). Patients with detectable anti-rhIL-18 antibodies at screening were not excluded from participation in the study. However, in that case, they were considered to have treatment-related anti-rhIL-18 antibodies only if their antibody level increased at least 2-fold following administration of rhIL-18. Patients were to be withdrawn if they developed detectable anti-rhIL-18 IgE antibodies at any time during the study. Antibody neutralizing activity was assessed using an in vitro bioassay, which measured inhibition of IL-18–mediated induction of IFN-γ in KG-1 cells in the presence of anti-rhIL-18 antibodies as described previously (12).
Criteria for response evaluation.
Tumor measurements were obtained within 28 d of the first dose of rhIL-18 and approximately every 8 wk while patients were receiving study drug. Tumor responses were assessed using Response Evaluation Criteria in Solid Tumors criteria.
Results
Patient characteristics.
Nineteen patients were enrolled on study, including 13 men and 6 women. The median age was 55 years (range, 41–69 years). The patient demographics were similar in groups A and B. In group A, there were six men and three women enrolled with a median age of 53 years (range, 41–67), whereas in group B there were seven men and three women with a median age of 56.5 years (range, 41–69). The primary tumor was melanoma in 10 patients and renal cell carcinoma in 9 patients. Of the 10 patients with melanoma, 5 (50%) had received prior treatment with IFN, 5 (50%) with chemotherapy, 3 (30%) with IL-2, and 2 (20%) with radiation therapy. Of the nine patients with renal cell carcinoma, eight (89%) had undergone prior nephrectomy and four (44%) had received prior treatment with IFN, three (33%) with IL-2, one (11%) with chemotherapy, and one (11%) with radiation therapy. Common sites of metastatic disease at the time of enrollment on study included lung in 15 (79%), lymph nodes in 10 (53%), liver in 5 (26%), and adrenal gland in 4 (21%) patients.
Administration of rhIL-18.
The doses of rhIL-18 administered in 28-day cycles to patients in group A were 100 μg/kg (n = 3 patients), 500 μg/kg (n = 3 patients), and 1,000 μg/kg (n = 3 patients). The median number of cycles of treatment received by patients in group A was 6 (range, 2–12). Due to disease progression, three patients did not receive the planned six cycles of treatment. After routine restaging evaluations revealed progressive disease, two patients were taken off study following completion of cycles 2 and 4, respectively. One patient was found to have a new brain metastasis after the day 4 infusion of rhIL-18 in cycle 6; the planned day 5 infusion in that cycle was held and the patient was taken off study.
The doses of rhIL-18 administered weekly to patients in group B were 100 μg/kg (n = 4 patients), 1,000 μg/kg (n = 3 patients), and 2,000 μg/kg (n = 3 patients). The median number of weekly infusions of rhIL-18 received by patients in group B was 15.5 (range, 7–32). Due to disease progression, six patients did not receive the planned 24 weeks of treatment. These patients were taken off study after 7, 7, 8, 8, 15, and 16 weeks of treatment, respectively. No patient in either group had to be taken off study due to toxicity of rhIL-18. One patient in group B required a dose reduction due to toxicity as described below.
Toxicity and laboratory abnormalities during study drug administration.
Common side effects associated with rhIL-18 administration included grade 1 to 2 chills, fever, headache, fatigue, and nausea (Table 1). Fever and chills were readily ameliorated with standard supportive care measures (acetaminophen and meperidine as needed). Common laboratory abnormalities associated with rhIL-18 administration included grade 1 to 4 hyperglycemia, grade 1 to 3 alkaline phosphatase elevations, grade 1 to 2 hypoalbuminemia and BUN elevations, and grade 1 elevations in liver transaminases (Table 2). Although cases of grade 3 (two patients) and grade 4 (one patient) hyperglycemia occurred only in patients with preexisting diabetes mellitus, there were cases of grade 1 to 2 hyperglycemia that occurred in patients without a history of diabetes. Generally, the hepatic chemistry abnormalities were grade 1 and none resulted in dose interruption. Common hematologic abnormalities associated with study drug administration included grade 1 to 3 lymphopenia, anemia, and neutropenia (Table 2). Grade 3 anemia and neutropenia were observed in only one patient, and no patient experienced infection attributed to neutropenia. No thrombocytopenia was observed in this study.
Table 1.
Adverse events experienced by more than two patients
| Most frequently reported nonhematologic toxicities (at least two patients) regardless of causality (all cycles, worst toxicity grade by patient) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Adverse event | No. patients with grade 1–2/3* adverse events | All cohorts | |||||
|
| |||||||
| Group A (daily × 5) | Group B (weekly) | ||||||
|
|
|
||||||
| Dose level (μg/kg) | |||||||
|
| |||||||
| 100 | 500 | 1,000 | 100 | 1,000 | 2,000 | ||
|
|
|
|
|
|
|
||
| n = 3 | n = 3 | n = 3 | n = 4 | n = 3 | n = 3 | N = 19 | |
| Chills | 2/0 | 3/0 | 3/0 | 3/0 | 2/0 | 3/0 | 16/0 |
| Fever | 2/0 | 3/0 | 1/1 | 3/0 | 2/1 | 2/0 | 13/2 |
| Headache | 1/1 | 3/0 | 3/0 | 2/0 | 1/0 | — | 10/1 |
| Fatigue | 1/0 | 2/0 | 2/0 | 2/0 | 1/0 | 2/0 | 10/0 |
| Pain (back/extremity) | 3/0 | 5/0 | 1/0 | — | 1/0 | — | 10/0 |
| Pruritus/pruritic rash | 1/0 | 3/0 | 2/0 | 2/0 | — | 1/0 | 9/0 |
| Nausea | 2/0 | 2/0 | 2/0 | 1/0 | 1/0 | — | 8/0 |
| Pain | 1/0 | 2/0 | 2/0 | 2/0 | — | 1/0 | 8/0 |
| Myalgia/arthralgia | 2/0 | 1/0 | 1/0 | — | 1/0 | 3/0 | 8/0 |
| Anorexia | 1/0 | 2/0 | — | 1/0 | 2/0 | 1/0 | 7/0 |
| Vomiting | — | 1/0 | 1/0 | 2/0 | 1/0 | 1/0 | 6/0 |
| Diarrhea | 1/0 | 1/0 | 2/0 | 1/0 | 1/0 | — | 6/0 |
| Cough | 1/0 | 1/0 | 2/0 | — | 1/0 | 1/0 | 6/0 |
| Dizziness | 1/0 | 2/0 | — | 1/0 | 1/0 | 1/0 | 6/0 |
| Insomnia | 2/0 | 2/0 | 1/0 | — | — | — | 5/0 |
| Injection site/skin reaction | 1/0 | — | 3/0 | 1/0 | — | — | 5/0 |
| Abdominal pain/distension | 3/0 | — | 1/0 | — | — | 1/0 | 5/0 |
| Chest pain | 1/0 | — | — | 1/0 | 1/0 | 2/0 | 5/0 |
| Rash/urticaria | 1/0 | 2/0 | 1/0 | — | — | 1/0 | 5/0 |
| Paresthesia/hypoesthesia | 1/0 | 1/0 | 1/0 | — | — | 1/0 | 4/0 |
| Hypotension | 1/0 | 1/0 | — | — | 0/1 | 1/0 | 3/1 |
| Hypertension | — | — | 2/0 | — | — | — | 2/0 |
No grade 4 adverse events were reported.
Table 2.
Common laboratory abnormalities seen during rhIL-18 administration
| Common laboratory abnormalities (all cycles, worst toxicity grade by patient) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Laboratory abnormality | No. patients with grade 1–2/3–4* adverse events | All cohorts | |||||
|
| |||||||
| Group A (daily × 5) | Group B (weekly) | ||||||
|
|
|
||||||
| Dose level (μg/kg) | |||||||
|
| |||||||
| 100 | 500 | 1,000 | 100 | 1,000 | 2,000 | ||
|
|
|
|
|
|
|
||
| n = 3 | n = 3 | n = 3 | n = 4 | n = 3 | n = 3 | N = 19 | |
| Lymphopenia | 1/2 | 0/2 | 2/1 | 0/4 | 0/3 | 0/2 | 3/14 |
| Anemia | 2/0 | 2/0 | 3/0 | 3/1 | 3/0 | 1/0 | 14/1 |
| Leukopenia | 2/0 | 1/0 | 2/0 | 3/0 | 2/0 | 1/0 | 11/0 |
| Neutropenia | 1/0 | 1/0 | 2/0 | 1/0 | 1/1 | — | 6/1 |
| Hyperglycemia | 1/0 | 2/1 | 2/1 | 2/1† | 2/0 | 1/0 | 10/3 |
| Hypoalbuminemia | — | 2/0 | 2/0 | 2/0 | 2/0 | 3/0 | 11/0 |
| Elevated alkaline phosphatase | 2/0 | 1/0 | 1/1 | 1/0 | 2/0 | 2/0 | 9/1 |
| Elevated AST | 2/0 | — | 2/0 | 2/0 | — | 2/0 | 8/0 |
| Elevated creatinine | 1/0 | 1/0 | — | 3/0 | 2/0 | 1/0 | 8/0 |
| Hyponatremia | 1/0 | 1/0 | — | 1/0 | 1/0 | 1/0 | 5/0 |
| Elevated ALT | 1/0 | — | 1/0 | — | — | 2/0 | 4/0 |
| Elevated bilirubin | — | — | 1/0 | 1/0 | 1/0 | 1/0 | 4/0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
All grade 3 with the exception of single episode of grade 4 hyperglycemia.
Grade 4 hyperglycemia.
Only one serious adverse event was attributed to rhIL-18 in this study. A patient with renal cell cancer enrolled in the 1,000 μg/kg dose cohort of group B developed grade 3 hypotension and grade 2 serum creatinine elevation several hours after the week 6 infusion of rhIL-18 had been completed. Hypotension resolved within 48 h after administration of i.v. fluids and the serum creatinine elevation resolved within 7 days. rhIL-18 was held on week 7 and resumed on week 8 with a reduction in dose to 100 μg/kg. The patient tolerated the 100 μg/kg dose well but was taken off study shortly thereafter due to disease progression. At the time the serious adverse event occurred, enrollment in the 2,000 μg/kg cohort of group B had already occurred as per the study design. None of the three patients enrolled in the 2,000 μg/kg cohort experienced DLT. A MTD of rhIL-18, as given by either schedule of administration used in this study, was not determined.
Pharmacokinetic results.
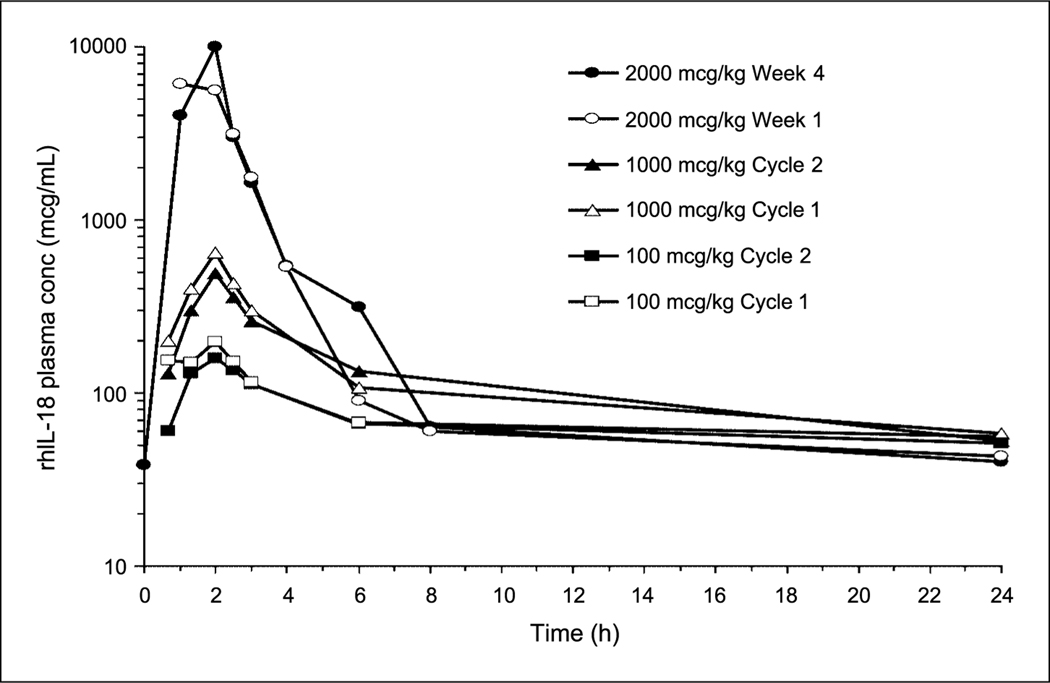
Plasma concentrations of rhIL-18 in group A during the 5 days of dosing in cycle 1 closely resembled those observed at corresponding doses in a previous single-cycle study (12). In addition, plasma concentrations at each dose on day 1 of cycles 1 and 2 were similar, although accumulation over the 5 dosing days in cycle 2 (2-fold) was slightly less than in cycle 1 and the previous study (2.5-fold). In group B, plasma concentrations at each dose were essentially superimposable between weeks 1 and 4 due to complete elimination within the 7 days preceding the next dose. Therefore, systemic exposure to rhIL-18 was comparable between cycles 1 and 2 and between weeks 1 and 4 of dosing (Fig. 1).
Fig. 1.
Representative median plasma rhIL-18 concentration versus time profiles spanning the dose range, showing the similarity in systemic exposure over time, from day 1 of cycle 1 to day 1 of cycle 2 (group A) and from week 1 to week 4 (group B).
Biological effects of rhIL-18.
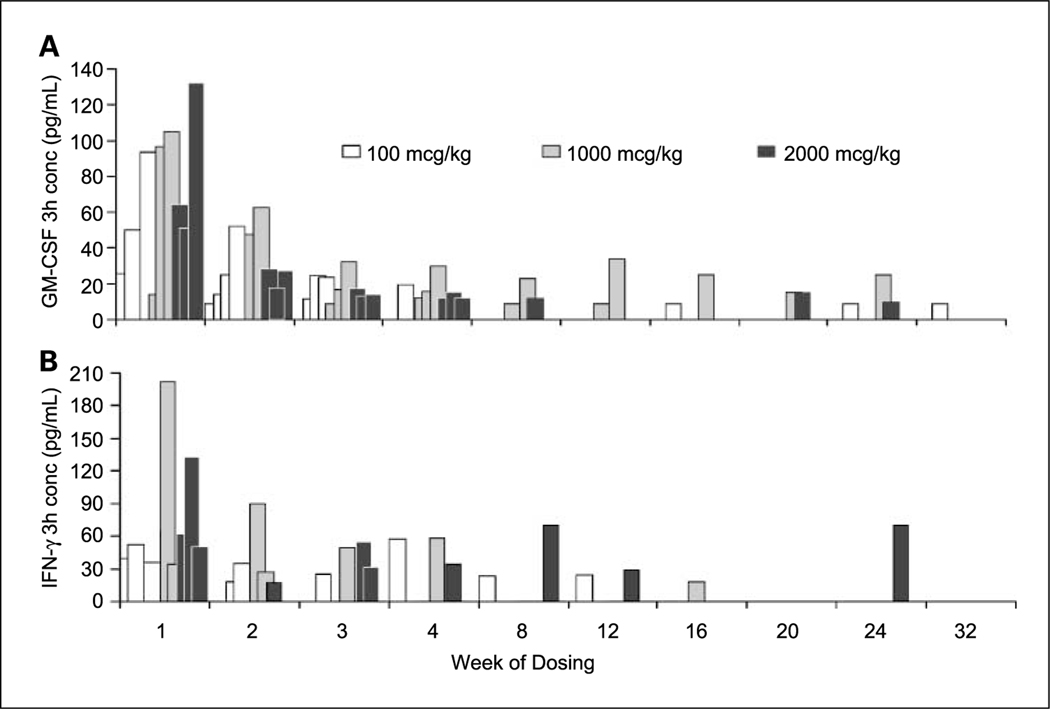
Administration of rhIL-18 produced elevated plasma concentrations of proinflammatory Th1 cytokines IFN-γ (up to 267 pg/mL), granulocyte macrophage colony-stimulating factor (up to 159 pg/mL), and tumor necrosis factor-α (up to 185 pg/mL); CXC chemokines IP-10 (up to 5.62 ng/mL) and MIG (up to 2.59 ng/mL), known to play an important role in the recruitment and migration of activated Th1 lymphocytes; and the CC chemokine MCP-1 (up to 23.4 ng/mL), important in recruiting macrophages and monocytes to sites of inflammation. However, all of these effects were attenuated on average 22% to 84% over the first 28 days (cycle 2 in group A, week 4 in group B) and remained attenuated to a similar extent throughout the subsequent 28 weeks of observation in group B as shown for granulocyte macrophage colony-stimulating factor and IFN-γ in Fig. 2A and B, respectively. The magnitude of attenuation for IFN-γ was greater in group A than in group B, in contrast to the other cytokines that displayed greater attenuation in group B. In general, the magnitude of attenuation corresponded to the magnitude of induction. rhIL-18 modestly increased plasma concentrations of the Th2 cytokines (in descending order) IL-8, IL-6, IL-10, and IL-4 in both groups, although they were generally lower and often unmeasurable in group A. These cytokines were also attenuated by day 28 to an extent that corresponded to the extent of their induction in each group.
Fig. 2.
Attenuation of granulocyte macrophage colony-stimulating factor (GM-CSF; A) and IFN-γ response (B) to 32 weekly doses of rhIL-18 in individual patients (group B).
IL-18 BP was induced after the first dose of rhIL-18 to a similar extent across all dose levels (Fig. 3). There was an approximate 20% attenuation in the induction of IL-18 BP on day 1 of cycle 2 (group A) and day 1 of week 4 (group B) relative to what was observed after the initial dose, although variability was greater in group B. The predose concentrations of IL-18 BP on day 1 of week 4 (group B) remained elevated compared with day 1 of cycle 2 (group A), likely reflecting the shorter washout period with weekly dosing (Fig. 3).
Fig. 3.
Induction and persistence of IL-18 BP represented by predose and 24-h postdose concentrations on day 1 of cycles 1 and 2 (group A) and weeks 1 and 4 (group B) at all doses.
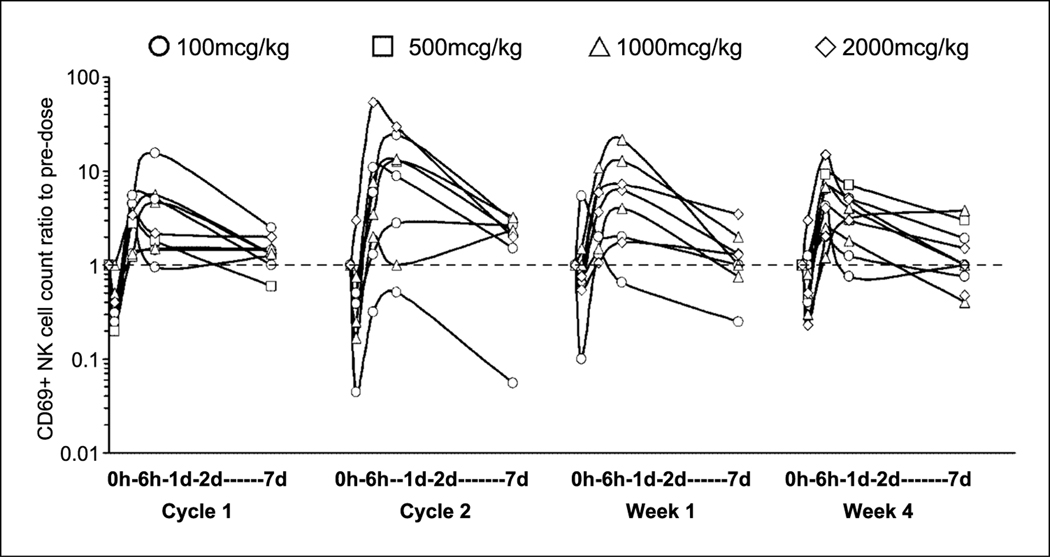
Administration of rhIL-18 produces transient lymphopenia involving CD4+ T cells, CD8+ T cells, and natural killer cells, shown previously to begin before the end of the 2-h infusion (12). In this study, circulating lymphocytes declined ∼4-fold below predose levels by 6 h and then recovered ∼3-fold within 24 h and had completely recovered on day 7. The decline in circulating cells occurred simultaneously with an increase in CD69 expression indicating cellular activation. Changes in circulating CD69+ CD4 and CD8 cell numbers essentially paralleled the changes in total lymphocyte counts, with 3- and 2-fold shifts, respectively. CD69+ natural killer cells declined on average ∼1.5-fold by 6 h followed by average 5- to 10-fold rebounds within 24 h that remained ∼2-fold above predose levels 7 days after the start of dosing (Fig. 4). In contrast to the attenuation of all cytokine responses regardless of the administration schedule, cellular activation and changes in circulating cell counts were not diminished in cycle 2 (group A) or week 4 (group B) despite induction of inhibitors such as IL-18 BP and Th2 cytokines such as IL-10 (Fig. 4).
Fig. 4.
Change in circulating CD69+ natural killer (NK) cell count relative to predose at all doses.
Development of antibodies to rhIL-18.
Treatment-related anti-rhIL-18 antibodies were observed in 6 (32%) of the 19 patients receiving rhIL-18. Anti-rhIL-18 antibodies were first detected in four of these six patients within the first month of treatment. Three additional patients were antibody positive at the screening visit. Two of these patients remained at baseline levels or showed a decrease in anti-rhIL-18 antibodies from screening levels during rhIL-18 therapy. In the third patient (group A, 100 μg/kg cohort), anti-rhIL-18 levels peaked at approximately three times above baseline during cycle 4 but returned to baseline levels when the patient was taken off study due to disease progression at cycle 6. Antibodies to rhIL-18 were detected among all dose groups, and there was no clear relationship between dose and development of treatment-related antibodies. There was no obvious difference in the frequency or severity of adverse events comparing patients with detectable anti-rhIL-18 antibodies to patients with no detectable antibodies. Anti-rhIL-18 IgE antibody responses were not detected in any patient during the study.
Neutralizing activity was detected in serum samples obtained from one patient (group A, 1,000 μg/kg cohort) on days 8, 15, and 29 of cycle 4. Although this patient developed treatment-related anti-rhIL-18 antibodies during cycles 1 to 3, these samples were negative for neutralizing activity. Moreover, antirhIL-18 antibody levels were below the limits of detection for the assay by cycle 4. This patient did not experience any serious adverse events or hypersensitivity-related adverse events during the study but did develop disease progression at the end of cycle 4.
Tumor response.
No objective complete or partial responses according to Response Evaluation Criteria in Solid Tumors criteria were observed during this study. Of the six patients in group A that completed at least six cycles of rhIL-18 treatment, one patient went off study with progressive disease after cycle 6 and five patients had stable disease after cycle 6. Two of the latter went on to receive 10 and 12 cycles, respectively, before developing disease progression. Of the four patients in group B who completed at least 24 weeks of rhIL-18 treatment, all had stable disease after 24 weeks of treatment and one patient received 32 weeks of treatment before going off study for disease progression. One of these subjects at the 1,000 μg/kg dose level experienced a delayed, near-complete clinical response of her skin-only disease documented 15 months after study in the absence of other therapies.
Discussion
IL-18 is an immunostimulatory cytokine that has antitumor activity in preclinical animal models (7–11). In the first clinical trial of rhIL-18, patients with advanced cancer received a single cycle of treatment consisting of five consecutive daily i.v. infusions of the cytokine (12). Repeated cycles of rhIL-18 therapy were not permitted on that study because preclinical primate toxicology studies indicated that high doses (30 mg/kg/d) of i.v. rhIL-18 in the presence of elevated levels (>2 mg/mL) of circulating treatment-related anti-IL-18 antibodies could induce anaphylactic reactions on reexposure to rhIL-18.5 Doses of rhIL-18 from 3 to 1,000 μg/kg were given in the prior phase I clinical trial; only one serious adverse event (hypotension) related to rhIL-18 was observed. In that study, the MTD was not determined and the magnitude of evaluated biological responses declined at higher doses. Treatment-related antibodies to rhIL-18 were detectable in 38% of evaluable patients but did not seem to alter toxicity, pharmacokinetic, or pharmacodynamic variables. Biological activity was seen in patients receiving doses of rhIL-18 as low as 10 μg/kg and delayed, unconfirmed partial responses were observed in two patients after the completion of rhIL-18 therapy (12).
The primary objective of this subsequent dose-escalation clinical trial in patients with advanced cancer was to determine the safety of administering i.v. infusions of rhIL-18 for 5 consecutive days repeated every 28 days. During conduct of this study, preliminary analysis of pharmacokinetic and pharmacodynamic data from group A suggested that five daily doses might not be the optimal dosing schedule. Cumulative induction of IL-18 BP and attenuation of cytokines suggested that less intensive dosing might produce stronger biological responses. In particular, the apparent half-life of elimination indicated that weekly dosing would allow for nearly complete elimination of rhIL-18, which was anticipated to allow for recovery of attenuated responses. Therefore, the study was amended to allow patients in group B to receive once weekly infusions of rhIL-18.
In group A, the repetitive 5-day cycles of rhIL-18 in doses as high as 1,000 μg/kg/d were well tolerated by patients. Despite receiving up to 12 cycles of rhIL-18 therapy, no patient in group A experienced an anaphylactic reaction or any other serious adverse event. Toxicities of rhIL-18 in group A patients were similar to those experienced by patients receiving comparable doses of rhIL-18 as a single 5-day cycle (12). Moreover, patients in group B also tolerated rhIL-18 very well with only one serious adverse event observed in these patients. Indeed, no MTD of rhIL-18 was identified for either schedule of administration in this study. Therefore, the potential safety issues raised by preclinical toxicology studies were not borne out in our clinical experience.
In the first human trial, the pharmacokinetics of rhIL-18 exhibited concentration and time dependence due to binding and induction of IL-18 BP. This dependence was evident in the current study as slightly lower accumulation with five daily doses, consistent with lower concentrations of IL-18 BP. Apart from this minor distinction, systemic exposure to rhIL-18 was generally similar over time at a given dose. Therefore, the attenuation in cytokine response seems to be pharmacodynamic and not pharmacokinetic in origin. As a specific feedback antagonist to the biological effects of rhIL-18, the induction and persistence of IL-18 BP are likely to contribute to the observed attenuation in cytokine responses. The induction of IL-18 BP and attenuation of IFN-γ (which mediates IL-18 BP induction) were greater with dosing over 5 consecutive days than with weekly dosing. However, persistence of IL-18 BP over the shorter washout periods between weekly doses, evident in higher IL-18 BP concentrations before the week 4 dose, suggests that dosing less frequently than weekly may be preferable. The emergence of inhibitory cytokines did not seem to modulate other biological responses to rhIL-18 in either a concentration- or time-dependent manner. Therefore, the apparent absence of attenuation in cellular responses suggests that this effect of rhIL-18 was preserved despite diminished systemic presence of proinflammatory cytokines and increased systemic presence of inhibitory cytokines and IL-18 BP.
The relatively minor toxicity of rhIL-18, compared with other immunostimulatory cytokines that have undergone clinical development, is remarkable. The MTD of rhIL-2 given by bolus i.v. injections every 8 h for 5 days is ∼500 μg/kg/dose (13). Even with aggressive supportive care measures, including vasopressor support in an intensive care unit setting, most patients cannot receive the planned 14 doses of rhIL-2 per cycle of treatment (14). Furthermore, the MTD of rhIL-12 given by daily i.v. infusion for 5 days is <2.5 μg/kg/dose (15). In contrast, rhIL-18 can be given in doses as high as 1,000 μg/kg/d for 5 days with relatively minor toxicity. It is currently not clear why rhIL-18 can be safely given in doses that are 10- to 1,000-fold higher than doses of rhIL-2 or rhIL-12 that are associated with substantial toxicity.
A plausible hypothesis is that IL-18 acts predominantly as a costimulatory cytokine to augment the response of immune effectors that have been stimulated by other cytokines (e.g., IL-12) or ligation of activating receptors (e.g., CD16 by antibodies or the T-cell receptor by cognate antigen/MHC complexes). If this hypothesis is correct, then optimal cancer immunotherapy may require administration of rhIL-18 in combination with monoclonal antibodies, tumor vaccines, and/or other immunostimulatory cytokines (10, 16). The very favorable toxicity profile of rhIL-18 as a single agent should facilitate its use with other biologically active agents. Further investigation of rhIL-18–based immunotherapy of cancer is warranted.
Acknowledgments
We thank Heather Blair, Angie Secrest, Deborah Jaworski, and Tim Hepburn for their assistance and support for the completion of this clinical trial.
Grant support: NIH grants RR00750–27S3 (M.J. Robertson) and MO1RR00750 (Indiana University General Clinic Research Center).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
K. Koch, L. Kirby, W. Bell, and M. Dar are employed by GlaxoSmithKline. M. Robertson received a research grant from GlaxoSmithKline and has a financial interest in Eli Lilly Corporation. The other authors have disclosed no potential conflicts of interest.
GlaxoSmithKline, unpublished data.
References
- 1.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 2001;19:423–74. [DOI] [PubMed] [Google Scholar]
- 2.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol 2003;73:213–24. [DOI] [PubMed] [Google Scholar]
- 3.Sims JE. IL-1 and IL-18 receptors, and their extended family. Curr Opin Immunol 2002;14:117–22. [DOI] [PubMed] [Google Scholar]
- 4.Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 1995;378:88–91. [DOI] [PubMed] [Google Scholar]
- 5.Robinson D, Shibuya K, Mui A, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity 1997;7:571–81. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimoto T, Takeda K, Tanaka T, et al. IL-12 upregulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J Immunol 1998;161:3400–7. [PubMed] [Google Scholar]
- 7.Micallef MJ, Tanimoto T, Kohno K, Ikeda M, Kurimoto M. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxicT lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res 1997;57:4557–63. [PubMed] [Google Scholar]
- 8.Osaki T, Peron J-M, Cai Q, et al. IFN-γ-inducing factor/IL-18 administration mediates IFN-γ- and IL-12-independent antitumor effects. J Immunol 1998;160: 1742–9. [PubMed] [Google Scholar]
- 9.Hashimoto W, Osaki T, Okamura H, et al. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas-Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol 1999;163:583–9. [PubMed] [Google Scholar]
- 10.Wigginton JM, Lee J-K, Wiltrout TA, et al. Synergistic enhancement of ineffective endogenous antitumor immune response and induction of IFN-γ and Fasligand-dependent tumor eradication by combined administration of IL-18 and IL-2. J Immunol 2002;169: 4467–74. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin CM, Salhany KE, Wysocka M, et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest 1998;101:1441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson MJ, Mier JW, Logan T, et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin Cancer Res 2006;12:4265–73. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994;271:907–13. [PubMed] [Google Scholar]
- 14.Siegel JP, Puri RK. Interleukin-2 toxicity. J Clin Oncol 1991;9:694–704. [DOI] [PubMed] [Google Scholar]
- 15.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-γ production. Blood 1997;90:2541–8. [PubMed] [Google Scholar]
- 16.Son Y-I, Dallal RM, Mailliard RB, Egawa S, Jonak ZL, Lotze MT. Interleukin-18 (IL-18) synergizes with IL-2 to enhance cytotoxicity, interferon-γ production, and expansion of natural killer cells. Cancer Res 2001;61:884–8. [PubMed] [Google Scholar]