Key Points
Question
What is the optimal sequence of chemoradiotherapy (CRT) and chemotherapy before total mesorectal excision surgery for total neoadjuvant therapy in rectal cancer?
Findings
In this secondary analysis of 311 patients treated within the CAO/ARO/AIO-12 randomized phase 2 clinical trial, CRT followed by consolidation chemotherapy led to higher rates of pathological complete response, the primary end point, without compromising disease-free survival, chronic toxicity, global health status, quality of life, or stool incontinence compared with induction chemotherapy followed by CRT and total mesorectal excision.
Meaning
These findings indicate that up-front CRT followed by consolidation chemotherapy is the preferred sequence for total neoadjuvant therapy if organ preservation is a priority.
This secondary analysis of a randomized clinical trial investigates the treatment sequence for total neoadjuvant therapy of chemoradiotherapy followed by consolidation chemotherapy before total mesorectal excision surgery in patients with rectal cancer.
Abstract
Importance
Total neoadjuvant therapy has been increasingly adopted for multimodal rectal cancer treatment. The optimal sequence of chemoradiotherapy (CRT) and chemotherapy needs to be established.
Objective
To report the long-term results of the secondary end points prespecified in the Randomized Phase 2 Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy (CAO/ARO/AIO-12 trial) for Locally Advanced Rectal Cancer.
Design, Setting, and Participants
This secondary analysis of a randomized clinical trial included 311 patients who were recruited from the accrued CAO/ARO/AIO-12 trial population from June 15, 2015, to January 31, 2018, from 18 centers in Germany. Patients with cT3-4 and/or node-positive rectal adenocarcinoma were included in the analysis. Data were analyzed from June 15, 2015, to January 31, 2018. The follow-up analysis was conducted between January 31, 2018, and November 30, 2020.
Interventions
Patients were randomly assigned to group A for 3 cycles of fluorouracil, leucovorin, and oxaliplatin before fluorouracil/oxaliplatin CRT (50.4 Gy), or to group B for CRT before chemotherapy. Total mesorectal excision was scheduled on day 123 after the start of total neoadjuvant therapy in both groups.
Main Outcomes and Measures
The end points assessed in this secondary analysis included long-term oncologic outcomes, chronic toxicity, patient-reported outcome measures for global health status (GHS) and quality of life (QoL), and the Wexner stool incontinence score.
Results
Of the 311 patients enrolled, 306 were evaluable, including 156 in group A (mean [SD] age, 60 [11] years; 106 men [68%]) and 150 in group B (mean [SD] age, 62 [10] years; 100 men [67%]). After a median follow-up of 43 months (range, 35-60 months), the 3-year disease-free survival was 73% in both groups (hazard ratio, 0.95; 95% CI, 0.63-1.45, P = .82); the 3-year cumulative incidence of locoregional recurrence (6% vs 5%, P = .67) and distant metastases (18% vs 16%, P = .52) were not significantly different. Chronic toxicity grade 3 to 4 occurred in 10 of 85 patients (11.8%) in group A and 8 of 66 patients (9.9%) in group B at 3 years. The GHS/QoL score decreased after total mesorectal excision but returned to pretreatment levels 1 year after randomization with no difference between the groups. Stool incontinence deteriorated 1 year after randomization in both groups and only improved slightly at 3 years, but never reached baseline levels.
Conclusions and Relevance
This secondary analysis of a randomized clinical trial showed that CRT followed by chemotherapy resulted in higher pathological complete response without compromising disease-free survival, toxicity, QoL, or stool incontinence and is thus proposed as the preferred total neoadjuvant therapy sequence if organ preservation is a priority.
Trial Registration
ClinicalTrials.gov identifier: NCT02363374
Introduction
Total neoadjuvant therapy (TNT) is the delivery of chemoradiotherapy (CRT) or short-course radiotherapy (SCRT) and chemotherapy before surgery (or as nonoperative management [NOM]) and has been increasingly adopted for multimodal rectal cancer treatment.1,2,3,4 Total neoadjuvant therapy offers several advantages compared with standard CRT/SCRT, surgery, and adjuvant chemotherapy, such as earlier administration of systemic treatment with less toxicity and better compliance, which can decrease distant metastases and improve disease-free survival (DFS). Moreover, TNT (and the longer interval from start of treatment to surgery) may also enhance local tumor regression, pathological complete response (pCR), and R0 (complete) resection rates and may guide patient selection for NOM or local excision. Caveats include the potential overtreatment and unnecessary toxicity of TNT in patients with low- or intermediate-risk rectal cancer and the substantial delay of curative surgery in nonresponding or poorly responding tumors.1,2,3,4
Two TNT sequences have emerged: induction chemotherapy followed by CRT/SCRT, and CRT/SCRT followed by consolidation chemotherapy. As demonstrated in the Partenariat de Recherche en Oncologie Digestive Group (PRODIGE23)5 and Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation (RAPIDO)6 randomized phase 3 trials, both sequences resulted in a significant improvement of their primary end points—DFS and disease-related treatment failure, respectively—compared with standard CRT and surgery with or without adjuvant chemotherapy. However, optimal scheduling of CRT/SCRT and chemotherapy remains a matter of debate.7 Indeed, a head-to-head comparison of both TNT sequences has, to the best of our knowledge, been investigated in only 2 randomized trials, the Organ Preservation in Rectal Adenocarcinoma (OPRA) in the US8,9 and the German Randomized Phase 2 Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer (CAO/ARO/AIO-12).10
First results of the CAO/ARO/AIO-12 were reported in 2019 and showed that up-front CRT followed by consolidation chemotherapy resulted in improved pCR (17% vs 25%; combined pCR and cCR: 21% vs 28%) and better compliance to CRT, but worse compliance to chemotherapy.10 We here present long-term outcomes of our trial after a median follow-up of 43 months (range, 35-60 months), including the secondary outcomes of DFS, chronic toxicity, quality of life (QoL), and stool incontinence.
Methods
Patient Selection
The CAO/ARO/AIO-12 was a multicenter, randomized, phase 2 trial.10 Inclusion criteria included patients aged 18 years or older with rectal adenocarcinoma up to 12 cm above the anal verge based on rigid rectoscopy; Eastern Cooperative Oncology Group (ECOG) performance status 0 to 1 and adequate organ function; cT3 tumor less than 6 cm from the anal verge, cT3 tumor in the middle third of the rectum (≥6-12 cm) with extramural tumor spread into the mesorectal fat of more than 5 mm (>cT3b), cT4 tumors, or lymph node involvement, based on magnetic resonance imaging that was mandatory. Computed tomography of the abdomen and chest was performed to exclude distant metastases. Data on race and ethnicity were not collected. The trial was conducted in Germany and included German citizens. Race and ethnicity documentation were not included in the trial protocol. The ethics committee of the University of Frankfurt approved the study, and all patients signed a consent form. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Treatment
Patients were randomized to group A for induction chemotherapy before CRT, or to group B for consolidation chemotherapy after CRT (eFigure 1 in Supplement 1). Compulsory, intensity-modulated radiotherapy to the primary tumor and to mesorectal, presacral, and internal iliac lymph nodes was prescribed to a total dose of 50.4 Gy in 28 fractions. Concurrent chemotherapy was administered with continuous infusion of fluorouracil (250 mg/m2) on days 1 to 14 and days 22 to 35, with a 2-hour infusion of oxaliplatin (50 mg/m2) on days 1, 8, 22, and 29 of radiotherapy, based on a previous phase 3 trial (CAO/ARO/AIO-04).11,12 Induction and consolidation chemotherapy was administered using oxaliplatin (100 mg/m2) as a 2-hour infusion, followed by a 2-hour infusion of leucovorin (400 mg/m2), followed by a continuous 46-hour infusion of fluorouracil (2400 mg/m2), repeated on day 15 for a total of 3 cycles (trial protocol is provided as Supplement 2). Total mesorectal excision (TME) surgery was mandatory independently of tumor response and was scheduled on approximately day 123 after initiation of TNT. Nonoperative management was considered a protocol violation but was chosen by a subset of patients (n = 10) with clinical complete response (cCR) who refused TME. Adjuvant chemotherapy after TME was not recommended.
Objectives
The primary end point, pathological complete response (pCR), has been reported before.10 Details on surgical and pathology objectives are shown in the eMethods in Supplement 1. Secondary end points included in the present analysis were DFS, cumulative incidence of locoregional recurrence and distant metastases, overall survival (OS), and chronic toxicity graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Assessment of QoL was based on the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires QLQ-C30 and CR29 as patient-reported outcome measures (PROMs). Stool incontinence was graded with the Wexner score13 and measured at baseline, before and after surgery, and at 6, 12, 24, and 36 months after randomization.
Statistical Analysis
A detailed description of statistical analyses of the primary and secondary end points, randomization and definition of DFS, cumulative incidence of locoregional recurrence and distant metastases, and OS are shown in the eMethods in Supplement 1. Chronic toxicity, QoL, and stool incontinence are reported in patients who received protocol-specified treatment and were disease-free at the time of assessment. The combined global health status (GHS)/QoL score was assessed by QLQ-C30 (questions 29-30), whereas a detailed analysis of all PROMs will follow elsewhere. The EORTC guidelines were used for analyses of missing data, whereas item responses were converted from a 7-point Likert-type scale with linear transformation onto a 0 to 100 scale, and the mean (SD) was calculated.14 Patients with a stoma were excluded from analysis of chronic diarrhea and stool incontinence. The Wexner stool incontinence score is shown as a box plot (Tukey definition; described in the eMethods in Supplement 1). Statistical analyses were performed using R software, version 3.6 (R Foundation). In the exploratory analyses presented here, a 2-sided P < .05 was considered significant. Statistical analyses were conducted from June 15, 2015, to January 31, 2018. The follow-up analysis was conducted between January 31, 2018, and November 30, 2020.
Results
Accrual and Patient Characteristics
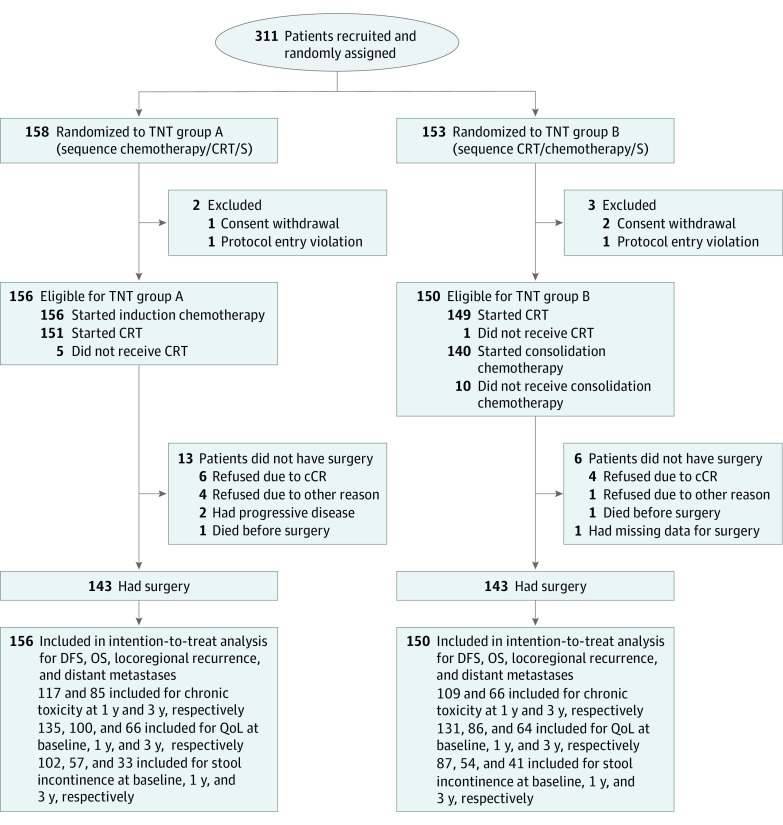
A total of 311 patients were recruited from June 15, 2015, to January 31, 2018, in 18 centers in Germany (eMethods in Supplement 1). Five patients were ineligible after enrollment owing to consent withdrawal or protocol entry violation. Of the remaining 306 eligible patients, 156 patients were randomized to group A (mean [SD] age, 60 [11] years; 106 men [68%] and 50 women [32%]), which included the treatment sequence chemotherapy, CRT, and surgery, and 150 patients were randomized to group B (mean [SD] age, 62 [10] years; 100 men [67%] and 50 women [33%]), which included the treatment sequence CRT, chemotherapy, and surgery (Figure 1). Generally, baseline characteristics were well balanced between the 2 groups; however, more patients had ECOG performance status 1 in group B (32 of 156 [20%] in group A vs 48 of 150 [32%] in group B), whereas mesorectal fascia involvement (≤1 mm) was slightly more present in group A (48 of 156 [31%] vs 33 of 150 [22%] in group B). Tumor location from the anal verge also showed some imbalances (Table 1). In group A, 156 patients started induction chemotherapy, 151 (97%) proceeded to CRT, and 143 (92%) underwent surgery. In group B, 149 (99%) received CRT, 140 (93%) started consolidation CT, and 143 (95%) underwent surgery (Figure 1).
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Diagram.
Patients who were disease-free after protocol-specified treatment as well as patients with clinical complete response (cCR) who rejected surgery (S) and had nonoperative management were included in the assessment of toxicity and quality of life (QoL) assessment. For the assessment of stool incontinence, patients who were disease-free and stoma-free as well as patients with cCR who rejected surgery and had nonoperative management were included. CRT indicates chemoradiotherapy; DFS, disease-free survival; OS, overall survival; TNT, total neoadjuvant therapy.
Table 1. Baseline Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| TNT group A (n = 156) | TNT group B (n = 150) | |
| Age, y | ||
| Mean (SD) | 60 (11) | 62 (10) |
| Median (IQR) | 62 (55-67) | 61 (54-70) |
| Sex | ||
| Male | 106 (68) | 100 (67) |
| Female | 50 (32) | 50 (33) |
| ECOG performance status | ||
| 0 | 118 (76) | 100 (67) |
| 1 | 32 (20) | 48 (32) |
| Missing | 6 (4) | 2 (1) |
| Clinical T categorya | ||
| cT2 | 6 (4) | 4 (3) |
| cT3 | 132 (84) | 118 (78) |
| cT4 | 18 (12) | 27 (18) |
| Missing | 0 | 1 (1) |
| Clinical N categoryb | ||
| cN0 | 16 (10) | 14 (9) |
| cN1-2 | 134 (86) | 135 (90) |
| Missing | 6 (4) | 1 (1) |
| Clinical disease stagec | ||
| Stage II | 16 (10) | 14 (9) |
| Stage III | ||
| cT1-2 N1-2 | 5 (3) | 5 (3) |
| cT3-4 N1-2 | 129 (83) | 130 (87) |
| Missing | 6 (4) | 1 (1) |
| Distance of tumor to mesorectal fascia, mm | ||
| ≤1 | 48 (31) | 33 (22) |
| >1 | 108 (69) | 117 (78) |
| Location from anal verge, cm | ||
| 0-5 | 64 (41) | 62 (41) |
| >5-10 | 67 (43) | 73 (49) |
| >10 | 15 (10) | 11 (7) |
| Missing | 10 (6) | 4 (3) |
| Histology | ||
| Adenocarcinoma | 152 (97) | 143 (96) |
| Mucinous adenocarcinoma | 1 (1) | 5 (3) |
| Signet ring cell carcinoma | 1 (1) | 0 |
| Otherd or missing | 2 (1) | 2 (1) |
| Tumor differentiation | ||
| Well differentiated (grade 1) | 6 (4) | 12 (8) |
| Moderately differentiated (grade 2) | 125 (80) | 113 (76) |
| Poorly differentiated (grade 3) | 11 (7) | 8 (5) |
| Missing data | 14 (9) | 17 (11) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; TNT, total neoadjuvant therapy.
T categories are as follows: cT2, tumor invades the muscularis propria; cT3, tumor invades through the muscularis propria into the pericolorectal tissues; cT4, tumor invades the visceral peritoneum or invades or adheres to adjacent organs or structures.
N categories are as follows: cN0, no regional lymph node metastasis; cN1-2, regional lymph node metastases.
Stage definitions: stage II, cT3-4b N0; stage III, cT1-2 N1-2 or cT3-4b N1-2.
Rare histologies were reported as “other” by the pathologist of the participating institution.
Efficacy
Early efficacy results, including pCR, R0 resection, acute toxicity, surgical morbidity, and treatment compliance have been reported before.10 The median follow-up was 43 months (range, 35-60 months; IQR, 35-49 months). Of note, we aimed to have a minimum follow-up of 36 months for all patients, whereas the follow-up period of the last recruited patient was 35 months. Among deaths (for groups A and B, respectively) 2 (1 and 1) were treatment-related, 14 (8 and 6) were rectal cancer–related, 1 (0 and 1) was due to second primary tumor, and 12 (5 and 7) were due to other causes (1 nephritis, 1 heart attack, 1 cardiac arrhythmia, 1 nonocclusive mesenteric ischemia, 3 multiorgan failure, 2 suicide, and 3 unknown). The 3-year DFS was 73% (95% CI, 66%-80%) for group A and 73% (95% CI, 66%-80%) for group B (HR, 0.95; 95% CI, 0.63-1.45; P = .82). We also performed a separate multivariable Cox proportional hazard model analysis for DFS by including both TNT groups and baseline parameters with potential imbalances between the 2 groups (ECOG performance status, distance of tumor to mesorectal fascia, and location from anal verge) (Table 1); we failed to detect any differences in DFS between the 2 TNT groups in the separate multivariable analysis (eTable 1 in Supplement 1). The 3-year cumulative incidence of locoregional recurrence was 6% (95% CI, 2%-10%) for group A and 5% (95% CI, 1%-9%) for group B (HR, 0.81; 95% CI, 0.30-2.18; P = .67), and of distant metastasis was 18% (95% CI, 12%-24%) for group A and 16% (95% CI, 9%-22%) for group B (HR, 0.84; 95% CI, 0.50-1.43; P = .52). Overall survival was similar in both groups, at 92% (95% CI, 88%-97%) in group A and 92% (95% CI, 88%-97%) in group B (HR, 1.10; 95% CI, 0.53-2.27; P = .81) (Figure 2). Subgroup analyses of DFS in the intention-to-treat population according to baseline characteristics did not identify subsets of patients who significantly benefited from one TNT sequence over the other (eFigure 2 in Supplement 1).
Figure 2. Long-term Oncologic Outcomes.
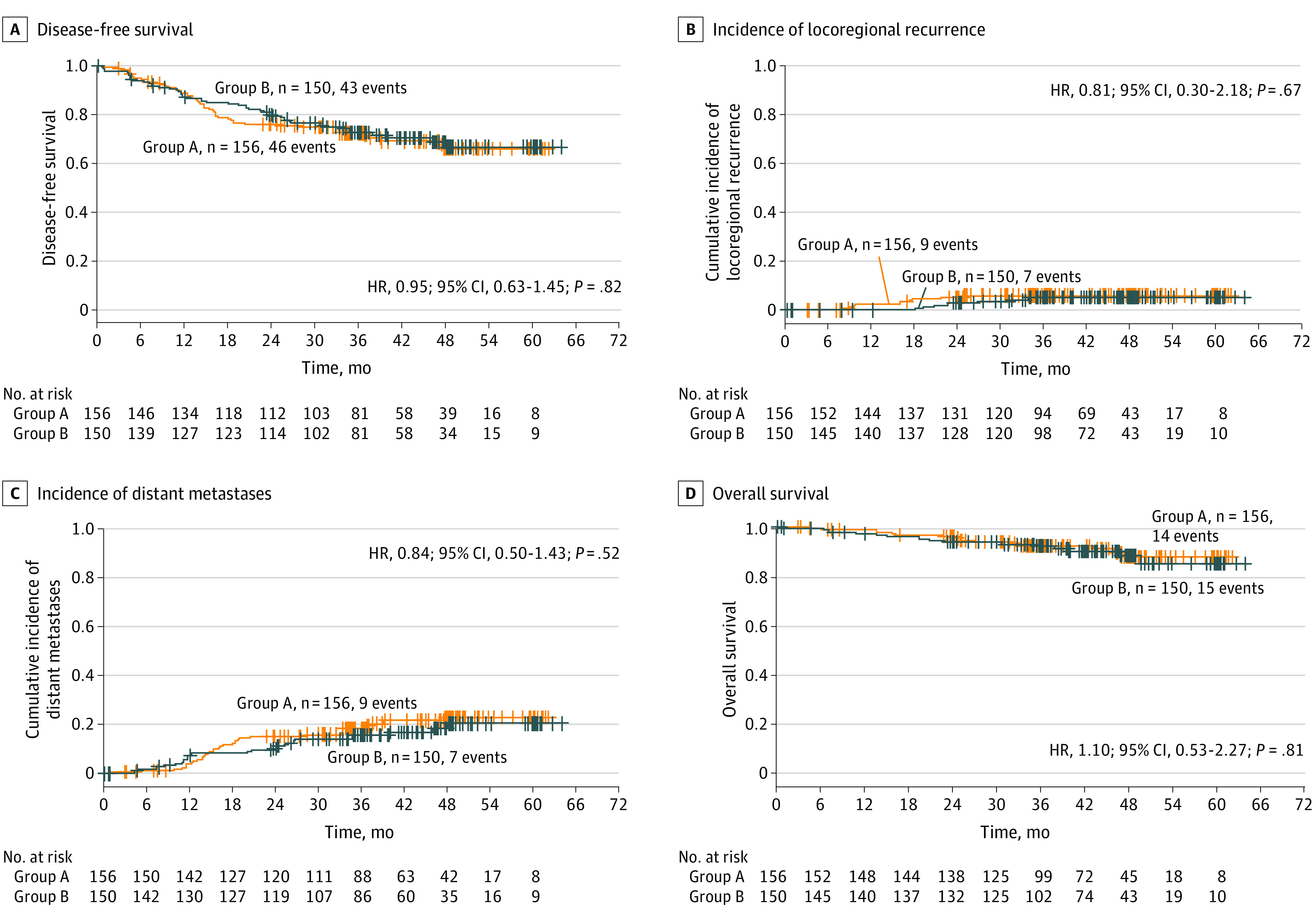
A, Disease-free survival; B, cumulative incidence of locoregional recurrence after R0-1 resection; C, cumulative incidence of distant metastases; D, overall survival. HR indicates hazard ratio.
Six of 156 patients (4%) in group A and 4 of 150 patients (3%) in group B had cCR at restaging and refused surgery. Their clinical outcome is summarized in eFigure 3 in Supplement 1. Of those patients, 1 in group A developed endoluminal tumor regrowth 3 months later that was salvaged (R0) by an abdominoperineal resection and remained disease-free during further follow-up. One patient in group B was diagnosed with local regrowth and distant metastases (liver and lung) at 27 months after TNT start and received palliative chemotherapy. The other 8 patients had sustained cCR and remained tumor-free by the time of last follow-up examination.
Chronic Toxicity, GHS/QoL, and Wexner Incontinence Score
The rates of total chronic toxicity grade 3 to 4 were 18 of 117 (15.4%) in group A and 19 of 109 (17.4%) in group B at 12 months and 10 of 85 (11.8%) in group A and 8 of 66 (9.9%) in group B at 36 months (Table 2). Oxaliplatin-induced grade 3 to 4 neurotoxicity according to the Wasserman score15 was reduced from 11 of 117 (9.4%) in group A and 10 of 109 (9.2%) in group B at 12 months to 1 of 85 (1.2%) in group A and 2 of 66 (2.5%) in group B at 36 months. Of note, only 1 patient (group A) had grade 4 neurotoxicity at 12 months, whereas no grade 4 toxicity occurred at 36 months in either group (Table 2).
Table 2. Chronic Toxicity at 12 and 36 Months After Completion of Treatment.
| Variable | Patients, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Toxicity at 12 mo | Toxicity at 36 moa | |||||||
| TNT group A (n = 117) | TNT group B (n = 109) | TNT group A (n = 85) | TNT group B (n = 66) | |||||
| Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | |
| Total | 72 (61.5) | 18 (15.4) | 67 (61.5) | 19 (17.4) | 43 (50.6) | 10 (11.8) | 52 (64.2) | 8 (9.9) |
| Gastrointestinal | ||||||||
| Anastomotic stenosis | 3 (4.8) | 1 (1.6) | 1 (1.5) | 0 | 2 (3.5) | 0 | 2 (3.3) | 0 |
| Diarrhea | 23 (36.5) | 0 | 29 (43.9) | 1 (1.5) | 18 (31.6) | 2 (3.5) | 26 (42.6) | 3 (4.9) |
| Proctitis | 4 (6.3) | 0 | 4 (6.1) | 0 | 4 (4.7) | 1 (1.8) | 3 (4.9) | 2 (3.3) |
| Rectal pain | 5 (7.9) | 0 | 1 (1.5) | 0 | 2 (3.5) | 0 | 1 (1.6) | 0 |
| Intestinal stoma site bleeding | 0 | 0 | 0 | 0 | 0 | 1 (1.2) | 0 | 0 |
| Intestinal stoma prolapse | 1 (0.9) | 1 (0.9) | 1 (0.9) | 0 | 0 | 1 (1.2) | 0 | 0 |
| Genitourinary | ||||||||
| Voiding disorder | 5 (4.3) | 0 | 5 (4.6) | 1 (0.9) | 3 (3.5) | 0 | 1 (1.2) | 0 |
| Cystitis | 2 (1.7) | 0 | 5 (4.6) | 0 | 2 (2.4) | 0 | 1 (1.2) | 0 |
| Erectile dysfunction | 6 (7.5) | 2 (2.5) | 12 (15.6) | 2 (2.6) | 5 (8.8) | 2 (3.5) | 11 (20.4) | 1 (1.9) |
| Vaginal dryness | 0 | 0 | 2 (6.2) | 1 (3.1) | 0 | 0 | 0 | 0 |
| Neurologic | ||||||||
| Sensory neuropathy | 30 (25.6) | 0 | 26 (23.9) | 2 (1.8) | 15 (17.6) | 1 (1.2) | 22 (27.2) | 0 |
| Oxaliplatin neurotoxicity (according to Wasserman score) | 33 (28.2) | 11 (9.4)b | 24 (22.0) | 10 (9.2)c | 14 (16.5) | 1 (1.2) | 19 (23.5) | 2 (2.5) |
| Fistula | ||||||||
| Rectovesical | 0 | 1 (0.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Rectovaginal | 0 | 0 | 0 | 1 (3.1) | 0 | 0 | 0 | |
| Other | ||||||||
| Radiation dermatitis | 3 (2.6) | 0 | 3 (2.8) | 0 | 2 (2.4) | 0 | 0 | 0 |
| Various | 35 (29.9) | 5 (4.3) | 21 (19.3) | 4 (3.7) | 9 (10.6) | 3 (3.5) | 7 (8.6) | 2 (2.5) |
Abbreviation: TNT, total neoadjuvant therapy.
No grade 4 toxicity was documented in any of the 2 groups at 36 months.
Only 1 of the 11 patients in group A developed grade 4 neurotoxicity per Wasserman score at 12 months; the remaining patients had grade 3 neurotoxicity.
None of the 10 patients in group B had grade 4 neurotoxicity per Wasserman score at 12 months.
The rate of PROMs completion was 272 of 306 (89%) at baseline, 211 of 306 (69%) before surgery, 177 of 306 (58%) at treatment completion, 187 of 306 (61%) at 12 months, 165 of 306 (54%) at 24 months, and 125 of 306 (41%) at 36 months, with no difference in response rates or missing items between groups. There was no difference between group A and group B in GHS/QoL score, both of which decreased after surgery and returned to pretreatment levels 12 months (group A, mean [SD] 65.9 [15.8] points and group B, 65.9 [15.9] points) after randomization (Figure 3A).
Figure 3. Quality of Life (QoL) and Incontinence Changes Over Time in Both Treatment Groups .
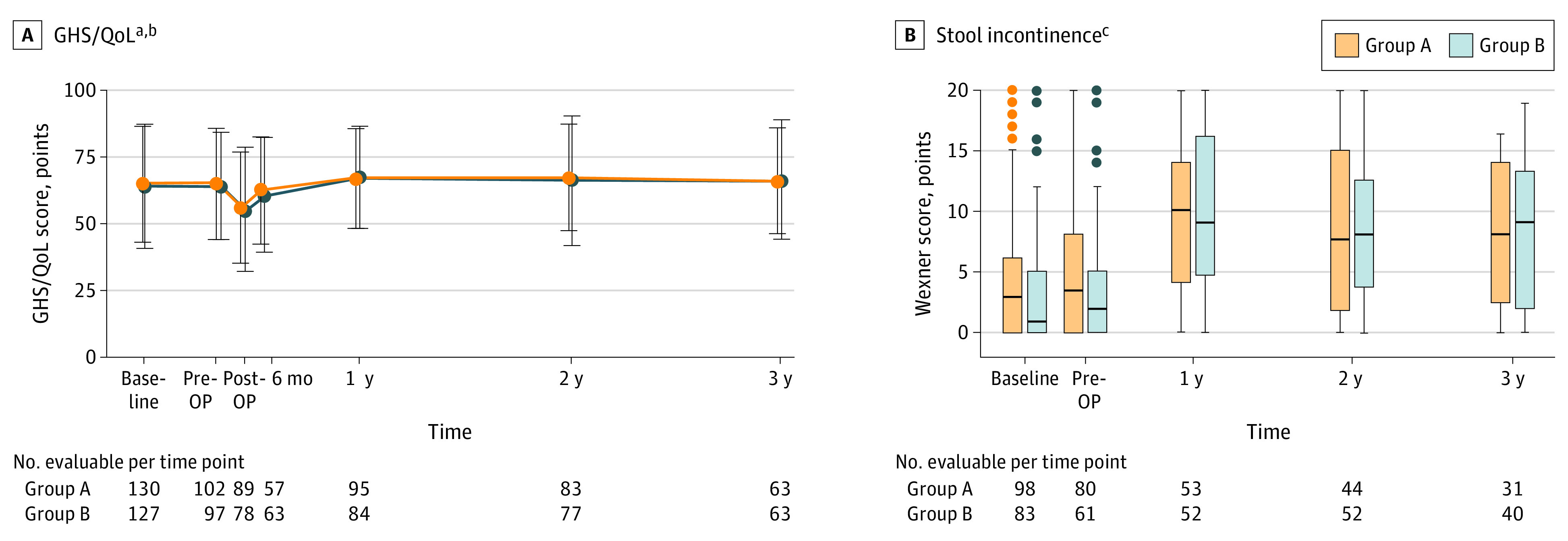
A, Global health status (GHS)/QoL score, assessed by the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire in the 2 groups, as indicated. B, Stool incontinence, assessed by the Wexner score. The Wexner score postoperatively and at 6 mo after randomization is not shown as most patients still had a protective ileostomy at this time point. A higher score represents worse incontinence status. OP indicates operation.
aOf note, only disease-free patients were included in the analysis, whereas patients who refused surgery due to clinical complete response were excluded (data shown separately in eFigures 4A and B in Supplement 1).
bData are shown as mean (SD) score. A higher mean score represents a better level of GHS/QoL status.
cBaseline patient numbers for the Wexner score are lower compared with those for GHS/QoL score in Figure 3A as patients who initially received a protective ileostomy were excluded from the stool incontinence analysis. Similarly, patients with cCR were excluded (data shown separately in eFigures 4C and D in Supplement 2).
Stool incontinence as assessed by the Wexner questionnaire (eTable 2 in Supplement 1) was not different between the 2 groups at any point. Notably, stool incontinence scores were worse in both groups at 12 months and only improved slightly at 24 months and 36 months but never reached the baseline levels (Figure 3B). The severity of stool incontinence was measured as previously reported (eTable 3 in Supplement 1).16 In group A at 12 months, normal stool continence was observed in 6 of 53 patients (11.3%), minor stool incontinence was observed in 18 of 53 patients (34%), average stool incontinence was observed in 17 of 53 patients (32.1%), and complete stool incontinence was observed in 12 of 53 patients (22.6%). In group B at 12 months, 5 of 2 (9.6%) had normal stool continence, 19 of 52 (36.5%) had minor stool incontinence, 13 of 52 (25%) had average stool incontinence, and 15 of 52 (28.8%) had complete stool incontinence. The median stool incontinence score at 12 months in group A was 10 points (IQR, 4-14 points) and in group B was 9 points (IQR, 4.8-16.1 points).
Chronic toxicity in the 8 patients with sustained cCR after NOM is provided in eTable 4 in Supplement 1. The mean (SD) GHS/QoL score for the 8 patients was 87 (6) points at 12 months (eFigure 4A and B in Supplement 1); normal stool continence was observed in 1 of 6 patients (16.7%) and minor stool incontinence was observed in 5 of 6 patients (83.3%) at 1 year, whereas neither average nor complete stool incontinence occurred. The median stool incontinence score was 2.5 points (IQR, 1.2-3 points) at 12 months, with no differences between groups (eFigure 4C and D in Supplement 1).
Discussion
The first report of CAO/ARO/AIO-1210 showed that up-front CRT followed by chemotherapy and TME was associated with higher pCR, better compliance with CRT, and worse compliance with chemotherapy compared with group A. Acute grade 3 to 4 toxicity occurred in 37% and 27% in group A and B, respectively, during CRT, and in 22% (both groups) during chemotherapy. The longer interval of CRT to TME in group B did not increase surgical morbidity.10 Here, we present the long-term clinical outcomes of both TNT sequences after a median follow-up of 43 months. Results of this secondary analysis did not reveal a significant difference in oncologic end points, chronic toxicity, GHS/QoL, or stool incontinence between the 2 groups.
The Organ Preservation in Rectal Adenocarcinoma (OPRA) randomized phase 2 trial8,9 used a design similar to ours and assigned patients with locally advanced rectal cancer, requiring abdominoperineal resection or coloanal anastomosis at baseline, to fluoropyrimidine-based CRT with either induction or consolidation chemotherapy (8 cycles of folinic acid, fluorouracil, and oxaliplatin [FOLFOX] or 6 cycles of capecitabine and oxaliplatin [CAPOX]). That trial included the option of NOM/local excision for patients with near cCR or cCR. In line with our experiences, compliance with up-front CRT was better, and compliance with chemotherapy was slightly worse compared with the chemotherapy/CRT sequence. Grade 3 to 4 toxicity occurred in 45.5% and 49% in the CRT/chemotherapy and chemotherapy/CRT groups, respectively. After a median follow-up of 25 months, the 3-year DFS was 78% and 77% in the CRT/CT and CT/CRT groups, respectively. The 3-year metastases-free survival was not different. The CRT/CT sequence was associated with a superior 3-year TME-free survival.9 Thus, in both trials that tested the optimal sequence of TNT, up-front CRT (and the associated longer interval to restaging or surgery) resulted in better pCR (CAO/ARO/AIO-1210) or sustained cCR/organ preservation (OPRA9) without compromising DFS and metastases-free survival.
Regarding TNT using SCRT, the Polish II trial compared SCRT followed by 3 cycles of FOLFOX vs fluorouracil/oxaliplatin–based CRT.17,18 In the initial report, OS was better in favor of TNT with comparable R0 resection rates (primary end point), pCR, local control, and DFS rates; however, no difference in OS was shown in the updated report.18 The RAPIDO phase 3 trial6 randomly assigned patients with high-risk rectal cancer (cT4, mesorectal fascia involvement, extramural vascular invasion, cN2, and lateral node positive) to capecitabine-based CRT followed by surgery with optional adjuvant chemotherapy vs SCRT followed by consolidative chemotherapy (6 cycles of CAPOX or 8 cycles of FOLFOX) and surgery. Treatment in the experimental group significantly improved 3-year disease-related treatment failure, the primary end point, compared with the control group, mainly owing to a decrease in the 3-year distant metastases rate, with comparable OS. The experimental group resulted in doubling of the pCR.6
Induction chemotherapy with fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX), followed by CRT, has been tested within the PRODIGE23 phase 3 trial against standard CRT followed by surgery and adjuvant chemotherapy.5 The primary end point, 3-year DFS, was met in favor of the experimental compared with the control group, mainly owing to an increase in the 3-year distant metastases-free survival rate. The Grupo Cancer de Recto 3 (GCR-3) phase 2 trial19 randomized patients to induction chemotherapy with 4 cycles of CAPOX before CRT or as adjuvant chemotherapy. Grade 3 to 4 acute toxicity and compliance with chemotherapy were better in the TNT group; however, no differences in pCR rates and 5-year DFS were observed.19,20 In the NRG-GI002 phase 2 trial, addition of the poly(ADP-ribose) polymerase inhibitor veliparib to CRT after induction chemotherapy failed to improve the primary end point, neoadjuvant rectal score21; in the subsequent study, addition of the programmed cell death 1 immune checkpoint inhibitor pembrolizumab to TNT was safe but did not improve neoadjuvant rectal score.22
Thus, data for an increased efficacy of both TNT sequences vs standard CRT followed by TME with or without adjuvant chemotherapy have been provided in randomized trials. Which TNT sequence is preferred remains a matter of debate and might be guided by either different pretreatment factors or treatment goals.1,2 Exploratory subset analysis of DFS according to pretreatment characteristics in our trial did not identify subsets of patients who significantly benefited from one TNT sequence over the other, but this analysis may lack adequate statistical power. It has been hypothesized that earlier onset and better compliance of induction chemotherapy may achieve better control of micrometastatic disease.1,2 However, the rates of DFS and metastases-free survival were similar between the 2 groups in our CAO/ARO/AIO-12 trial and the OPRA trial.9 Conversely, induction chemotherapy may help to enable selective omission of CRT based on treatment response, as tested in the PROSPECT trial (results pending),23 whereas up-front CRT/SCRT followed by consolidation chemotherapy may be the preferred TNT approach if NOM and organ preservation is the goal.
Data on chronic toxicity, PROMs/QoL, and functional outcomes after TNT with or without organ preservation remain limited and should be a priority in further reporting and trial design. Chronic toxicity grade 3 to 4 did not differ between the 2 groups in our study. We failed to identify any differences in stool incontinence between the 2 groups that expectedly deteriorated after surgery and only improved slightly at 36 months but never reached baseline levels, whereas GHS/QoL score returned to pretreatment levels 12 months after randomization.
In the PRODIGE23 trial, GHS improved with time in both groups, whereas a trend toward better global QoL was demonstrated for TNT; incontinence was not reported.5 In the RAPIDO trial, GHS and global QoL were similar between groups, as was the low anterior resection syndrome score6; chronic toxicity data were not provided.6,24 Results of the OPRA trial8,9 regarding QoL and functional outcomes comparing patients who underwent TME and NOM after TNT are pending. Interestingly, in our trial, GHS/QoL and stool incontinence scores of the 8 patients with NOM compare favorably with the scores of patients who underwent TME; however, conclusions cannot be drawn owing to the small patient number.
Limitations
Our study has several limitations. First, the trial was not designed to demonstrate differences in long-term oncologic end points, toxicity, QoL, and functional outcomes, as pCR constituted the primary end point. Despite the median follow-up of 43 months, further assessment of the oncologic outcome after longer follow-up will be important to see whether any differences between the groups are observed. Second, NOM and organ preservation for patients with cCR, as performed in the OPRA study,8,9 were not part of the study protocol. Third, patient compliance with collection of PROMs and stool incontinence data was reduced over time. Fourth, although a complete case analysis was conducted for QoL, this measure was purely descriptive, as we refrained from any formal comparison between the groups.
Conclusions
To our knowledge, this is the first randomized clinical trial to report full data on long-term clinical outcome after a head-to-head comparison of both TNT sequences. Long-term oncologic outcomes did not differ between both groups. We suggest that up-front CRT followed by consolidation chemotherapy may be the preferred TNT sequence if organ preservation is a priority. This sequence resulted in higher rates of pCR without compromising DFS, toxicity, or QoL. The TNT regimen as tested in group B of the CAO/ARO/AIO-12 has, upon adaptation, been selected for comparison against TNT according to the RAPIDO protocol in the ongoing ACO/ARO/AIO-18.1 trial,25 which uses 3-year organ preservation rate as the primary end point.
eMethods.
eTable 1. Multivariable Cox Proportional Hazard Model for DFS Including TNT Group and Baseline Parameters With Potential Imbalances
eTable 2. Wexner Incontinence Score
eTable 3. Severity of Stool Incontinence Based on the Wexner Score
eTable 4. Chronic Toxicity at 12 Months and 36 Months in Patients With Clinical Complete Response (cCR) That Refused Surgery
eFigure 1. Treatment Schedule
eFigure 2. Forest Plot of the Effect of Treatment on Disease-Free Survival (DFS) According to Pretreatment Characteristics
eFigure 3. Clinical Outcome in the 10 Patients With Clinical Complete Response (cCR) That Denied Surgery and Were Managed With a “Watch and Wait” Strategy
eFigure 4. Quality of Life and Incontinence Changes Over Time in the 10 Patients With Clinical Complete Response (cCR) That Denied Surgery and Were Managed With a “Watch and Wait” Strategy
eReferences
Trial Protocol
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Kasi A, Abbasi S, Handa S, et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12):e2030097. doi: 10.1001/jamanetworkopen.2020.30097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440-448. doi: 10.1097/SLA.0000000000003471 [DOI] [PubMed] [Google Scholar]
- 3.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6):e180071. doi: 10.1001/jamaoncol.2018.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fokas E, Glynne-Jones R, Appelt A, et al. Outcome measures in multimodal rectal cancer trials. Lancet Oncol. 2020;21(5):e252-e264. doi: 10.1016/S1470-2045(20)30024-3 [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Bosset JF, Etienne PL, et al. ; Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group . Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702-715. doi: 10.1016/S1470-2045(21)00079-6 [DOI] [PubMed] [Google Scholar]
- 6.Bahadoer RR, Dijkstra EA, van Etten B, et al. ; RAPIDO collaborative investigators . Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29-42. doi: 10.1016/S1470-2045(20)30555-6 [DOI] [PubMed] [Google Scholar]
- 7.Shi DD, Mamon HJ. Playing with dynamite? a cautious assessment of TNT. J Clin Oncol. 2021;39(2):103-106. doi: 10.1200/JCO.20.02199 [DOI] [PubMed] [Google Scholar]
- 8.Smith JJ, Chow OS, Gollub MJ, et al. ; Rectal Cancer Consortium . Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. doi: 10.1186/s12885-015-1632-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Aguilar J, Patil S, Kim JK, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2020;38(15 suppl):4008-4008. doi: 10.1200/JCO.2020.38.15_suppl.4008 [DOI] [Google Scholar]
- 10.Fokas E, Allgäuer M, Polat B, et al. ; German Rectal Cancer Study Group . Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37(34):3212-3222. doi: 10.1200/JCO.19.00308 [DOI] [PubMed] [Google Scholar]
- 11.Rödel C, Liersch T, Becker H, et al. ; German Rectal Cancer Study Group . Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679-687. doi: 10.1016/S1470-2045(12)70187-0 [DOI] [PubMed] [Google Scholar]
- 12.Rödel C, Graeven U, Fietkau R, et al. ; German Rectal Cancer Study Group . Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979-989. doi: 10.1016/S1470-2045(15)00159-X [DOI] [PubMed] [Google Scholar]
- 13.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77-97. doi: 10.1007/BF02050307 [DOI] [PubMed] [Google Scholar]
- 14.Fayers P AN, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual. European Organisation for Research and Treatment of Cancer; 1999. [Google Scholar]
- 15.Wasserman E, Cuvier C, Lokiec F, et al. Combination of oxaliplatin plus irinotecan in patients with gastrointestinal tumors: results of two independent phase I studies with pharmacokinetics. J Clin Oncol. 1999;17(6):1751-1759. doi: 10.1200/JCO.1999.17.6.1751 [DOI] [PubMed] [Google Scholar]
- 16.Dulskas A, Kavaliauskas P, Pilipavicius L, Jodinskas M, Mikalonis M, Samalavicius NE. Long-term bowel dysfunction following low anterior resection. Sci Rep. 2020;10(1):11882. doi: 10.1038/s41598-020-68900-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bujko K, Wyrwicz L, Rutkowski A, et al. ; Polish Colorectal Study Group . Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834-842. doi: 10.1093/annonc/mdw062 [DOI] [PubMed] [Google Scholar]
- 18.Ciseł B, Pietrzak L, Michalski W, et al. ; Polish Colorectal Study Group . Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol. 2019;30(8):1298-1303. doi: 10.1093/annonc/mdz186 [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26(8):1722-1728. doi: 10.1093/annonc/mdv223 [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28(5):859-865. doi: 10.1200/JCO.2009.25.8541 [DOI] [PubMed] [Google Scholar]
- 21.George TJ, Yothers G, Hong TS, et al. NRG-GI002: A phase II clinical trial platform using total neoadjuvant therapy (TNT) in locally advanced rectal cancer (LARC)—first experimental arm (EA) initial results. J Clin Oncol . 2019;37(15):3505. [Google Scholar]
- 22.Rahma OE, Yothers G, Hong TS, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol. 2021;7(8):1225-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrag D, Weiser M, Saltz L, et al. Challenges and solutions in the design and execution of the PROSPECT Phase II/III neoadjuvant rectal cancer trial (NCCTG N1048/Alliance). Clin Trials. 2019;16(2):165-175. doi: 10.1177/1740774518824539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Valk MJM, Marijnen CAM, van Etten B, et al. ; Collaborative investigators . Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer—results of the international randomized RAPIDO-trial. Radiother Oncol. 2020;147:75-83. doi: 10.1016/j.radonc.2020.03.011 [DOI] [PubMed] [Google Scholar]
- 25.Short-course radiotherapy versus chemoradiotherapy, followed by consolidation chemotherapy, and selective organ preservation for MRI-defined intermediate and high-risk rectal cancer patients. ClinicalTrials.gov identifier: NCT04246684. Updated June 16, 2021. Accessed July 24, 2021. https://clinicaltrials.gov/ct2/show/NCT04246684
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Multivariable Cox Proportional Hazard Model for DFS Including TNT Group and Baseline Parameters With Potential Imbalances
eTable 2. Wexner Incontinence Score
eTable 3. Severity of Stool Incontinence Based on the Wexner Score
eTable 4. Chronic Toxicity at 12 Months and 36 Months in Patients With Clinical Complete Response (cCR) That Refused Surgery
eFigure 1. Treatment Schedule
eFigure 2. Forest Plot of the Effect of Treatment on Disease-Free Survival (DFS) According to Pretreatment Characteristics
eFigure 3. Clinical Outcome in the 10 Patients With Clinical Complete Response (cCR) That Denied Surgery and Were Managed With a “Watch and Wait” Strategy
eFigure 4. Quality of Life and Incontinence Changes Over Time in the 10 Patients With Clinical Complete Response (cCR) That Denied Surgery and Were Managed With a “Watch and Wait” Strategy
eReferences
Trial Protocol
Nonauthor Collaborators
Data Sharing Statement