Abstract
This study aimed to calculate the seroconversion rate and IgG antibody dynamic range of the CoronaVac vaccine in healthcare workers (HCWs) after immunization. Serum samples from 133 HCWs from Southern Brazil were collected 1 day before (Day 0) and +10, +20, +40, + 60, +110 days after administering the vaccine's first dose. Immunoglobulin G (IgG) was quantified using immunoassays for anti-N-protein (nucleocapsid) antibodies (Abbott, Sligo, Ireland) and for anti-S1 (spike) protein antibodies (Euroimmun, Lübeck, Germany). Seroconversion by day 40 occurred in 129 (97%) HCWs for the S1 protein, and in 69 (51.87%) HCWs for the N protein. An absence of IgG antibodies (by both methodologies), occurred in 2 (1.5%) HCWs undergoing semiannual rituximab administration, and also in another 2 (1.5%) HCWs with no apparent reason. This study showed that CoronaVac has a high seroconversion rate when evaluated in an HCW population.
Keywords: Vaccine, Immunization, Public health, Immunoglobulin G, CoronaVac, Pandemic
1. Introduction
By July 5, 2021, approximately 1 year after the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, confirmed cases of infection worldwide numbered 183,560,151 people, including 3,978,581 deaths (World Health Organization (WHO) 2021). After the description of this new human coronavirus in December 2019, there was a global effort by researchers, public and private companies in the search for an effective vaccine to control this pandemic (Angeli et al., 2021; Golob et al., 2021; Kumar et al., 2021). These studies resulted in late 2020, with the first doses of immunization in the population, and there are currently 2,988,941,529 doses of the vaccine administered until July 5, 2021 (WHO, 2021).
Many SARS-CoV-2 proteins can induce an immune response, amongst them: M (membrane), E (envelope), N (nucleocapsid), and S (spike) (Zeng et al., 2020). However, the S and N proteins are the most responsive to infection, which induces high titers of anti-SARS-CoV-2 IgM and IgG antibodies. S protein has been more studied for vaccines because it participates in the virus entry mechanism through the connection of the S1 region receptor-binding domain (S1-RBD) in virus particles with the angiotensin-converting enzyme 2 (ACE 2) in the host cell (Barchuk et al., 2021; Saelens and Schepens, 2021). Then, the antibodies binding in this region can cause viral neutralization. Both S and N proteins have also been used for diagnosis, S protein is more specific despite being a more variable portion. In contrast, N protein is a more preserved region, including high homology with N protein SARS-CoV (>90%), but both may have false-positive results (Jiang et al., 2020). To evaluate the neutralization antibody activity, the gold-standard assay is the plaque reduction neutralization test (PRNT) that involves the measurement of the ability of patient sera to prevent infection (Murray et al., 2021). However, since this assay is time-consuming and requires higher levels of biological safety, multiple groups proposed anti-RBD ELISA assays as a reliable tool to predict neutralization (Murray et al., 2021; Padoan et al., 2021; Papenburg et al., 2021).
Worldwide efforts resulted in several vaccines against SARS-CoV-2 with distinct antigen platforms systems (nonreplicating viral vector, protein subunit, inactivated virus, and mRNA), with the main antigenic focus on S protein (Golob et al., 2021; Kumar et al., 2021).
The vaccination in Brazil started with CoronaVac (Sinovac Life Sciences, Beijing, China) in January 2021, and until June 2021, 2 other vaccines come into use in the country. However, CoronaVac (Sinovac Life Sciences, Beijing, China) remains the most administered in Brazilian territory (Brasil, Ministério da Saúde 2021), using the inactivated virus as a component of the vaccine (Golob et al., 2021; Kumar et al., 2021). In phase I/II studies, this vaccine was safe, tolerable, presented high immunogenicity, and had uncommon adverse reactions. A similar response was observed for both tested concentrations (3 μg and 6 μg), and 97% of seroconversion occurred in the participants with 18 to 59 ages (Padoan et al., 2021). In phase III trials, carried out with health care workers, this vaccine presented 50.7%, 83.7%, and 100% efficacy against symptomatic disease, cases requiring assistance, and severe cases, respectively (Zhang et al., 2021a, Zhang et al., 2021b). Phase III also tested some serum samples against the B.1.1.28, gamma (P.1), and zeta (P.2) variants, showing great antibody response (Palacios et al., 2021).
As the vaccine has been administered to people with different ethnicities, comorbidities, and ages, the results of pre-approval clinical trials for its use may not perfectly reflect the response to the vaccine. Thus, vaccine response analyses, either by seroconversion or by neutralizing antibody titration, are essential to assess the possible impacts of this immunization on the population and must be monitored so that the humoral response time can be defined. In this context, this study aimed to identify the seroconversion rate and antibody dynamic range after vaccination with SARS-CoV-2 (CoronaVac) in healthcare workers (HCWs) 40 days after its application.
2. Methods
2.1. Participants
In total, 170 participants were recruited at the Complexo Hospital de Clínicas, UFPR, Clinical Laboratory, Curitiba, Brazil, during the vaccination of HCWs in this city. The Institutional Ethical Committee approved the study (CAAE: 31687620.2.0000.0096), and all participants signed their consent.
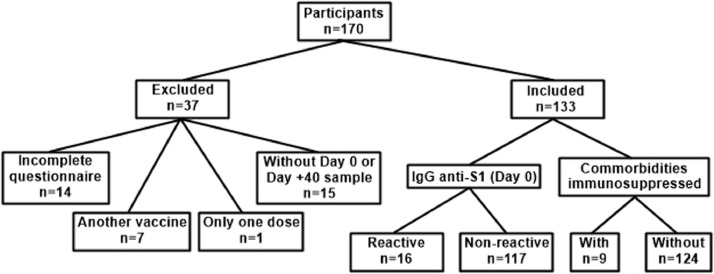
The inclusion criteria were as follows: answering the questionnaire, being vaccinated with 2 doses of CoronaVac, and providing serum samples. Fourteen participants were excluded because they did not complete the questionnaire. In addition, 7 participants took another vaccine, 1 participant did not have the second dose, and 15 participants did not provide a sample on days 0 (previous vaccination) or +40 (post-vaccination) (Fig. 1 ).
Fig. 1.
Participants included and excluded in the study and division of groups for analysis. Comorbidities (immunosuppressive) included: Immunosuppressive drugs use, Crohn's disease, bariatric surgery, HIV and Diabetes.
Serum samples of 133 healthcare workers included in this study were collected on days 0 (previous first dose application), +10, +20, +40, +60, and +110 after the first dose. On day 0 and +40, 133 serum samples were analyzed, and on day +10, +20, +60 and +110, 123, 119, 114 and 132 serum samples were analyzed, respectively. All samples were stored at −20 °C until analysis.
The participants were divided into 2 groups based on day 0 serology according to anti-spike-1 (anti-S1) immunoglobulin G (IgG) (Dutta et al., 2020, Fergie and Srivastava, 2021, Zeng et al., 2020): reactive (n = 16) and nonreactive (n = 117). The participants were also sorted according to the presence of comorbidities into 2 divisions: immunosuppressed (n = 9) or not (n = 124) (Fig. 1; Table 1 ). The immunosuppressed group consisted in participants who presented comorbidity associated with compromised humoral or cellular immune response or those who used immunosuppressive drugs, such as HIV infection, use of chemotherapy or steroids (prednisone at a dose of 20 mg/day or equivalent).
Table 1.
Demographics characteristics of participants included in the study for each respective group.a
| IgG Anti-S1 (Day 0) |
Comorbidities immunosuppressiveb |
|||||
|---|---|---|---|---|---|---|
| Reactive | Nonreactive | With | Without | |||
| n (%) | n (%) | P value | n (%) | n (%) | P value | |
| Total | 16 | 117 | 9 | 124 | ||
| Female | 13 (81.25) | 93 (79.49) | 1.0000 | 6 (66.67) | 100 (80.64) | 0.5636 |
| Median Age (IQR) | 44 (25.25−52.75) | 49 (39.50−53.50) | 0.2225 | 51 (45.50−54.50) | 48 (38.25−53.75) | 0.2297 |
Information on the handling of special cases: 2 immunosuppressed (Rituximab 1400 mg/semiannually), 1 myasthenia gravis (Pyridostigmine 120 mg/day), 1 Crohn's disease ostomized 22 years ago (Azathioprine 100 mg/day), 2 participants with bariatric surgery (11 and 12 years), and 1 HIV+ (Tenofovir 300 mg, Lamivudine 300 mg + Dolutegravir 50 mg/day; CD4+ 541/µL).
Comorbidities (immunosuppressive) included: Immunosuppressive drugs use, Crohn's disease, bariatric surgery, HIV and Diabetes. The patient with Myasthenia gravis is not included here because the treatment used was not immunosuppressive.
2.2. Seroconversion evaluation
Semi-quantitative assays were performed to detect anti-SARS-CoV-2 IgG. For all serum samples, assays used the Chemiluminescent Microparticle Immunoassay (CMIA) Architect-I System for anti-nucleocapsid protein (anti-N) IgG (Abbott, Sligo, Ireland). Additionally, for serum samples from days 0, +40 and +110, assays used the Enzyme-Linked Immunosorbent Assay (ELISA) for IgG anti-S1 spike-protein receptor-binding domain (RBD) (Euroimmun, Lübeck, Germany).
Samples were tested in duplicate, following the manufacturer's instructions. Results with a variation coefficient greater than 15.0% were repeated.
2.3. Statistical analysis
According to the distribution of seroconversion at day +40, the category variables were evaluated using Pearson's chi-squared test with Yates’ continuity correction. The age variable was evaluated using the Wilcoxon signed rank sum test with continuity correction. Samples paired over time were evaluated using the Friedman ANOVA test (as implemented in the rstatix package), followed by the Wilcoxon signed rank test as a post hoc pairwise comparison. For samples without multiple observations over time, the Wilcoxon signed rank test was used. All statistical analyses were performed using R (R Core Team). P values less than 0.05 were considered significant.
3. Results
3.1. Seroconversion to S1 protein
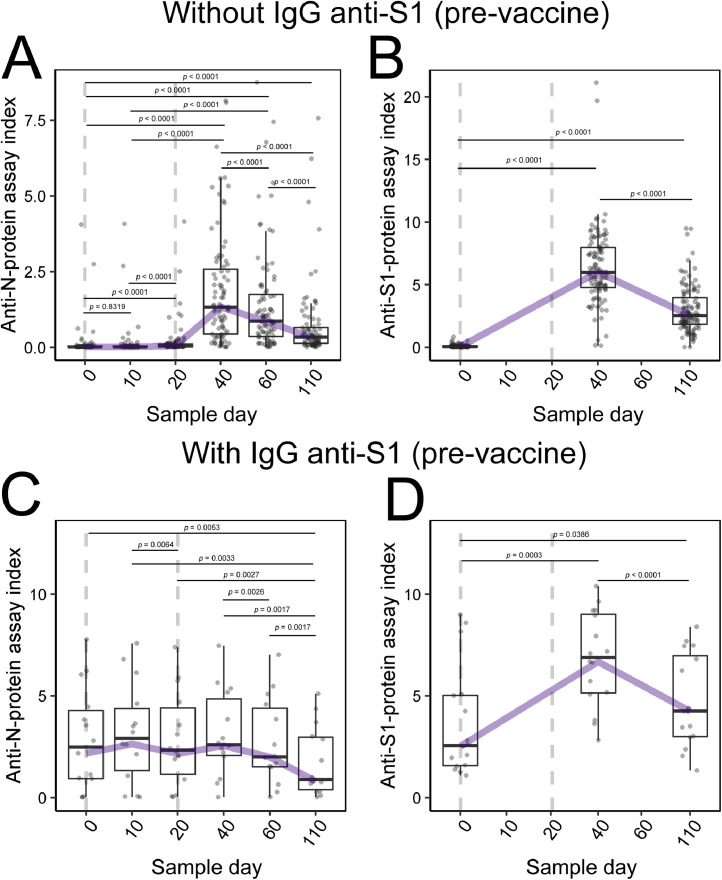
Robust production of anti-S1-protein IgG was observed by day +40 in 129 (97%) HCW participants by the index test result. Although the reactive (Fig. 2 D) and nonreactive (Fig. 2B) groups had different average index values for S1-protein IgG on day 0 (P < 0.0001), on day +40, the average index between the groups was not significantly different (P = 0.3704).
Fig. 2.
Antibody rates in the S1-protein IgG seroconverted/not seroconverted groups at day 0. Boxplot graph presents median (line dividing the box), interquartile range (box), maximum value (line above the box), and minimum value (line below the box). The line connecting the boxes represents the trend of the data. The dotted line represents the days of the vaccine application (2 doses). (A) N-protein IgG evaluation in S1-antibody nonreactive participants at day 0. (B) S1-protein IgG evaluation in S1-protein IgG nonreactive participants at day 0. (C) N-protein IgG evaluation in S1-protein IgG reactive participants at day 0. (D) IgG anti-S1 protein evaluation in anti-S1 protein IgG reactive participants at day 0.
3.2. Seroconversion to N protein
Distributing the data in the division of groups is possible to observe no significant production of the anti-N-protein IgG in nonreactive group participants 10 days after the first vaccine dose (P = 0.5027; Fig. 2A), and although there was a statistical difference in the sample on day +20 (P < 0.0001), there was no apparent seroconversion at that time. By contrast, there was a marked increase in N-protein IgG levels in 69 (51.87%) participants on day +40 (Fig. 2A). A significant difference was also observed in the average index for this antibody between the reactive (Fig. 2C) and nonreactive groups (Fig. 2A): day 0 (P < 0.0001) and day +40 (P = 0.0657).
3.3. Combined response
In the nonreactive group, better-developed antibody responses were observed for N and S1 proteins (P < 0.0001; Fig. 2A, B), while in the reactive group, the antibody response showed a significant difference (P < 0.0001) only for antibodies against S1 protein (Fig. 2D), increasing the level of circulating humoral response. No significant changes were observed in IgG anti-N protein analysis for the reactive group at days +10, +20, and +40 (P = 0.2231). The antibody index for IgG anti-N and anti-S1 presented at day +40 approximated mean of 2.0 and 6.0, respectively.
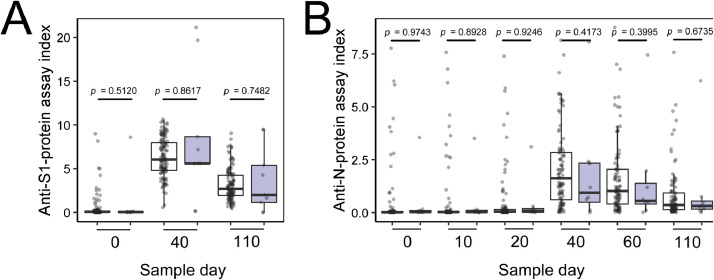
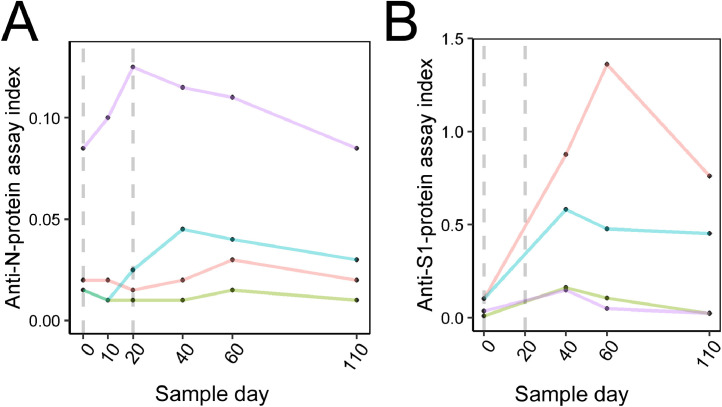
Comorbidities were reported by some HCWs, including Crohn's disease, prior bariatric surgery, HIV+, or diabetes. In general, the participants with comorbidities responded to the vaccine similarly to participants without any comorbidities (Fig. 3 ). However, 2 cases in the immunosuppressed group did not undergo seroconversion. Furthermore, 2 other HCWs (not in the immunosuppressed group) did not seroconvert by day +40; both had no apparent cause. These 4 HCWs without seroconversion were re-evaluated at +60 and +110 days. One participant presented seroconversion of the S1 protein in a sample of +60 days (Fig. 4 ).
Fig. 3.
Antibody rates for participants with and without immunosuppression. White boxes indicate nonimmunosuppressed participants. Gray boxes indicate immunosuppressed participants. (A) S1-protein IgG evaluation. (B) N-protein IgG evaluation.
Fig. 4.
Antibody rates for participants without seroconversion on day +40. Purple and green lines represent the participants with Rituximab treatment. The dotted line represents the days of the vaccine application (2 doses). (A) N-protein IgG evaluation. (B) S1-protein IgG evaluation (color version of figure is available online).
In the anti-S1 reactive group on day 0, 6 (37.50%) participants did not have a previous SARS-CoV-2 diagnosis, possibly due to asymptomatic infection. Furthermore, in the anti-S1 nonreactive group, 7 (5.98%) participants had symptoms suggestive of SARS-CoV-2 (fever, dry cough, tiredness, loss of taste or smell, aches and pains, headache, sore throat, nasal congestion, red eyes, diarrhea, or a skin rash) (WHO, 2021), although we did not have information about nasopharyngeal RT-PCR or immunological rapid-test detection. Demographic data according to immunologic response and comorbidities, are shown in Table 1.
3.4. Antibodies level range
Overall, it is observed that the antibody index showed a decrease in the comparison between days +40 vs +110. However, this antibody index in this last sample collection is still significantly higher when comparing days 0 vs +110 (all P < 0.0001) for both participants without (Fig. 2A and 2B) and those with (Fig. 2C and 2D) immunity before vaccination.
4. Discussion
The seroconversion rate of 97% for the anti-S1 IgG observed in HCWs is important data and corroborates the results of phase I/II trials of CoronaVac vaccine (Zhang et al., 2021a). However, it should be noted that the necessary antibody titers for protection are not entirely known. Furthermore, in the clinical trials carried out previously to vaccine registration, the primary outcome was disease severity, so it cannot be affirmed so far whether seroconversion or antibody titers are associated with protection from infection.
Several mutations in the RBD region of the S1 protein have been shown, giving rise to the viral variants of concern, as previously described: gamma (P.1), zeta (P.2), beta (B.1.351), alpha (B.1.1.7), and B.1.325 (Claro et al., 2021, Sabino et al., 2021, Tegally et al., 2021). Such mutations confer the potential for the virus to escape the humoral immune response produced due to the disease or to viral vectors or mRNA vaccines (Garcia-beltran et al., 2021). Thus, studies that evaluate vaccine efficacy against these new strains are valuable (Madhi et al., 2021).
Seroconversion rates observed for anti-N protein IgG could be valuable with the emergence of SARS-CoV-2 variants, considering the lower mutation levels in this protein (Dutta et al., 2020), compared to the high mutation levels in the S1 protein (Fergie and Srivastava, 2021). Thus, seroconversion of N-protein antibodies may be an alternative for the vaccine industry to produce efficient vaccines for circulating strains, including those that may arise in the future. However, more studies are needed to understand the impact of antibodies against other viral proteins in the protection against infection.
In this study, there was no difference in the analysis for the anti-N protein IgG in the reactive group, possibly due to the antibody levels present at day 0 in this group; the vaccine has not interfered in the humoral response; the group remained at the same average index. A total of 5.98% of the participants without seroconversion reported they had been previously infected by SARS-CoV-2. All of them presented seroconversion after the complete vaccination. Moreover, whether the person had experienced the disease or not, the levels of antibodies at day +40 post-vaccine were the same. This finding agrees with Krammer et al., 2021 in a study of individuals with and without previous COVID-19, given the mRNA vaccine. This same response level implies the same antigen concentration, showing no difference in individual antibody response regardless of the previous infection.
Higher index of anti-S1 antibodies were observed in comparison to the response of anti-N antibodies, corroborating what was exposed by Jiang et al., 2020. The Khoury et al., 2021 determination can be used to estimate the level of neutralizing antibodies; for a 50% protection caused by neutralizing antibodies, approximately 20% of the antibody levels observed in the ELISA assays correspond to this level of protection. And for 50% protection in severe cases, only 3% of antibody levels observed in ELISA assays correspond to such protection in severe cases (Khoury et al., 2021). Therefore, it is possible to estimate the index of neutralizing antibodies in this study.
In participants with immunosuppressive treatment (n = 2), the absence of the antibody response was probably due to rituximab having been administered approximately 1 month before the vaccine. In this situation, as described by Kado et al., 2016, there is a significant B lymphocytes decrease. Consequently, there is no production of antibodies until the B lymphocytes recover in 6 to 24 months. In such cases, the response must be evaluated after the repletion time, and re-vaccination considered with medical and clinical endorsement. Two other participants did not seroconvert on day +40. One of these had late-response seroconversion on day +60. No explanation was found for the other case, and more studies are needed to understand what interfered with the immune response.
As with the humoral response developed by other inactivated virus vaccines (Gresset-Bourgeois et al., 2017) and other vaccines for SARS-CoV-2 (Bayart et al., 2021), the dynamics of antibodies produced by CoronaVac in this study shows a peak in the antibody index followed by a sharp drop in that index. It is expected that even with these lower levels, memory B lymphocytes persist for a faster humoral response in cases of reinfection, resulting in less viral activity and minor damage to the host (Kurosaki et al., 2015). This lowest observed index has not yet been evaluated to verify whether the remaining humoral response is likely to generate a protective response against an infection.
The antibodies anti-SARS-CoV-2 produced by vaccine induction showed a significant decrease in the period of 3 to 6 months in other studies (Bayart et al., 2021, Yigit et al., 2021), as well as in this one, the need for a dose boosting has been recommended. Previous reports have already shown that the heterologous or homologous booster dose for SARS-CoV-2 vaccines (Ho et al., 2021), including CoronaVac (Keskin et al., 2021), have a surprising effect in the short term, even increasing the rate of effectiveness against the variants of concern (Yue et al., 2021). However, the antibody concentration needed to determine humoral protection remains unknown. However, it has been observed that about 6 months after completing the vaccination schedule, vaccinated individuals begin to show susceptibility to SARS-CoV-2 infection.
The immune response developed by vaccination depends not just on antibodies but primarily on neutralizing antibodies (Kurosaki et al., 2015). Both natural infection and vaccination act on the immune system in complex ways, stimulating the production of nonneutralizing antibodies (with their specific actions) and TCD4+ and TCD8+ T cells, which also act to protect against COVID-19, as shown by Tarke et al., 2021. That study evaluated the immune response to the SARS-CoV-2 variants and showed that cellular immunity-unlike the humoral response, is little affected by the virus variants. In addition to the specific immune response, innate immunity is another essential protection mechanism against infections (Kurosaki et al., 2015).
The present study has some limitations: the humoral immunity was studied semi-quantitatively, there was no quantification and titration of anti-SARS-CoV-2 antibodies, and no testing for neutralizing antibodies. The total number of participants was small, and immunosuppressed comorbidities were low in number and had diverse etiologies. More studies are needed to elucidate the vaccine response in these specific groups. However, this is the first study to evaluate the dynamics of IgG anti-N and anti-S1 production after CoronaVac immunization in the community.
The results of seroconversion have shown the importance of 2 doses for this vaccine as, until the second dose was applied, there was no change in the production of N-protein IgG, as previously described by Zhang et al., 2021 in phase I/II tests for this vaccine, with the antibody response detectable just 14 days after the second dose. The second dose is important for several types of vaccines, including mRNA vaccines, as described by Dörschug et al., 2021, resulting in a significant increase in antibody levels. Therefore, with SARS-CoV-2, there would be no difference at this point.
In conclusion, significant antibody production was observed 40 days after the first CoronaVac dose in the large majority of study participants, independent of comorbidities. The anti-N protein and anti-S1 protein antibody responses of participants without prior SARS-CoV-2 infection were comparable with those of the previously infected group, in which the immune response was maintained or optimized, with no decrease in levels.
Acknowledgments
We would like to thank all participants who agreed to participate in this study, those involved in the collection and storage of samples, and the Immunochemistry Laboratory section of Complexo Hospital de Clínicas, UFPR, and CAPES.
Funding
This work was supported by the PROPLAN/Federal University of Paraná, Curitiba-Paraná, Brazil; FINEP, Funder of Studies and Projects, Ministry of Science, Technology and Innovation, Brazil Institutional Network, Project: Laboratories for Diagnostic Tests for COVID-19 (grant number 0494/20).
Declaration of competing interest
The authors declare that there is no conflict.
Authors’ contributions
LBB: data collection, data analysis and interpretation, drafting the article, final approval. SMA: data analysis and interpretation, drafting the article, critical revision, final approval. SMR: data analysis and interpretation, drafting the article, critical revision, final approval. DA: data analysis and interpretation, drafting the article, critical revision, final approval. LLMA: data collection, drafting the article, final approval. SC: data collection, final approval. MBN: conception, data analysis and interpretation, drafting the article, critical revision, final approval, funding acquisition.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.diagmicrobio.2021.115597.
Appendix. Supplementary materials
References
- Angeli F, Spanevello A, Reboldi G, Visca D, Verdecchia P. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88(March):1–8. doi: 10.1016/j.ejim.2021.04.019. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchuk A, Shirokov D, Sergeeva M, Tursunzade R, Dudkina O, Tychkova V, et al. Evaluation of the performance of SARSCoV-2 antibody assays for the longitudinal populationbased study of COVID19 spread in St. Petersburg, Russia. J Med Virol. 2021;93:5846–5852. doi: 10.1002/jmv.27126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayart J, Douxfils J, Gillot C, David C, Mullier F, Elsen M, et al. Waning of IgG, total and neutralizing antibodies 6 months post-vaccination with BNT162b2 in healthcare workers. Vaccines. 2021;9:1092. doi: 10.3390/vaccines9101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil, Ministério da Saúde, COVID-19 Vacinação doses Aplicadas. [cited 2021 Jun 24] Available from:https://viz.saude.gov.br/extensions/DEMAS_C19Vacina/DEMAS_C19Vacina.html
- Claro IM, da Silva Sales FC, Ramundo MS, Candido DS, Silva CAM, de Jesus JG, et al. Local transmission of SARS-CoV-2 lineage B.1.1.7, Brazil, December 2020. Emerg Infect Dis. 2021;27(3):970–972. doi: 10.3201/eid2703.210038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörschug A, Frickmann H, Schwanbeck J, Yilmaz E, Mese K, Hahn A, et al. Comparative assessment of sera from individuals after S-Gene RNA-based SARS-CoV-2 vaccination with spike-protein-based and nucleocapsid-based serological assays. Diagnostics. 2021;11(3):426. doi: 10.3390/diagnostics11030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta NK, Mazumdar K, Gordy JT. The nucleocapsid protein of SARS–CoV-2: a target for vaccine development. J Virol. 2020;94(13):1–2. doi: 10.1128/JVI.00647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergie J, Srivastava A. Immunity to SARS-CoV-2: lessons learned. Front Immunol. 2021;12(March):1–12. doi: 10.3389/fimmu.2021.654165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-beltran WF, Lam EC, Denis KS, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob JL, Lugogo N, Lauring AS, Lok AS. SARS-CoV-2 vaccines: a triumph of science and collaboration. JCI Insight. 2021;6:1–11. doi: 10.1172/jci.insight.149187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresset-Bourgeois V, Leventhal PS, Pepin S, Hollingsworth R, Kazek-Duret M-P, De Bruijn I, et al. Quadrivalent inactivated influenza vaccine (VaxigripTetra™) Expert Rev Vaccines. 2017:1–11. doi: 10.1080/14760584.2018.1407650. [DOI] [PubMed] [Google Scholar]
- Ho T, Chen Y, Chan H, Chang C, Chuang K, Lee C, et al. the effects of heterologous immunization with prime-boost COVID-19 vaccination against SARS-CoV-2. Vaccines. 2021;9:1163. doi: 10.3390/vaccines9101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Li Y, Zhang H, Wang W, Yang X, Qi H, et al. SARS-CoV-2 proteome microarray for global profiling of COVID-19 specific IgG and IgM responses. Nat Commun. 2020;11(1):1–11. doi: 10.1038/s41467-020-17488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado R, Sanders G, Joseph, McCune W. Suppression of normal immune responses after treatment with rituximab. Curr Opin Rheumatol. 2016;28(3):251–258. doi: 10.1097/BOR.0000000000000272. [DOI] [PubMed] [Google Scholar]
- Keskin AU, Bolukcu S, Ciragil P, Topkaya AE. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J Med Virol. 2021 doi: 10.1002/jmv.27350. [DOI] [PubMed] [Google Scholar]
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SU, Priya NM, Priyanka SRN, Nikita K, Thirumal JD. A review of novel coronavirus disease (COVID ‑ 19): based on genomic structure, phylogeny, current shreds of evidence, candidate vaccines, and drug repurposing. 3 Biotech. 2021;11 doi: 10.1007/s13205-021-02749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol. 2015;15:149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, McIntosh M, Atkinson C, Mahungu T, Wright E, Chatterton W, et al. Validation of a commercially available indirect assay for SARS-CoV-2 neutralising antibodies using a pseudotyped virus assay. J Infect. 2021;82(5):170–177. doi: 10.1016/j.jinf.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoan A, Bonfante F, Cosma C, Di Chiara C, Sciacovelli L, Pagliari M, et al. Analytical and clinical performances of a SARS-CoV-2 S-RBD IgG assay: comparison with neutralization titers. Clin Chem Lab Med. 2021;59:1444–1452. doi: 10.1515/cclm-2021-0313. [DOI] [PubMed] [Google Scholar]
- Palacios R, Batista AP, Albuquerque CSN, Patiño EG, Santos J do P, Tilli Reis Pessoa Conde M, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in brazil: the PROFISCOV study. SSRN Electron J. 2021;21 doi: 10.1186/s13063-020-04775-4. [DOI] [Google Scholar]
- Papenburg J, Cheng MP, Corsini R, Caya C, Mendoza E, Manguiat K, et al. Evaluation of a commercial culture-free neutralization antibody detection kit for severe acute respiratory syndrome-related coronavirus-2 and comparison with an antireceptor-binding domain enzyme-linked immunosorbent assay. Open Forum Infect Dis. 2021;8(6) doi: 10.1093/ofid/ofab220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino EC, Buss LF, Carvalho MPS, Prete CA, Crispim MAE, Fraiji NA, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397(10273):452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelens X, Schepens B. Single-domain antibodies make a difference. Science (80-) 2021;371(6530):681–682. doi: 10.1126/science.abg2294. [DOI] [PubMed] [Google Scholar]
- Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, Goodwin B, et al. Impact of SARS-CoV-2 variants on the total CD4 + and CD8 + T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), Coronavirus disease (COVID-19) advice for the public. [cited 2021 Jun 24]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public
- World Health Organization (WHO), WHO coronavirus (COVID-19) dashboard. [cited 2021 Jul 5]. Available from: https://covid19.who.int/
- Yigit M, Ozkaya-Parlakay A, Cosgun Y, Ince YE, Bulut YE, Senel E. Should a third booster dose be scheduled after two doses of CoronaVac? A single-center experience. J Med Virol. 2021:1–4. doi: 10.1002/jmv.27318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Zhou J, Zhou Y, Yang X, Xie T, Yang M, et al. Antibody response elicited by a third boost dose of inactivated SARS-CoV-2 vaccine can neutralize SARS-CoV-2 variants of concern. Emerg Microbes Infect. 2021 doi: 10.1080/22221751.2021.1996210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Liu G, Ma H, Zhao D, Yang Y, Liu M, et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochem Biophys Res Commun. 2020;527(3):618–623. doi: 10.1016/j.bbrc.2020.04.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis [Internet] 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. https://linkinghub.elsevier.com/retrieve/pii/S1473309920308434 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.