Abstract
The efficacy of sodium lauryl sulfate (SLS), a sulfated anionic chaotropic surfactant, and dextran sulfate (DS), a polysulfated carbohydrate, against herpes simplex virus (HSV) and human immunodeficiency virus (HIV) infections was evaluated in cultured cells and in different murine models of HSV infection. Results showed that both SLS and DS were potent inhibitors of the infectivities of various HSV-1 and HSV-2 strains. Pretreatment of HIV-1 (strain NL4-3) with SLS also reduced its infectivity to 1G5 cells. DS prevented the binding of HSV to cell surface receptors and therefore its entry into cells. Pretreatment of HSV-1 (strain F) with 50 μM SLS resulted in a complete loss of virus infectivity to Vero cells. However, viruses were able to enter into cells and to produce in the nuclei capsid shells devoid of a DNA core. The amount of the glycoprotein D gene produced in these cells remained unchanged compared to controls, suggesting that SLS could interfere with the maturation of the virus. At a higher SLS concentration (100 μM), HSV was highly damaged by SLS pretreatment and only a few viral particles could enter into cells to produce abnormal capsids. Although DS was a more potent inhibitor of HSV infectivity in vitro, it was unable to provide any protection in murine models of HSV infection. However, SLS conferred a complete protection of animals infected cutaneously with pretreated viruses. In addition, skin pretreatment of mice with a polymer formulation containing SLS completely prevented the development of cutaneous lesions. More interestingly, intravaginal pretreatment of mice with SLS in a buffered solution also completely protected against lethal HSV-2 infection. Taken together, our results suggest that SLS could thus represent a candidate of choice as a microbicide to prevent the sexual transmission of HIV, HSV, and possibly other pathogens that cause sexually transmitted diseases.
The global incidence, morbidity, and mortality of sexually transmitted diseases (STDs) caused by Human immunodeficiency virus (HIV), Herpes simplex virus (HSV), and other pathogens are very significant. Several hundred million individuals are infected worldwide with pathogens causing STDs (17). In fact, 5 of the 10 most commonly reported infectious diseases are sexually transmitted (13). Young women are biologically more susceptible to sexually transmitted infections because of their immature cervical epithelialization. Underlying gender power inequalities may also limit women's ability to negotiate condom use with their partners, especially if domestic violence or economic abandonment are present (12). The development of safe topical microbicides under women's control is actually a very high priority for the World Health Organization, the National Institutes of Health, and the Centers for Disease Control and Prevention in the field of prevention of STDs and HIV.
A topical microbicide is often composed of an active ingredient and a vehicle (11). Active ingredients may act via a variety of mechanisms, including (i) disrupting the organism cell membrane, envelope or capsid lipid or protein constituents (e.g., detergent-type spermicides and/or microbicides such as nonoxynol-9); (ii) blocking the receptor-ligand interactions essential for infectivity (e.g., microbial adhesion inhibitors such as sulfated compounds); (iii) inhibiting the intracellular or extracellular replication of the pathogen (e.g., antimicrobial drugs); (iv) altering the vaginal environment and reducing susceptibility to infection (e.g., buffering agents and products that maintain normal vaginal flora and environment); or (v) enhancing local immune responses (e.g., immune response modifiers) (34).
Most currently available vaginal formulations use the spermicide nonoxynol-9, a nonionic surfactant, as a microbicide. In vitro, nonoxynol-9 inactivates enveloped viruses, such as HSV, HIV, and other microorganisms, including Chlamydia trachomatis and Neisseria gonorrhoeae (1, 7, 14, 22, 41). However, the potential efficacy of nonoxynol-9 against HIV has never been clearly established, and the results of clinical trials are controversial (14, 23, 33, 41, 42). A recent controlled trial conducted among 1,292 HIV-negative female sex workers in Cameroon showed that the use of a vaginal film containing 70 mg of nonoxynol-9, inserted intravaginally before intercourse, did not reduce the rate of new HIV, gonorrhea, or chlamydia infection (33). The frequent use of nonoxynol-9 was also associated with an increased incidence of vulvar ulcers and vulvitis which could increase the risk of HIV infection (23, 38, 42). Consequently, there is an urgent need to develop novel compounds that can efficiently reduce sexually transmitted infections.
To initiate an infection, an obligate intracellular pathogen must attach to and enter the cell through specific receptor-ligand interactions (35). The adherence of C. trachomatis, N. gonorrhoeae, and herpesviruses to host cells involves a common cell surface receptor, heparan sulfate glycosaminoglycans (35). In vitro, sulfated carbohydrate compounds that mimic heparan sulfate potentially interfere with the attachment of pathogens to cells (19). Moreover, in vitro studies have shown that polysulfated carbohydrates also inhibit HIV binding, replication, and syncytium formation, probably because they interfere with the ionic interaction between cell surface components such as CD4 or sulfated polysaccharides and positively charged amino acids concentrated in the V3 region of HIV gp120 (2, 3, 5, 21, 26).
Sodium lauryl sulfate (SLS) is a sulfated surfactant that denatures membrane proteins of cells and pathogens. It thus has a dual action as a detergent and as a chaotropic agent. Previous studies from our laboratory have demonstrated that in vitro SLS inhibited the infectivity of HSV-1 to Vero cells at quite low concentrations, suggesting that SLS could be a potential candidate for use as a topical microbicide (J. Piret, A. Désormeaux, P. Gourde, and M. G. Bergeron, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H8, 1998). On the other hand, dextran sulfate (DS) is a polysulfated carbohydrate which has been shown to inhibit in vitro the infectivities of HIV and herpesviruses. In this study, we have evaluated the efficacy of SLS and DS against HSV and HIV infections in vitro and in various animal models of HSV infections. The mechanism of action of these two compounds was also examined in vitro.
MATERIALS AND METHODS
Materials.
SLS and DS were obtained from Sigma Chemical Co. (St. Louis, Mo.). 35S-labeled-methionine was purchased from Amersham Canada, Ltd. (Oakville, Ontario, Canada).
Cell lines.
Vero cells (African green monkey kidney cells; ATCC CCL-81; Rockville, Md.) were cultivated in Eagle minimal essential medium (EMEM; Life Technologies, Burlington, Ontario, Canada) supplemented with 5% heat inactivated fetal bovine serum (FBS; Life Technologies), sodium bicarbonate (0.22%), penicillin-streptomycin (100 U/ml), and l-glutamine (2 mM). 1G5 cells, a derivative of Jurkat E6-1 cells that contain a stable integrated HIV-1–LTR–luciferase construct, were provided by the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases. 1G5 cells were cultured in RPMI 1640 medium (Life Technologies) supplemented with 10% FBS, penicillin G (100 U/ml), streptomycin (100 mg/ml), and l-glutamine (2 mM). The human embryonic kidney cell line 293T expressing the simian virus 40 T antigen was maintained in Dulbecco modified Eagle medium (DMEM; Life Technologies) supplemented with 10% FBS, penicillin G (100 U/ml), streptomycin (100 mg/ml), and l-glutamine (2 mM). Cultures were maintained at 37°C in a 5% CO2 atmosphere.
HSV strains.
HSV-1 strain F (ATCC VR-733), HSV-2 strain 333 (kindly provided by Lawrence R. Stanberry, Children's Hospital Medical Center, Cincinnati, Ohio), wild-type HSV-2 strain 22, HSV-2 strain 6 resistant to acyclovir (thymidine kinase deficient), and HSV-2 strain 15589 resistant to foscarnet (kindly provided by Guy Boivin, Centre de Recherche en Infectiologie, Laval University, Ste-Foy, Québec, Canada) were propagated in Vero cells.
Concentrating HSV.
HSV-1 (strain F) was propagated in Vero cells by using EMEM–2% FBS as maintenance medium. At approximately 80 to 90% cell lysis, cells were scraped off from the dishes by using a sterile cell scraper. The cellular suspension was centrifuged (1,450 × g for 10 min at 4°C), and the supernatant was retained. The pellet was submitted to three freeze-thaw cycles by using dry ice in methanol and then centrifuged again. Supernatants were pooled, filtered on a 0.45-μm (pore-size) Durapore low-binding membrane (Millipore Co., Bedford, Mass.), and centrifuged (100,000 × g for 2 h 40 min at 4°C with slow deceleration). The supernatant was discarded, and the pellet was resuspended in EMEM–2% FBS overnight at 4°C and stored at −80°C in small aliquots. The viral titer determined in Vero cells was 3.15 × 108 PFU/ml.
Preparation of radiolabeled HSV.
Vero cells were incubated with HSV-1 (strain F) at a multiplicity of infection of 0.1 for 1 h at 37°C to allow virus adsorption. The medium was removed, and cell sheets were washed twice with methionine-free DMEM, 10% regular DMEM, and 4% dialyzed FBS. Cells were then incubated with the above-described medium containing 25 μCi of [35S]methionine/ml for 2 days at 37°C. Cells and medium were collected, frozen at −80°C, and thawed at 37°C. The suspension was centrifuged (600 × g for 10 min at 4°C) to pellet the cell debris, and the supernatant (10 ml) was layered over a 3-ml cushion of 15% sucrose in a 15-ml polyallomer bell-top Quick-Seal centrifuge tube (Beckman Instruments, Inc., Palo Alto, Calif.). Samples were centrifuged (100,000 × g for 2 h at 37°C) to pellet the virus which was resuspended in 1 ml of phosphate-buffered saline (PBS; pH 7.4) overnight on ice at 4°C. The specific activity of the virus was approximately 0.12 cpm/PFU.
Production of HIV-1 strain NL4-3 stocks.
All transfections were performed by following a modification of the calcium phosphate transfection protocol of Chen and Okayama (10). A typical transfection experiment was carried out with 10 μg of pNL4-3. Plasmidic preparations were mixed with 25 μl of 2.5 μM CaCl2, and the volume was completed to 250 μl of 2× HBS buffer (280 mM NaCl, 50 mM HEPES, 1.5 mM Na2HPO4; pH 7.05) and incubated at room temperature for 4 min. The solution was overlaid onto a semiconfluent monolayer of 293T cells that had been seeded (5 × 105 cells/well in 3 ml of DMEM plus 10% FBS) 24 h before initiation of transfection in a six-well plate (Falcon; Becton Dickinson, Lincoln Park, N.J.). At 16 h posttransfection, cells were washed twice with 3 ml of PBS and incubated for 24 h in 3 ml of DMEM–10% FBS. Virion-containing supernatants were filtered through a 0.45-μm-pore-size cellulose acetate membrane (Millipore), aliquoted in 500-μl fractions, and frozen at −85°C until use. All virus stocks underwent one freeze-thaw cycle before initiation of infection studies. Virus stocks were normalized for virion content by using a p24 commercial assay (Organon Teknika, Durham, N.C.). The standardization of p24 content is based on the observation that, in such virus preparations, the great majority of viral p24 is part of complete HIV-1 particles (15).
Preparation of polymer formulations.
In some experiments, a polymer composed of polyoxypropylene and polyoxyethylene suspended in phosphate buffer (200 mM, pH 6.0) at a concentration of 18% (wt/wt) was used as a vehicle. For the formulations containing SLS or DS, each of these products was first dissolved in phosphate buffer at concentrations of 5% for SLS and 1% for DS. These solutions were then mixed under agitation with the polymer powder to get a final polymer concentration of 18% (wt/wt).
HSV infectivity to Vero cells.
Vero cells were seeded in 24-well plates. Prior to infection, the virus was either suspended in PBS or diluted with SLS (6.25 to 100 μM) or DS (0.1 to 50 nM) at the desired concentration in PBS and preincubated for 1 h at 37°C in a water bath. Confluent cells were infected with HSV (approximately 50 to 100 PFU/500 μl), and plates were centrifuged (750 × g for 45 min at 20°C). Virus was removed by aspiration, and cell sheets were overlaid with 0.5 ml of EMEM plus 2% FBS containing 0.6% SeaPlaque agarose (Marine Colloids, Rockland, Maine). The plates were incubated for 2 days at 37°C in a 5% CO2 atmosphere. Cells were then fixed with 10% formaldehyde in PBS for 20 min, washed with deionized water, and stained with 0.05% methylene blue. Virus infectivity was evaluated following the determination of PFU.
Plaque reduction assay.
Confluent Vero cells seeded in 24-well plates were infected with approximately 100 PFU of HSV-1 (strain F) in 0.5 ml of EMEM plus 2% FBS for 2 h at 37°C. The infected medium was removed, and cell sheets were overlaid with 0.5 ml of 0.6% SeaPlaque agarose in EMEM plus 2% FBS containing increasing concentrations of SLS or DS. The plates were incubated for 2 days at 37°C, and cells were fixed with 10% formaldehyde in PBS for 20 min, washed with deionized water, and stained with 0.05% methylene blue. Virus-induced cytopathic effect (CPE) was evaluated by determination of PFUs.
HIV-1 infectivity to 1G5 cells.
HIV-1 (strain NL4-3) was pretreated with 500 μM SLS for 1 h at 37°C in a final volume of 100 μl of complete culture medium. 1G5 cells were then infected with equal amounts of pretreated NL4-3 (10 ng of p24) for 2 h at 37°C in a final volume of 200 μl. Cells were then washed with PBS, resuspended in 200 μl of complete culture medium, and transferred in 96-well flat-bottom tissue culture plates. After an incubation period of 72 h at 37°C, luciferase activity was monitored as described previously (6). In brief, 100 μl of cell-free culture supernatant was withdrawn from each well, and 25 μl of 5× cell culture lysis (125 mM Tris-phosphate [pH 7.8], 10 nM dithiothreitol, 5% Triton X-100, 50% glycerol) was added before incubation at room temperature for 30 min. Thereafter, an aliquot of this cell lysate (20 μl) was mixed with 100 μl luciferase assay buffer [20 mM tricine, 1.07 mM (MgCO3)4 · Mg(OH)2 · 5H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 270 μM coenzyme A, 470 μM luciferin, 530 μM ATP, 33.3 mM dithiothreitol) to evaluate activity from a microplate luminometer (MLZ; Dynex Technologies, Chantilly, Va.).
Cellular viability.
At confluency, cells were incubated with PBS (Vero cells), RPMI 1640 medium (1G5 cells), SLS, or DS at the desired concentrations prepared in the corresponding medium for 1 h at 37°C in a 5% CO2 atmosphere. Cell sheets were washed twice with the corresponding medium. Cell viability was then determined by using a tetrazolium salt in the presence of phenazine methosulfate-based colorimetric assay as previously described by Buttke et al. (9).
Binding of radiolabeled HSV to Vero cells.
Confluent monolayers of Vero cells seeded in 96-well plates were first incubated for 30 min at 4°C with PBS plus 1% bovine serum albumin (BSA) in order to block nonspecific virus adsorption. The radiolabeled virus was diluted with PBS–1% BSA containing various concentrations of SLS or DS and incubated for 1 h at 37°C. Cells were then incubated with pretreated virus for 1 h at 4°C with gentle agitation. Cells were washed three times with cold PBS and lysed with 1% SDS–1% Triton X-100, and the amount of radioactivity was determined by liquid scintillation counting.
Electron microscopy.
HSV-1 (strain F) was pretreated with various SLS concentrations (50, 75, and 100 μM) in EMEM–2% FBS for 1 h at 37°C in a water bath. Vero cells (80 to 90% confluent) were then infected with the virus (approximately 70 PFU/ml in 14 ml) for 2 days at 37°C. Cells were scraped off from the dishes and resuspended in culture medium. Cells were centrifuged (515 × g for 10 min at 4°C). The supernatant was decanted, and the cells were resuspended in approximately 500 μl of medium. Cells were transferred in an Eppendorf tube and centrifuged (10,000 × g for 5 min at 4°C). The supernatant was completely removed, and the pellet was resuspended in approximately 200 μl of 20% BSA. Then, 15 to 100 μl of 25% glutaraldehyde (depending upon the size of the pellet obtained) was added to the mixture, and the samples were immediately put in an ice bath to allow polymerization. The pellet was cut into 1-mm3 samples, which were treated in 2% glutaraldehyde in PBS for 1 h, postfixed with 1% OsO4 in PBS for 1 h, and then postfixed with 0.1% tannic acid in PBS for 30 min. Samples were rinsed three times in PBS for 5 min between each step. Samples were stained with 2% uranyl acetate in 10% ethanol for 30 min. Samples were dehydrated and embedded in Epon according to routine procedures. Sections (approximately 75-nm thickness) were mounted on copper grid (200 mesh). Specimens were stained with uranyl acetate, counterstained with lead citrate, and observed with a JEOL 1010 electron microscope (JEOL Canada, Inc., St-Hubert, Québec, Canada).
Quantification of HSV glycoprotein D gene in Vero cells.
HSV-1 (strain F) was pretreated with various SLS concentrations (12.5, 25, 50, 75, and 100 μM) in EMEM–2% FBS for 1 h at 37°C in a water bath. Vero cells (80 to 90% confluent) were infected with the virus (100 PFU/ml in 20 ml) for 2 days at 37°C. The culture medium was removed, and the cell sheets were washed twice with sterile Hanks' balanced salt solution. Cells were scraped off from the dishes and resuspended in EMEM–2% FBS. Total DNA was extracted by using the standard phenol-chloroform procedure (36). Quantification of total DNA was achieved by using the Burton procedure (8). BglII-fragmented DNA aliquots (325 ng) were applied to 0.8% agarose gel, transferred to a nylon membrane, and hybridized with the 32P-labeled glycoprotein D probe. The probe used for this study corresponds to a part of glycoprotein D of HSV-2 (strain 333), generated by PCR using P1 (5′-GCCACCATGGGGCGTTTGACC-3′) and P2 (5′-AAACTCAGTTATCTAGTCCTCGGGGTC-3′) primers and was 32P-labeled by random priming. Hybridization was performed at 65°C in 0.25 M Na2HPO4 (pH 6.8 with orthophosphoric acid) and 7% sodium dodecyl sulfate (SDS). Washes were done in 40 mM Na2HPO4 (pH 6.8 with orthophosphoric acid) and 1% SDS for 20 min at 65°C, followed by treatment for 20 min at 25°C.
HSV-1 cutaneous infections.
Female hairless mice (SKH1; Charles River Breeding Laboratories, Inc., St-Constant, Québec, Canada), 5 to 6 weeks old, were used for this study. Mice were anesthetized by intraperitoneal injection of a 70-mg/kg ketamine hydrochloride and 11.5-mg/kg xylazine mixture. The virus was inoculated on the lateral side of the body in the left lumbar skin area. Two different protocols for induction of cutaneous lesions were used. A first protocol was used for the evaluation of the infectivity of HSV-1 (strain F) pretreated with SLS or DS. Prior to infection, the virus was diluted with PBS, SLS (6.25, 25, or 100 μM), or DS (0.25, 1, or 10 nM) prepared in PBS to obtain a viral inoculum of 3 × 105 PFU/50 μl and then incubated for 1 h at 37°C. The skin was scratched six times in a crossed-hatched pattern with a 27-gauge needle. Viral suspension (50 μl) was deposited onto the scarified area and rubbed for 10 to 15 s with a cotton tipped applicator saturated with the different solutions.
The second protocol was used to evaluate the capacity of formulations containing SLS or DS to protect against the development of cutaneous infection. In this case, 50 μl of the polymer formulations were applied on the left lateral side of mice, and the treated area was protected by using a Tegaderm patch (3M Canada, London, Ontario, Canada). After 5 min or 1 h, the Tegaderm patch was removed, and the skin was scratched only once with a 27-gauge needle held vertically. Viral suspension (3.15 × 108 PFU/ml) was deposited on the pretreated skin area. In this model, the viral inoculum used needed to be higher than that previously mentioned to obtain a complete zosteriform rash in almost all mice. However, the mortality associated with infection was low and could not be used as a criteria to evaluate the efficacy of pretreatments. In both cases, the scarified area was protected with a corn cushion (Schering-Plough Canada, Inc., Mississauga, Ontario, Canada), which was attached to the mouse body with surgical tape. The aperture of the corn cushion was also closed with surgical tape. Mice were returned to their cages and observed daily for signs of cutaneous infections and death for a period of 15 days.
HSV-2 intravaginal infection.
Female BALB/c mice (Charles River Breeding Laboratories, Inc.), 4 weeks old, were used for this study. On days 7 and 1 prior to infection, mice were injected subcutaneously in the neck region with 150 mg of progestogen (Pharmacia and Upjohn, Don Mills, Ontario, Canada) per ml. Mice were anesthetized by intraperitoneal injection of a mixture containing 70 mg of ketamine hydrochloride and 11.5 mg of xylazine per kg. The vaginal secretions were removed by turning a calcium alginate swab five times into the vagina. Mice were pretreated with 15 μl of a pH 4.0 citrate buffer alone or containing SLS or DS administered into the vagina by using a micropipette. Mice were maintained for 5 min on their back, and 1.2 × 105 PFU of HSV-2 (strain 333) per 5 μl was inoculated into the vagina while the micropipette was moved up and down five times to simulate coitus. Mice were returned to their cages and examined daily for symptoms of vaginal infections and death for a period of 15 days. The criteria used for the evaluation of herpetic genital infections were the degree of redness or swelling in the perineal region (ranked 1 for minimal, 2 for moderate, and 3 for marked) and death.
RESULTS
Infectivity of HSV-1 and HSV-2 strains pretreated with SLS or DS.
Table 1 shows that pretreatment of various HSV-1 and HSV-2 strains with SLS or DS for 1 h at 37°C decreased, in a concentration-dependent manner, their infectivities to Vero cells. HSV-1 (strain F) infectivity was reduced to 21% when viral particles were pretreated with 25 μM SLS. The infectivities of all HSV-2 strains were between 51 and 73% after preincubation with 25 μM SLS. A complete loss of infectivity of all strains tested was obtained after pretreatment of the viruses with 50 μM SLS. The concentrations of DS required to inhibit 50 and 100% of the viral infectivities of HSV-1 (strain F) and HSV-2 (strain 22) were 1 and 50 nM, respectively. Preincubation of Vero cells for 1 h at 37°C with SLS concentrations ranging from 6.25 to 100 μM prior to their infection with HSV-1 (strain F) did not result in a loss of infectivity of the virus (data not shown).
TABLE 1.
Infectivity of HSV-1 and HSV-2 strains pretreated with different concentrations of SLS and DS for 1 h at 37°C
| Compound and concn | PFU (% of control) for:
|
||||
|---|---|---|---|---|---|
| HSV-1 (F)a | HSV-2 (333)a | HSV-2 (22)a | HSV-2 (6)b | HSV-2 (15589)c | |
| SLS (μM) | |||||
| 6.25 | 101.1 ± 7.0d | 102.9 ± 23.5 | 128.0 ± 18.5 | 105.3 ± 12.4 | 108.7 ± 22.2 |
| 12.5 | 79.2 ± 36.4 | 115.4 ± 17.0 | 103.4 ± 14.9 | 82.1 ± 40.7 | 115.1 ± 17.5 |
| 25 | 21.2 ± 18.0 | 72.9 ± 9.1 | 63.8 ± 11.9 | 51.1 ± 30.1 | 59.0 ± 24.0 |
| 50 | 0 | 0 | 0 | 0 | 0 |
| DS (nM) | |||||
| 0.1 | 88.4 ± 23.7 | 69.2 ± 1.4 | |||
| 0.25 | 72.6 ± 30.2 | 63.1 ± 6.2 | |||
| 0.5 | 58.8 ± 21.2 | 55.2 ± 3.1 | |||
| 1 | 49.3 ± 22.7 | 43.5 ± 3.1 | |||
| 10 | 15.6 ± 9.1 | 3.2 ± 1.5 | |||
| 50 | 0 | 0 | |||
Wild-type strain.
Acyclovir-resistant strain.
Foscarnet-resistant strain.
Results are the mean (± the SD) of four independent experiments.
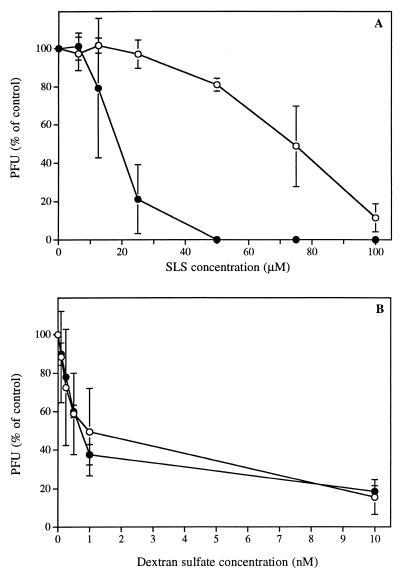
Figure 1 shows the effect of time of pretreatment of HSV-1 (strain F) with different concentrations of SLS (Fig. 1A) or DS (Fig. 1B) on its infectivity to Vero cells. When SLS was immediately added to Vero cells following their infection, the loss of viral infectivity was less dramatic compared to that obtained for virus pretreated for 1 h at 37°C with the same SLS concentrations. After pretreatment, a loss of 50% of viral infectivity was observed at a concentration of 20 μM compared to a concentration of 75 μM when the virus was not pretreated. Moreover, although a complete inhibition of viral infectivity was obtained following preincubation with 50 μM SLS, the inhibition was not complete even at 100 μM without pretreatment. Similarly, the time of pretreatment of HSV-2 (strain 333) with SLS also influenced the infectivity of this strain (data not shown). On the other hand, DS reduced the infectivity of the virus independent of the time of pretreatment. In this case, a loss of 50% of viral infectivity was observed at a concentration of about 1 nM. No signs of cytotoxicity have been observed in the range of SLS and DS concentrations used (data not shown).
FIG. 1.
Infectivity of HSV-1 (strain F) to Vero cells after pretreatment of the virus with different concentrations of SLS (A) or DS (B) for 1 h at 37°C (●) or after the addition of SLS or DS to viruses without pretreatment (○). PFU are expressed as a percentage of the control. Results are the mean ± the standard deviation (SD) of four independent experiments.
In vitro infectivity of HIV-1 (strain NL4-3) pretreated with SLS.
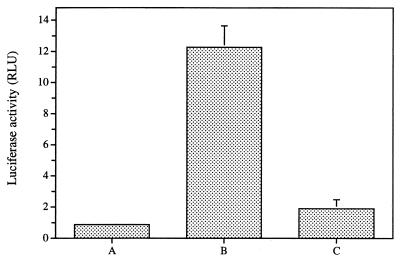
The effect of pretreating HIV-1 (strain NL4-3) with SLS on its infectivity to 1G5 cells has been also evaluated. Results from this set of experiments clearly showed that pretreatment of this HIV-1 strain with 500 μM SLS for 1 h at 37°C almost completely inhibited HIV-1 infectivity to 1G5 cells (Fig. 2).
FIG. 2.
Effect of pretreating HIV-1 (strain NL4-3) with 500 μM of SLS for 1 h at 37°C on its infectivity of 1G5 cells. (A) Uninfected untreated cells. (B) Cells infected with untreated virus. (C) Cells infected with virus pretreated with 500 μM. Values represent the mean ± the SD of three determinations.
INfluence of SLS and DS on HSV-1 (strain F)-induced CPE.
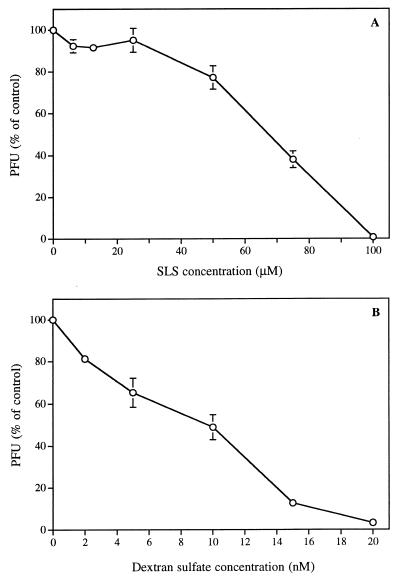
Figure 3A shows that the concentrations of SLS needed to inhibit 50 and 100% of HSV-1 (strain F)-induced CPE in Vero cells were 70 and 100 μM, respectively, as evaluated by plaque reduction assay. DS also inhibited virus-induced CPE, with 50 and 100% inhibitory effects at 10 and 20 nM, respectively. Thus, the 50% effective dose of DS was 7,000-fold lower than that of SLS.
FIG. 3.
Influence of SLS (A) or DS (B) on HSV-1 (strain F)-induced CPE in Vero cells. PFU are expressed as the percentage of the control. Results are the mean ± the SD of three independent experiments.
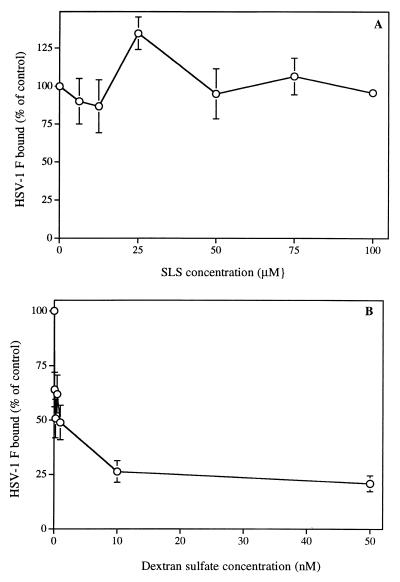
Binding of HSV-1 (strain F) pretreated with SLS or DS to Vero cells.
Figure 4A shows that the binding of [35S]methionine-labeled HSV-1 (strain F) to Vero cells was not influenced by the pretreatment of virus for 1 h at 37°C with concentrations ranging from 6.25 to 100 μM SLS. In contrast, pretreatment of HSV-1 (strain F) with DS markedly reduced the binding of the virus to cells in the range of concentrations altering virus infectivity. A reduction of 50% of the binding was obtained when the virus was pretreated with 1 nM DS.
FIG. 4.
Binding of [35S]methionine-labeled HSV-1 (strain F) pretreated with SLS (A) or DS (B) to Vero cells. Binding was expressed as the percentage of counts per minute in the control (without SLS or DS added). Results are the mean ± the SD of three independent experiments.
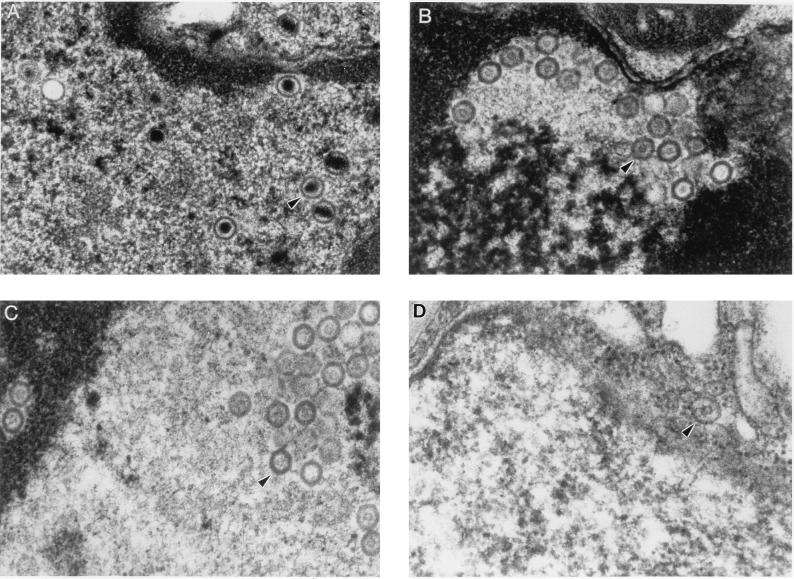
Electron microscopy of Vero cells infected with HSV-1 (strain F) pretreated with SLS.
Figure 5A shows the normal appearance of HSV-1 (strain F) in the nuclei of Vero cells. Viral particles were composed of a capsid, hexagonal in shape and containing an electron-dense DNA core. Complete viral particles formed by a nucleocapsid surrounded by an envelope were also found in the cytoplasm of most of these cells. In Vero cells infected with viruses pretreated with 50, 75, and 100 μM SLS (Fig. 5B to D, respectively), viral particles could be recovered in the nuclei but not in the cytoplasm of cells. No mature nucleocapsid could be observed in the nuclei, but viral particles were constituted of capsids containing a discrete accumulation of electron-dense material. The number of empty capsids found in nuclei of cells infected with viruses pretreated with SLS decreased with increasing concentrations of SLS used for the pretreatment. In cells infected with viruses pretreated with 100 μM SLS, only a few cells with empty capsids in the nuclei could be detected. No viral particles could be recovered in Vero cells infected with HSV-1 (strain F) pretreated with 50 nM DS (data not shown).
FIG. 5.
Electron micrographs of Vero cells infected with HSV-1 (strain F) pretreated for 1 h at 37°C with 50 μM (B), 75 μM (C), and 100 (D) μM SLS. Cells infected with HSV-1 (strain F) in the absence of SLS were used as the control (A). Magnification, ×52,500.
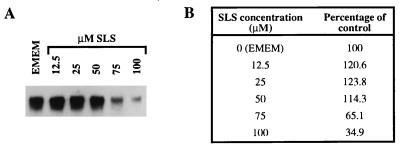
Quantification of HSV glycoprotein D gene.
Figure 6A shows the quantification of the glycoprotein D gene of HSV-1 (strain F) pretreated with various concentrations of SLS in Vero cells 48 h after their infection. No major modification in the amount of the viral glycoprotein D gene could be observed in cells infected with HSV-1 (strain F) pretreated with 12.5, 25, or 50 μM SLS compared to the control. Quantitative measurements of HSV-1 glycoprotein D gene obtained by scanning densitometry of the autoradiogram were similar (Fig. 6B). However, when the virus was pretreated with higher concentrations of SLS (75 and 100 μM), a marked reduction in the amount of the glycoprotein D gene was observed, as indicated by a reduction in the hybridization signal intensity to 65.1 and 34.9% of control values, respectively.
FIG. 6.
(A) Quantification of glycoprotein D gene of HSV-1 (strain F) pretreated for 1 h at 37°C with 12.5, 25, 50, 75, or 100 μM SLS in Vero cells. Cells infected with HSV-1 (strain F) in EMEM plus 2% FBS were used as a control. (B) Quantitative measurements of HSV-1 glycoprotein D gene levels obtained by scanning densitometry of the autoradiogram by using an AlphaImager. Values are expressed as a percentage of the hybridization signal intensity compared to that of the control.
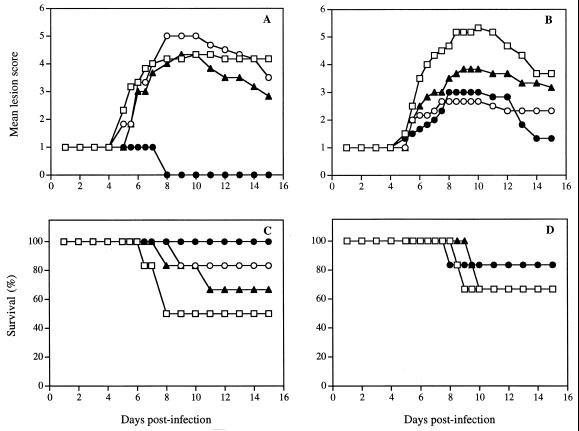
In vivo infectivity of HSV-1 (strain F) pretreated with SLS or DS (cutaneous model).
Figure 7A shows the time evolution of the mean lesion score of mice infected cutaneously with HSV-1 (strain F) pretreated with various SLS concentrations for 1 h at 37°C. The evaluation of the lesion score was performed according to the criteria presented in Table 2. In infected untreated mice, no pathological signs of cutaneous infection were visible during the first 4 days postinfection, and only the scarified area remained visible. On days 5 and 6, herpetic skin lesions began to appear in the form of small vesicles distant from the inoculation site. On day 7, almost all untreated mice developed herpetic skin lesions in the form of a 4- to 5-mm-wide band extending from the spine to the anterior midline of the affected dermatome, a result similar to zoster-like infections. The maximal mean lesion score was observed on day 10. Mice infected with the virus pretreated with 6.25 and 25 μM SLS did not demonstrate a significant reduction of the mean lesion score. Of prime interest, mice infected with a viral inoculum pretreated with 100 μM SLS did not show any signs of cutaneous lesions throughout the study period. A modest reduction of the mean lesion scores was observed in mice infected with a viral inoculum pretreated with 0.25, 1, or 10 nM DS (Fig. 7B).
FIG. 7.
Time evolution of mean lesion score and survival of mice infected cutaneously with HSV-1 (strain F) pretreated for 1 h at 37°C with 6.25 (▴), 12.5 (○), and 100 (●) μM SLS (A and C) or 0.25 (▴), 1 (○), and 10 (●) nM DS (B and D). Mice infected with untreated virus were used as control (□). Results are expressed as the mean of six animals per group.
TABLE 2.
Criteria used for the evaluation of herpetic cutaneous lesionsa
| Score | Appearance of the lesion |
|---|---|
| 0 | No visible infection |
| 1 | Infection visible only at inoculation site and scarification area |
| 2 | Infection at inoculation site only with swelling, crust, and erythema |
| 3 | Infection at inoculation site with discrete lesions forming away from inoculation site |
| 4 | Rash visible around half of body but not yet confluent |
| 5 | Rash confluent but not yet necrotic or ulcerated |
| 6 | Complete rash with necrosis or ulceration, hind limb paralysis, bloating, or death |
Adapted from Lobe et al. (24).
Figure 7C shows the corresponding survival of mice infected cutaneously with HSV-1 (strain F) pretreated with various SLS concentrations. Death by encephalitis occurred in 50% of the control infected mice between days 7 and 8. The survival rate was increased to 67 and 83% in mice infected with viral inocula pretreated with 6.25 and 25 μM SLS, respectively. Of prime interest, all mice infected with a viral inoculum pretreated with 100 μM SLS survived the infection. Figure 7D shows that in mice infected with a viral inoculum pretreated with DS (0.25, 1, and 10 nM), the mortality associated with the infection was not significantly different from that observed in the control.
In vivo prophylactic effect of formulations containing SLS or DS (cutaneous model).
Figure 8 shows the time evolution of the mean lesion score of untreated infected mice and mice pretreated with the polymer alone, polymer containing 5% SLS or polymer containing 1% DS either 5 min or 1 h prior to their cutaneous infection with HSV-1 (strain F). Control infected mice did not develop any visible signs of cutaneous infection until day 4 postinfection. On day 7 postinfection, almost all infected mice developed zosteriform-like lesions. Maximal mean lesion scores were observed from day 7 to day 8. Mice pretreated with the polymer alone 5 min or 1 h prior to infection were only slightly protected against the development of cutaneous lesions. Of prime interest, in mice pretreated both for 5 min and for 1 h with the polymer containing 5% SLS, a complete protection against the development of cutaneous lesions was observed. On the contrary, when mice were pretreated with a polymer containing 1% DS, no protection against the development of the cutaneous lesions could be observed irrespective of the delay of initiation of infection after the pretreatment.
FIG. 8.
Time evolution of mean lesion score of mice infected with HSV-1 (strain F) after pretreatment of mice with the polymer formulation alone (○ and ●), with polymer containing 5% SLS (▵ and ▴), or with polymer containing 1% DS (◊ and ⧫) for either 5 min (open symbols) or 1 h (filled symbols) prior to infection. Untreated infected mice were used as a control (□). Results are the mean of six animals per group.
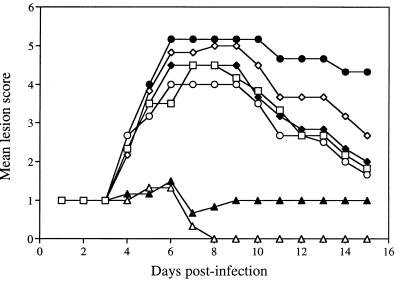
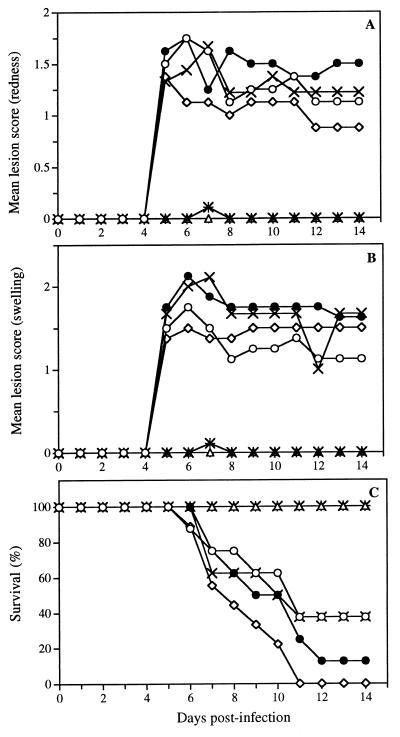
In vivo efficacy of SLS and DS to prevent HSV-2 (strain 333) infection (intravaginal model).
Figure 9 shows the time evolution of the mean lesion scores associated with redness (panel A), swelling (panel B), and survival (panel C) of untreated infected mice and mice pretreated with either 1 or 5% SLS or DS in a pH 4.0 buffered solution. Control infected mice did not develop any visible signs of redness or swelling in the perineal region until day 4. On day 5 postinfection, these symptoms appeared in almost all mice and were maintained up to day 14. Pretreatment of mice with buffer or with 1 or 5% DS in buffer did not prevent the appearance of redness and swelling in the perineal region. Of prime interest, pretreatment of mice with both concentrations of SLS in the same buffered solution completely protected mice from the appearance of these symptoms. Figure 9C shows that untreated infected mice died by encephalitis between day 6 and day 11. Pretreatment of mice with buffer alone or with 1 or 5% DS did not prevent the lethality associated with the infection. However, all mice pretreated with 1 or 5% SLS survived the infection.
FIG. 9.
Time evolution of mean lesion score and survival of mice infected intravaginally with HSV-2 (strain 333) after pretreatment of mice with pH 4.0 buffer (●), 1% SLS (×+), 5% SLS (▵), 1% DS (×), or 5% DS (◊) in buffer for 5 min prior to their intravaginal infection. Untreated infected mice were used as a control (○). Results are mean for eight animals per group.
DISCUSSION
There is great interest in the development of novel compounds to reduce the sexual transmission of HIV, HSV, and other pathogens causing STDs. More attention is now given to female-controlled methods for the prevention of HIV infection since many women are unable to negotiate condom use with their sexual partners (12). In the present study, we have evaluated the potency of SLS, a sulfated anionic chaotropic surfactant, and DS, a polysulfated carbohydrate, against HSV and HIV infections in cell culture models, as well as in murine models of HSV infection.
In vitro studies demonstrated that SLS is a potent inhibitor of the infectivity of various HSV-1 and HSV-2 strains as well as of HIV-1. Ward and Ashley have already reported that SLS inactivated rotavirus at quite low concentrations and under very mild conditions (40). Most of the proteins of the outer shell seemed to remain associated with the virions, and the decreased infectivity was attributed to an electrostatic effect due to the adsorption of SLS molecules on the virus surface (39). Previous studies from our laboratory have demonstrated that SLS inhibits in vitro the infectivity of HSV-1 (strain F) to Vero cells at quite low concentrations (Piret et al., 38th ICAAC). More recently, Howett et al. confirmed our findings that SLS is a potent inactivator of HSV-2 and HIV-1 (20). In addition, they have shown that SLS is also effective against rabbit, bovine, and human papillomaviruses (nonenveloped viruses) after brief treatment with low concentrations of the drug. They suggested that SLS denatures the capsid proteins of nonenveloped viruses, whereas both envelope disruption and denaturation of virus structural proteins occur simultaneously for enveloped viruses.
Our data showed that the time of the pretreatment of HSV-1 with SLS markedly influenced the inactivating potency of this compound on the virus, with a longer pretreatment period leading to a more pronounced loss of infectivity. We thus propose that the mode of action of SLS may be different than just a binding of SLS, via its sulfated moiety, to the virus, thus preventing its interaction with heparan sulfate glycosaminoglycans localized at the cell surface. Binding studies of radiolabeled HSV-1 to Vero cells in the presence of SLS have indeed demonstrated that SLS did not affect this parameter. We cannot, however, exclude the possibility that SLS could extract [35S]methionine-labeled proteins from the virus envelope for which we could actually measured the binding to cells rather than that of the complete virus.
Electron microscopic examination of Vero cells infected with HSV-1 pretreated with SLS revealed that at 50 μM, a concentration at which we observed a complete loss of virus infectivity, the virus was able to enter cells and initiate its replication to form complete capsid shells devoid of a DNA core. Lucin et al. have also reported that infection of murine embryonal fibroblasts, pretreated with both gamma interferon and tumor necrosis factor alpha, with a murine cytomegalovirus led to the production of capsids containing discrete accumulations of electron-dense material but no electron-dense DNA core (25). During a productive infection, three types of capsids can be isolated from cells infected with herpesviruses. They are visualized as light-scattering bands in sucrose gradients and are designated as A, B, and C (empty, intermediate and full) in order of increasing sedimentation distance (18). The shell structure is common to all three capsid types, but they differ in their protein and DNA compositions and in their eventual fate in the infected cell. C capsids contain the entire DNA genome and are probably identical to capsids found in native virions (29). In contrast, A and B capsids lack DNA and are present in the nuclei of infected cells (29, 31). B capsids can package DNA and mature into infectious virus, while A capsids cannot (30, 32, 37). A capsids are considered to result from abortive attempts to package DNA into B capsids. However, we cannot distinguish at this stage which types of capsids (A, B, or another type) are produced in cells infected with HSV-1 pretreated with SLS.
We demonstrated that, in Vero cells infected with HSV-1 pretreated with 50 μM SLS, the amount of the glycoprotein D gene of the virus was unchanged compared to control infected cells. These data suggest that SLS could interfere with the maturation of nucleocapsids either by reducing their rate of maturation or by interfering with the encapsidation of DNA into the capsid shell. At a higher SLS concentration (100 μM), only a few cells with empty capsids in the nuclei could be recovered. Moreover, the amount of the glycoprotein D gene was also markedly decreased in these cells, suggesting that viruses could be highly damaged during pretreatment with such a concentration of SLS and that only a few viruses were able to enter into cells and initiate their replication therein.
We also demonstrated that pretreatment of herpesviruses with 100 μM SLS, which completely inhibited viral infectivity in vitro, completely protected against the development of cutaneous herpetic infections. Of prime interest, skin pretreatment of mice with 5% SLS incorporated into a polymer formulation also completely protected against the development of cutaneous lesions. These results show the great potential of our formulations as a prophylactic approach to prevent infection with pathogens. Such a tool could indeed protect against the accidental infection of health care workers. More interestingly, the intravaginal pretreatment of mice with 1% SLS in a buffered solution was also effective in preventing the development of herpetic lesions and death of animals resulting from infection.
Our results also showed that DS was a potent inhibitor of the infectivity of HSV-1 and HSV-2 strains in vitro. A similar effect of DS was observed for various enveloped viruses, such as HSV, cytomegalovirus, vesicular stomatitis virus, and HIV (4, 28). Herold et al. have also reported that sulfated carbohydrates, which resemble heparan sulfate, could prevent in vitro HSV, C. trachomatis, and N. gonorrhoeae infections through competitive inhibition for their binding to heparan sulfate glycosaminoglycans on the host cell surface (19). Binding studies indeed revealed that radiolabeled HSV-1 pretreated with DS was less able to bind to Vero cells when the DS concentration in the preincubation medium was increased. In addition, no viral particles could be recovered in Vero cells infected with HSV-1 pretreated with DS, suggesting that no virus could enter into the cells under these conditions. Although the HIV and HSV proteins targeted by sulfated polymers are different, the mode of inhibition (i.e., competitive inhibition of virus binding to cells) is similar. In the case of HIV, sulfated carbohydrates are thought to interfere with the ionic interaction between cell surface CD4 or sulfated polysaccharides and the positively charged amino acids concentrated in the V3 region of HIV gp120 (2, 5, 21, 26).
Although DS was a 100-fold-more-potent inhibitor of the infectivity of HSV in vitro than SLS, this molecule was found to be of little interest in vivo. Indeed, we demonstrated that pretreatment of herpesviruses with DS at a concentration which gave an almost complete loss of virus infectivity in vitro could not protect mice against the development of cutaneous lesions and encephalitis in the cutaneous model. Similarly, skin pretreatment of mice with a polymer formulation containing 1% DS could not protect against the development of cutaneous lesions. An intravaginal pretreatment of mice with 1 or 5% DS in a buffered solution was also not effective in preventing the development of herpetic lesions and the lethality associated with the infection. Neyts and De Clercq also showed that intravaginal pretreatment of mice with DS was unable to provide protection against HSV-2 infection (27). These authors proposed that since the interaction between sulfated compounds and herpesviruses, as well as HIV-1, is reversible and nonvirucidal (4, 28), a release of the compound from the viral glycoproteins could occur once the virus-compound complex has reached the surrounding vaginal tissues.
Taken together, our results showed that SLS could represent a potent vaginal microbicide to protect against HIV, HSV, and possibly other microorganisms. The choice of the vehicle in which such molecules should be incorporated is also an important factor to take into account when setting up vaginal microbicides. Indeed, the vehicle has to minimize the potential toxicity of compounds for the vaginal mucosa while allowing the active ingredient to exert its effect against pathogens. In this respect, polymer composed of polyoxypropylene and polyoxyethylene, as used in this study, could be a good matrix for incorporating such molecules. Indeed, we have demonstrated that such polymers decreased markedly the toxicity of nonoxynol-9 to the vaginal mucosa of rabbits (16). Furthermore, in vivo efficacy studies showed that the polymer alone prevented the vaginal lethal infection of mice with HSV-2 compared to control infected mice. In conclusion, SLS could represent a candidate of choice as a microbicide to prevent the sexual transmission of HIV, HSV, and other pathogens causing STDs. Incorporation of SLS into this polymer formulation could represent an innovative approach to prevent sexually transmitted infections by acting both as a chemical and as a physical barrier against pathogens.
ACKNOWLEDGMENTS
This study was supported by a grant from Infectio Recherche, Inc.
We thank Guy Boivin and M. J. Tremblay for constructive comments and helpful discussions.
REFERENCES
- 1.Alexander N J. Future contraceptives. Sci Am. 1995;273:136–141. [PubMed] [Google Scholar]
- 2.Baba M, Pauwels R, Balzarini J, Arnout J, De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc Natl Acad Sci USA. 1988;85:6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba M, Schols D, De Clercq E, Pauwels R, Nagy M, Gyorgyi-Edelenyi J, Low M, Sandor G. Novel sulfated polymers as highly potent and selective inhibitors of human immunodeficiency virus replication and giant cell formation. Antimicrob Agents Chemother. 1990;34:134–138. doi: 10.1128/aac.34.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba M, Snoeck R, Pauwels R, De Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32:1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batinic D, Robey F A. The V3 region of the envelope glycoprotein of human immunodeficiency virus type 1 binds sulfated polysaccharides and CD4-derived synthetic peptides. J Biol Chem. 1992;267:6664–6671. [PubMed] [Google Scholar]
- 6.Bérubé P, Barbeau B, Cantin R, Sékaly R P, Tremblay M. Repression of human immunodeficiency virus type 1 LTR-driven gene expression by the binding of the virus to its primary cellular receptor, the CD4 molecule. J Virol. 1996;70:4009–4016. doi: 10.1128/jvi.70.6.4009-4016.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourinbaiar A S, Lee-Huang S. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9 and gossypol. Contraception. 1994;49:131–137. doi: 10.1016/0010-7824(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 8.Burton K. Methods in enzymology. In: Grosmann L, Moldave K, editors. vol. 12. New York, N.Y: Academic Press; 1968. pp. 163–166. [Google Scholar]
- 9.Buttke T M, McCubrey J A, Owen T C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphocyte-dependent cell lines. J Immun Methods. 1993;157:223–240. doi: 10.1016/0022-1759(93)90092-l. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Désormeaux A, Omar R F, Bergeron M G. Topical microbicides for the prevention of STDs/HIV. Can J Infect Dis. 1999;10(Suppl. C):41C–48C. [Google Scholar]
- 12.du Guerny J, Sjoberg E. Inter-relationship between gender relations and the HIV/AIDS epidemic: some possible considerations for policies and programmes. AIDS. 1993;7:1027. doi: 10.1097/00002030-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Eng T R, Butler W T, editors. The hidden epidemic: confronting sexually transmitted diseases. Washington, D.C.: National Academy Press; 1997. [PubMed] [Google Scholar]
- 14.Feldblum P J, Weir S S. The protective effect of nonoxynol-9 against HIV infection. Am J Public Health. 1994;84:1032–1034. doi: 10.2105/ajph.84.6.1032-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortin J-F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagné N, Cormier H, Omar R F, Désormeaux A, Gourde P, Tremblay M J, Juhász J, Beauchamp D, Rioux J E, Bergeron M G. Protective effect of a thermoreversible gel against the toxicity of nonoxynol-9. Sex Transm Dis. 1999;26:177–183. doi: 10.1097/00007435-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Gerbase A T, Rowley J T, Mertens T E. Global epidemiology of sexually transmitted diseases. Lancet. 1998;351(Suppl. III):2–4. doi: 10.1016/s0140-6736(98)90001-0. [DOI] [PubMed] [Google Scholar]
- 18.Gibson W, Roizman B. Proteins specified by herpes simplex virus: VIII. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972;10:1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herold B C, Siston A, Bremer J, Kirkpatrick R, Wilbanks G, Fugedi P, Peto C, Cooper M. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob Agents Chemother. 1997;41:2776–2780. doi: 10.1128/aac.41.12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howett M K, Neely E B, Christensen N D, Wigdahl B, Krebs F C, Malamud D, Patrick S D, Pickel M D, Welsh P A, Reed C A, Ward M G, Budgeon L R, Kreider J W. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob Agents Chemother. 1999;43:314–321. doi: 10.1128/aac.43.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito M, Baba M, Sato A, Pauwels R, De Clercq E, Shigeta S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antivir Res. 1987;7:361–367. doi: 10.1016/0166-3542(87)90018-0. [DOI] [PubMed] [Google Scholar]
- 22.Jennings R, Clegg A. The inhibitory effect of spermicidal agents on replication of HSV-2 and HIV-1 in vitro. J Antimicrob Chemother. 1993;32:71–82. doi: 10.1093/jac/32.1.71. [DOI] [PubMed] [Google Scholar]
- 23.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts P L, Ruminjo I, Sajabi R, Kimata J, Fleming T R, Anzala A, Holton D, Plummer F. Efficacy of nonoxynol-9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–482. [PubMed] [Google Scholar]
- 24.Lobe D C, Spector T, Ellis N. Synergistic topical therapy by acyclovir and A1110U for herpes simplex virus induced zosteriform rash in mice. Antivir Res. 1991;15:87–100. doi: 10.1016/0166-3542(91)90027-o. [DOI] [PubMed] [Google Scholar]
- 25.Lucin P, Jonjic S, Messerle M, Polic B, Hengle H. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumour necrosis factor. J Gen Virol. 1994;75:101–110. doi: 10.1099/0022-1317-75-1-101. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuya H, Looney D J, Kuno S, Ueno R, Woong-Staal F, Broder S. Dextran sulfate suppression of viruses in the HIV-family: inhibition of virion binding to CD4+ cells. Science. 1988;226:172–174. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- 27.Neyts J, De Clercq E. Effect of polyanionic compounds on intracutaneous and intravaginal herpesvirus infection in mice: impact on the search for vaginal microbicides with anti-HIV activity. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:8–12. [PubMed] [Google Scholar]
- 28.Neyts J, Snoeck R, Schols D, Balzarini J, Esko J D, Schepdael A V, De Clercq E. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology. 1992;189:48–58. doi: 10.1016/0042-6822(92)90680-n. [DOI] [PubMed] [Google Scholar]
- 29.Perdue M L, Cohen J C, Kemp M C, Randall C C, O'Callaghan D J. Characterization of three species of nucleocapsid of equine herpesvirus type-1 (EHV-1) Virology. 1975;64:187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- 30.Perdue M L, Cohen J C, Randall C C, O'Callaghan D J. Biochemical studies of the maturation of herpes virus nucleocapsid species. Virology. 1976;74:194–208. [PubMed] [Google Scholar]
- 31.Perdue M L, Kemp M C, Randall C C, O'Callaghan D J. Studies of the molecular anatomy of the L-M strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974;59:201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- 32.Preston V G, Rixon F J, McDougall I M, McGragor M, Al-Kobaisi M F. Processing of herpes simplex virus assembly protein ICP35 near its carboxy terminal end requires the product of the whole of the UL26 reading frame. Virology. 1992;186:87–93. doi: 10.1016/0042-6822(92)90063-u. [DOI] [PubMed] [Google Scholar]
- 33.Roddy R E, Zekeng L, Ryan K A, Tamoufé U, Weir S S, Wong E L. A controlled trial of nonoxynol-9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal S L, Cohen S S, Stanberry L R. Topical microbicides. Current status and research considerations for adolescent girls. Sex Transm Dis. 1998;25:368–377. doi: 10.1097/00007435-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sherman G, Bachenheimer S L. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology. 1988;163:471–480. doi: 10.1016/0042-6822(88)90288-7. [DOI] [PubMed] [Google Scholar]
- 38.Stafford M K, Ward H, Flanagan A, Rosenstein I J, Taylor-Robinson D, Smith J R, Weber J, Kitchen V S. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–331. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 39.Ward R L, Ashley C S. Comparative study on the mechanisms of rotavirus inactivation by sodium dodecyl sulfate and ethylediaminetetraacetate. Appl Environ Microbiol. 1980;39:1148–1153. doi: 10.1128/aem.39.6.1148-1153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward R L, Ashley C S. pH modification of the effects of detergents on the stability of enteric viruses. Appl Environ Microbiol. 1979;38:314–322. doi: 10.1128/aem.38.2.314-322.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weir S S, Feldblum P J, Zekeng L, Roddy R E. The use of nonoxynol-9 for protection against cervical gonorrhea. Am J Public Health. 1994;84:910–914. doi: 10.2105/ajph.84.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zekeng L, Feldblum P J, Oliver R M, Kaptue L. Barrier contraceptive use and HIV infection among high-risk women in Cameroon. AIDS. 1993;7:725–731. doi: 10.1097/00002030-199305000-00018. [DOI] [PubMed] [Google Scholar]