Capsule summary.
Airway sensory neuron-produced Substance P heightens allergy-induced goblet cell hyperplasia and hypersecretion of Muc5AC, electrically silencing these overreactive neurons reduced these components of lung type 2 allergic inflammatory response.
Keywords: Vagal sensory neurons, TRPV1, QX-314, Optogenetic, Mucous metaplasia, Goblet cell hyperplasia, Substance P, Muc5AC, Muc5B, Asthma
To the editor,
By trapping inhaled pathogens and toxic particulates, the mucus lining of the airways fulfills an important protective contribution to innate immune function (1). Mucus clearance or accumulation depends on the balance between mucin production and its elimination, providing either an effective defense barrier or disease favoring mucus excess (1). Mucus is largely composed of mucins which are high molecular weight heavily glycosylated proteins. They are encoded by MUC genes and segregate into three major families: secreted gel-forming (Muc5AC, Muc5B); membrane-associated; and non–gel-forming secreted mucin (2). While the functions of mucin subtypes are not well understood, membrane-associated mucins can act as receptors for invading pathogens and initiate innate immune responses, while secreted mucins prevent epithelial inflammation (2). Muc5AC contributes to airway hyperactivity (3), a key feature of asthma and Muc5B to mucociliary clearance, immune homeostasis, and airway inflammation resolution (4). In patients with severe asthma, lung airway Muc5B expression is decreased by up to 90%, while Muc5AC expression is highly upregulated (4).
Sensory neurons drive mucus hyperplasia.
The nature and mechanisms responsible for the protective role of mucins in preventing airway diseases in health, and the changes in secreted mucins and its clearance failure in disease, require further exploration. There are suggestions that the nervous system influences mucus production, and this may provide a way to intervene therapeutically. Circadian rhythms, through vagal nerve signaling, are primary regulators of submucosal gland activation (5). We previously uncovered that vagal sensory neurons amplify ILC2 and CD4+ T cells activation, driving a pro-inflammatory loop between these neurons and airway immune cells (6). To probe for a role of vagal sensory neurons in mucus production we used optogenetics via cre-loxp targeted channelrhodopsin (ChR2) expression in vagal sensory (TRPV1cre/wt∷ChR2fl/wt and Tac1cre/wt∷ChR2fl/wt) or motor neurons (ChATChR2-eYFP). Acute optogenetic stimulation of the vagus nerve trunk (3.5 ms, 5Hz, 473nm, 100 mW, giving approx. 2–6 mW/mm2 with a 0.39 NA fiber placed 5–10 mm from the nerve, for 30 min) in isoflurane-anesthetized mice enhanced the influx of CD45+ immune cells into BALF (p≤0.05; Fig. 1A), mucus metaplasia (Fig. 1B) and BALF mucin imbalance (Muc5AC/Muc5B; Fig. 1C). Optical stimulation did not affect goblet cell hyperplasia (Supplementary figure 1A) in littermate control mice with no channelrhodopsin (cre−/−ChR2fl/wt; Fig. 1A–C) or in mice where only vagal autonomic neurons were activated (data not shown). Activation of vagal sensory neurons is sufficient, therefore, to trigger both immune cell influx into the lung and mucin imbalance. This article’s Methods section is in the JACI Online Repository at www.jacionline.org.
Figure 1. Airway sensory neuron neurons reverse mucus metaplasia and mucin imbalance.
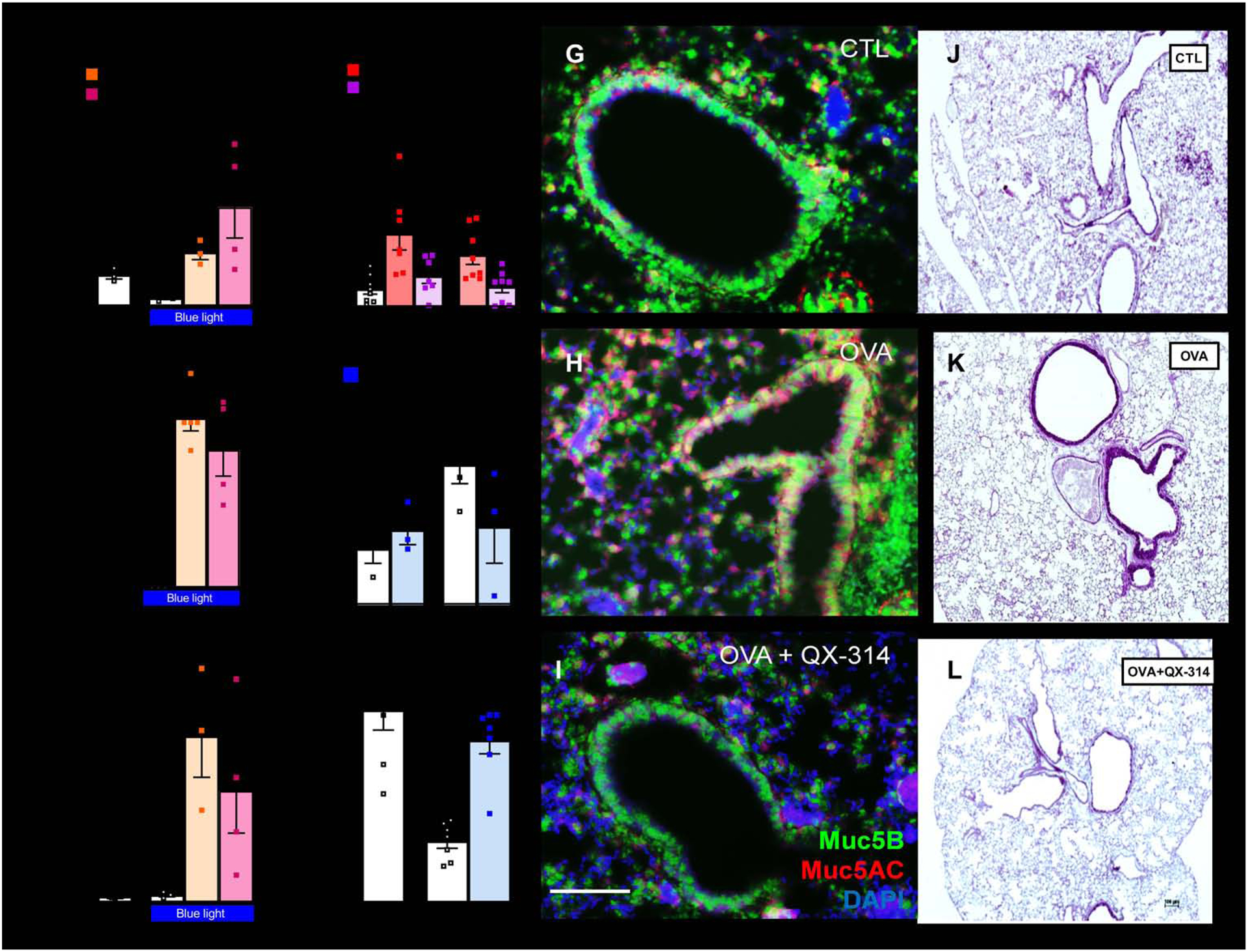
Optogenetic-stimulation of TRPV1cre/wt∷ChR2fl/wt and Tac1cre/wt∷ChR2fl/wt mice vagal sensory neurons increases BALF CD45+ cells (A), lung mucus deposition (B), and BALF Muc5AC/Muc5B ratio (C). In naïve C57BL6 mice, an acute capsaicin challenge (1 uM, i.n.) increased BALF levels of Muc5AC/Muc5B. Similar findings were observed in Rag1−/− mice, suggesting that these effects are independent of B or T cells. These consequences were absent when mice were co-treated with QX-314 (100 μM, intranasal, D) to silence sensory neurons. Allergen-challenges increased airway mucus deposition (E) as well as in situ expression of Muc5AC/Muc5B (F), effects that were reversed by sensory neuron silencing using QX-314 (100 uM, aerosolized). QX-314 had no detectable outcomes when given to naïve mice (E–F). Muc5AC (red) and Muc5B (green) transcript expression (G–I) visualized by in situ-hybridization-stained sections of naïve (G) and OVA-exposed (H, I) lungs treated with saline (G, I) or QX-314 (100 μM; H). Scale 100 μm. Mucus deposition (purple; J–L) in Periodic acid–Schiff-stained sections of naïve (J) and OVA-exposed (K, L) lungs treated with saline (J, K) or QX-314 (100 μM; L). DAPI-stained cell nucleus (blue; G–I). Scale 100 μm. Mean ± S.E.M; Two-tailed unpaired Student’s t-test.
Sensory neuron silencing strategy.
We exploited a nociceptor neuron blocking strategy to locally silence lung-innervating nociceptors and probe their role in driving pathological mucous cell metaplasia in allergic lung inflammation. This protocol uses large pore ion channels such as TRPV1, as cell-specific drug-entry ports that deliver a charged and membrane-impermeable form of lidocaine (QX-314) into sensory fibers to block sodium currents. During allergic airway inflammation, these ion channels on the surface of nociceptors open, allowing the small-size (263 Da) QX-314 to permeate into these neurons (6). This results in a highly targeted and long-lasting (>9h) electrical blockade of nociceptors, greatly exceeding efficacy of lidocaine (<1h). While QX-314 does not impact BALF immune cell function it reverses OVA-induced airway inflammation for up to 72h (6).
Sensory neuron-induced Muc5AC overproduction is independent of immune cells.
Mucin production and hypersecretion are influenced by inflammatory conditions, with immunocyte-produced cytokines modulating switches in mucin production (1). To test whether the effect of sensory neurons on mucin production is mediated directly or indirectly via airway-infiltrating leukocytes, we compared the effect of capsaicin-mediated activation of TRPV1+ lung sensory neurons on mucin production in wildtype and in adaptive immune cell deficient mice (Rag1−/−). Both wildtype and Rag1−/− mice treated with inhaled capsaicin to activate TRPV1+ sensory neurons, present similar increases in BALF Muc5AC/Muc5B secreted levels (Fig. 1D) and goblet cell (Muc2+) transcript expression of Muc5AC/Muc5B (Supplementary figure 1B). The effects of the capsaicin challenge were abolished when both sets of mice were co-treated with QX-314 (Fig. 1D, Supplementary figure 1B) which silences activity in these neurons by entry through activated large pore channels (6). Based on these results, we conclude that the sensory neuron-induced change in Muc5AC/Muc5B expression and release is independent of airway lymphocytes.
Sensory neurons control allergen-mediated mucin imbalance.
Mice were sensitized to ovalbumin (OVA) (intraperitoneally [i.p.] with aluminum hydroxide, days 0 and 7) as an allergen, followed by inhaled OVA challenges on days 14–17 (6). QX-314 (100 μM; 20 min aerosol; day 18) prevents the OVA-challenge induced mucus metaplasia (Fig 1E, J–L), and imbalance (Muc5AC/Muc5B) as well as the changes in transcribed mucins (Fig 1F–I; Supplementary figure 1C) measured on day 21. QX-314 inhalation had no effect when administered in non-inflamed conditions (Fig. 1E). These findings support sensory neuron silencing as a potential therapeutic strategy to reverse asthma-mediated mucin imbalance.
Sensory neuron-released SP drives goblet cell hyperplasia.
Substance P (SP) is a sensory neuropeptide which is increased in the sputum of asthmatic patients (7) and contributes to the neurogenic component of inflammation (8). Here, we found that the OVA-challenge increased BALF SP levels, in a manner that was prevented by sensory neuron silencing with QX-314 (Supplementary figure 2A); indicating that airway type 2 inflammation activates axonal terminal release of SP. Next, we explored if sensory neurons heighten tracheal mucus production in type 2 allergic lung inflammation via locally-secreted SP. To do this we engineered mice whose peptidergic and other sensory neurons are genetically ablated by expression of diphtheria toxin in TRPV1 lineage cells (TRPV1cre/wt∷DTAfl/wt). The stable NK-1R agonist [Sar9-Met-(O2)11]-SP directly drives mucus secretion in wildtype mice tracheas, as does the TRPV1 agonist capsaicin and the acetylcholine receptor agonist carbachol (Supplementary figure 2B). However, capsaicin-induced mucus secretion was not observed in sensory neuron ablated (TRPV1cre/wt∷DTAfl/wt) and Tac1 knockout (no SP) mice but the degree of carbachol-induced mucus secretion was similar between these mice and their littermate controls (Supplementary figure 2P). Of note, sensory neuron silencing with QX-314 also did not impact carbachol (100 μM, 30 min) induced mucus secretion from OVA-challenged mice trachea (Supplementary figure 2C). These data suggest that vagal sensory neuron-mediated mucus secretion in the inflamed mouse trachea depends on SP secretion.
The excessive mucus production seen in allergic asthma patients is the consequence of increased transdifferentiation of airway ciliated epithelial cells into mucin-producing goblet cells, a phenomenon known as goblet cell hyperplasia. These cells are often found near airway sensory neuron terminals and express receptors for various neuropeptides (9). To test the hypothesis that neurons drive goblet cell hyperplasia, we found that OVA-exposed littermate control mice develop goblet cells hyperplasia, detected by alcian blue histological staining, as compared to vehicle-treated mice (Fig. 2A–G). Sensory neuron silenced (QX-314), sensory neuron ablated (TRPV1cre/wt∷DTAfl/wt) or Tac1 knockout mice were protected from this effect (Fig. 2G). Daily intranasal injections of the NK-1R agonist [Sar9-Met-(O2)11]-SP to TRPV1cre/wt∷DTAfl/wt mice also partially rescued the goblet cells hyperplasia (Fig. 2A–G).
Figure 2: Allergic inflammation-mediated goblet cell hyperplasia and mucin imbalance are controlled by SP release from vagal sensory neurons.
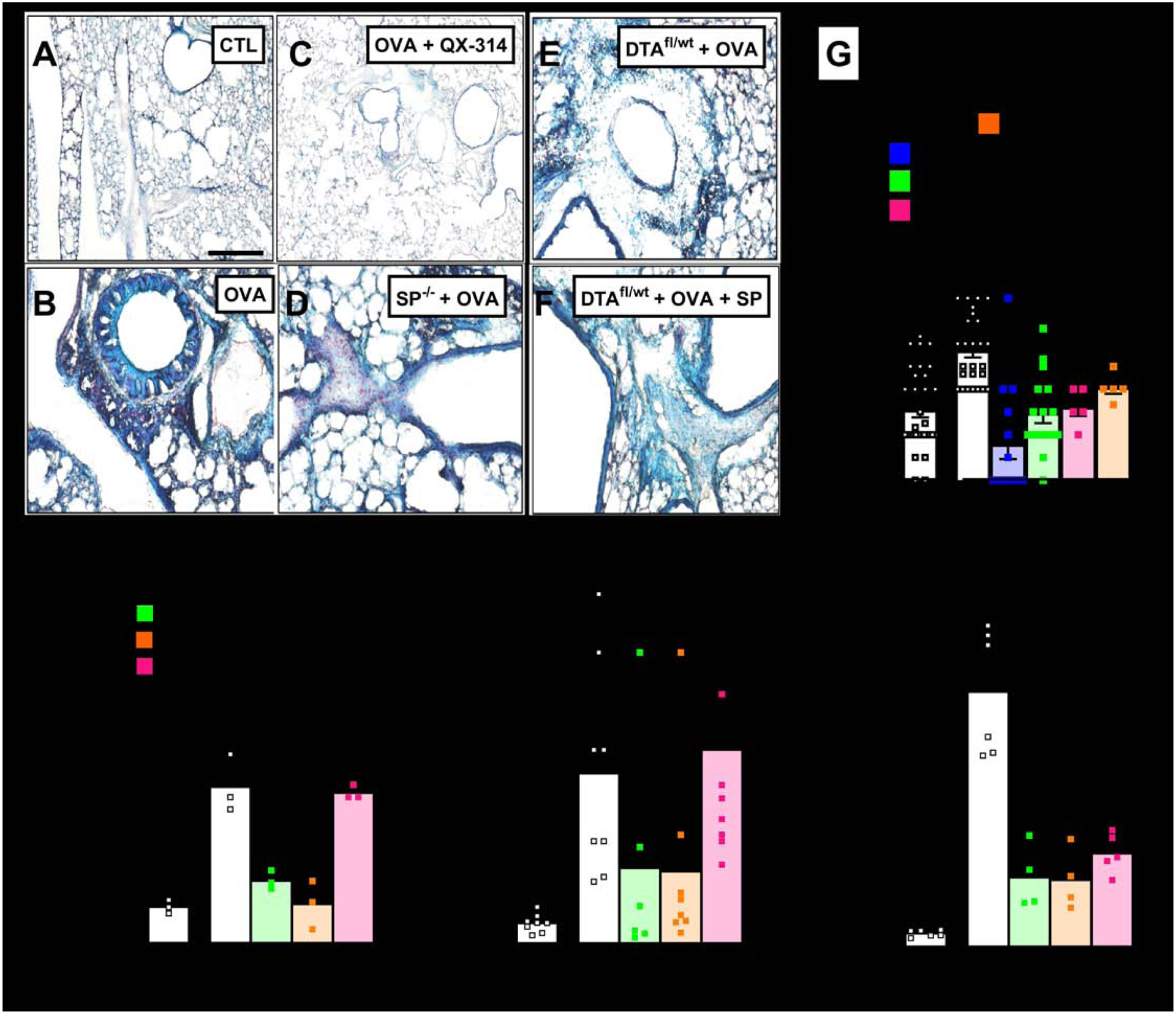
Goblet cell hyperplasia (blue; A–F) detected in Alcian Blue (AB)-stained sections of naïve (A) and OVA-exposed (B–F) lungs from littermate control (A–C), Tac1−/− (D), or sensory neuron ablated mice (E, F) treated with vehicle (A, B, D, E), QX-314 (100 uM; C) or [Sar9, Met(O2)11]-SP (F). Scale 100 μm. Littermate control mice challenged with OVA present a significant goblet cell hyperplasia (G), mucus metaplasia (H), BALF Muc5AC/Muc5B levels (I) as well as in situ expression of Muc5AC (J) relative to naïve mice. These effects were absent in sensory neuron silenced (G), ablated (G–I) or Tac1 knockout (G–I) mice, but partially rescued by daily intranasal administration of the stable substance P analog Sar9-Met-(O2)11]-SP (G–I). Mean ± S.E.M; Two-tailed unpaired Student’s t-test.
Relative to naïve animals, OVA-challenged control mice present with a significant increase in airway mucus deposition (Fig. 2H; Supplementary figure 2 E–I), as well as an increase in the BALF ratio of Muc5AC/Muc5B (Fig. 2I), in situ Muc5AC transcripts levels (Fig. 2J; Supplementary figure 2J–N) and the NK-1R+ goblet cell Muc5AC/Muc5B transcript ratio (Supplementary figure 2D). These effects were absent in Tac1−/− or TRPV1cre/wt∷DTAfl/wt mice (Fig. 2H–J) or blocked by sensory neuron silencing with QX-314 (Supplementary figure 2D). Daily intranasal injections of [Sar9-Met-(O2)11]-SP in TRPV1cre/wt∷DTAfl/wt mice increased these levels close to ones measured in mice with OVA-induced inflammation (Fig. 2H–J), supporting a direct role for SP in the changes.
Currently no therapies target the resolution of allergy-induced mucous cell metaplasia and hypersecretion of Muc5AC nor can rescue mucociliary transport in asthma, COPD or cystic fibrosis patients (3). Given the contribution that SP release from activated sensory neurons play in these changes, silencing these sensory neurons may constitute a viable treatment strategy for these pathologies.
Supplementary Material
Acknowledgments.
This work was partially supported by the National Institute of Health grants (CJW and BPB; PO1NS072040) and (BDL; HL122531); the Canadian Institutes of Health Research (ST), the Canada Research Chair program (ST), Natural Sciences and Engineering Research Council of Canada (ST) and the Brain Canada Foundation, Health Canada and the Azrieli Foundation (ST); as well as by a European Commission fellowship (LEB; 329202) and a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (LEB; 109372/Z/15/Z). JCW received a Bastable-Potts Graduate research award from the Canadian Allergy, Asthma and Immunology Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: ST, DR, BPB, BDL and CJW have an equity stake in Nocion Therapeutics.
References
- 1.Fahy JV, Dickey BF. Airway mucus function and dysfunction. The New England journal of medicine. 2010;363(23):2233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135(2):505–12. [DOI] [PubMed] [Google Scholar]
- 3.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nature communications. 2015;6:6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505(7483):412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bando H, Nishio T, van der Horst GT, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(16):4359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87(2):341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomaki M, Ichinose M, Miura M, Hirayama Y, Yamauchi H, Nakajima N, et al. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am J Respir Crit Care Med. 1995;151(3 Pt 1):613–7. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. Neurogenic inflammation and asthma. J Asthma. 1992;29(3):165–80. [DOI] [PubMed] [Google Scholar]
- 9.Rogers DF. Motor control of airway goblet cells and glands. Respir Physiol. 2001;125(1–2):129–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


