Abstract
Invasive fungal infections remain highly problematic for human health. Collectively, they account for more than 1 million deaths a year in addition to more than 100 million mucosal infections and 1 billion skin infections. To be able to make progress it is important to understand the pathobiology of fungal interactions with the immune system. Here, we highlight new advancements pointing out the pivotal role of fungal cell wall components (β-glucan, mannan, galactosaminogalactan and melanin) in modulating host immunity and discuss how these open new opportunities for the development of immunomodulatory strategies to combat deadly fungal infectious diseases.
Keywords: Innate immunity, Trained immunity, Inflammasome, Cell death, Pyroptosis, PANoptosis, Immunometabolism, Polysaccharides, Melanin, Cell wall
The incidence of invasive fungal infections on human health represents a major worldwide health burden accounting for more than 1 million attributed deaths a year. These deep-seated infections, mostly caused by Candida, Cryptococcus and Aspergillus species, are associated with mortality rates that may be 20 to 50% in immunocompromised patients (Bongomin et al., 2017, Papon et al., 2013, Rokas et al., 2020). For this reason, research to better understand fungal pathophysiology is essential for the development of new strategies for early diagnosis and antifungal therapies. Fungi have a complex cell wall, mostly composed of polysaccharides, such as glucans (β- and α- linked), chitin, chitosan, mannans, galactosaminogalactan (Garcia-Rubio et al., 2019, Gow et al., 2017, Latgé et al., 2017). In the past two decades, unprecedented progress has been made in understanding the roles of the major cell wall components in activation of the host immune system. These components represent the predominant pathogen-associated molecular patterns (PAMPs) that orchestrate the antifungal immune response. The last few years have led to several conceptual breakthroughs that have pointed out the pivotal role of fungal cell wall components in modulating host immunity. Here, we highlight a selection of benchmark articles published in this domain in recent years and discuss how these contribute to a better comprehension of molecular events governing host-fungus interactions, and how this may pave the way for rational developments in innovative therapeutic approaches for combating life-threatening fungal infections. Our current issue does not cover all aspects of fungal polysaccharides and readers should refer to other reviews or recent primary articles for more comprehensive details concerning chitin, chitosan, or other polysaccharides (Garcia-Rubio et al., 2019, Gow et al., 2017, Latgé et al., 2017, Fuchs et al., 2018, Moeller et al., 2019, Vendele et al., 2020, Wagener et al., 2017).
β-glucans train the immune cells to rheostat the inflammation
Candidiasis is responsible for 250–400,000 deaths each year, and remains the leading cause of invasive fungal infection (Campion et al., 2015). The cell wall of Candida albicans is mainly composed of mannans and β-glucans (Garcia-Rubio et al., 2019) which are the major fungal PAMPs that activate diverse pattern recognition receptors (PRRs) including C-type lectin receptors (CLRs), Dectin-1 and Toll-like receptors (TLRs), TLR-2 and TLR-4, but also the complement receptor 3 (CR3) (Erwig and Gow, 2016). Fungal β-glucans are also known to mediate trained immunity, which results in functional reprogramming of innate immune cells enabling adaptive responses following secondary exposures to pathogens (Netea et al., 2020). In a recent report, Camilli and colleagues demonstrate that β-glucan-mediating trained immunity inhibits inflammasome activation (Fig. 1A) (Camilli et al., 2020). The inflammasome is a multimeric protein complex that induces inflammatory responses via caspase-1 activation, leading to proteolytic maturation of pro-inflammatory cytokines IL-1β, IL-18 and the inflammatory cell death executioner gasdermin D, that mediates pyroptosis (Xue et al., 2019). Recent studies have also shown that fungi can also induce PANoptosis, a unique form of inflammatory cell death regulated by the PANoptosome complex, which provides a molecular scaffold for contemporaneous engagement of key molecules from pyroptosis, apoptosis, and necroptosis (Banoth et al., 2020). Interestingly, the macrophages from patients with the cryopyrin-associated periodic syndrome (CAPS) have an auto-activation of the NLR family pyrin domain-containing 3 (NLRP3) inflammasome. However, the β-glucan training of CAPS macrophages drastically reduces the auto-activation of inflammasome and the release of pro-inflammatory cytokines (Camilli et al., 2020). This supports previous observations on the innate immune training of microglia with LPS, which induces tolerance to ischemic shock and reduces IL-1β release (Wendeln et al., 2018). These reports bring together two crucial innate immune mechanisms that are induced during fungal infections and suggest a pivotal role of the fungal cell wall in controlling infection-mediated inflammation. These observations also raise some questions that require reconciliation. β-glucans are known to directly trigger NLRP3 inflammasome (Briard et al., 2019, Lamkanfi et al., 2009), but trained immunity inhibits inflammasome activation (Camilli et al., 2020). What therefore is the net effect of trained immunity on inflammasome activation during fungal infection? Might β-glucan mediate counterbalance immune responses via trained immunity that can offset the detrimental hyper-inflammation effects due to inflammasome activation? What is the exact mechanism mediating NLRP3 inflammasome inhibition? Does β-glucan reprogramming inhibit other inflammasomes (Non-canonical NLPR3, NLRC4, AIM2,…)? Further investigations are therefore needed on the relationship between trained immunity and inflammasome activation.
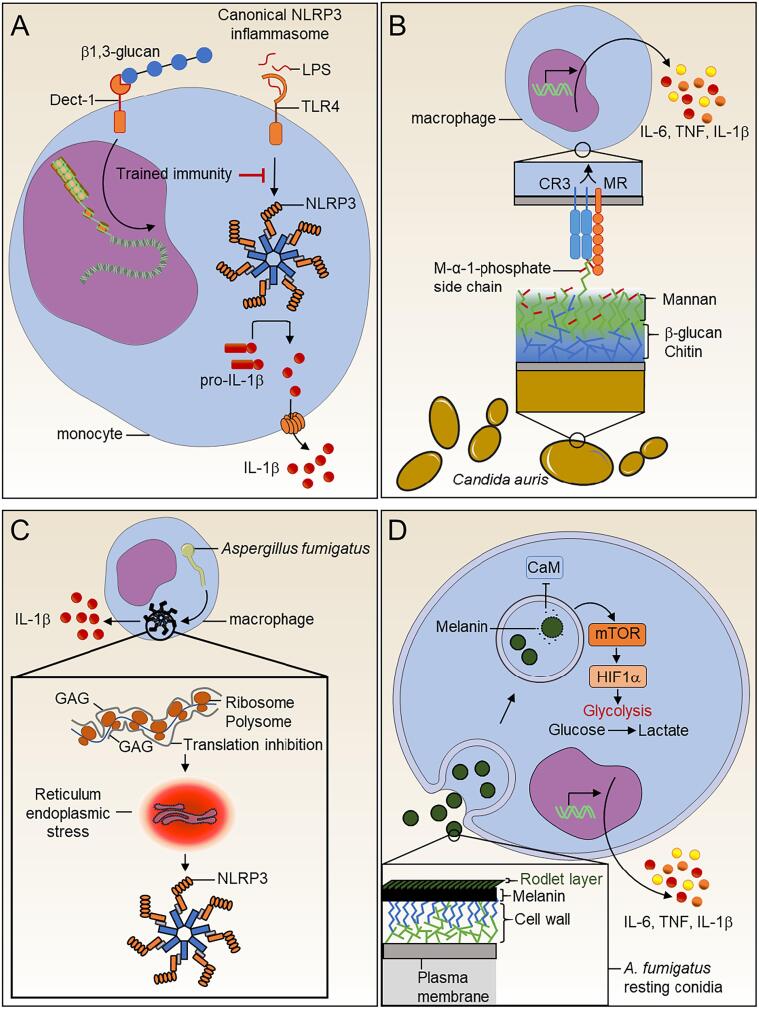
Fig. 1.
Fungal cell wall metabolites modulate our immune system. (A) Fungal β-glucan mediates trained immunity to inhibit canonical NLRP3 inflammasome (B) Host immune system-Candida auris interplay. The C. auris cell surface harbors mannoproteins with specific α-1,2-mannose-phosphate (M-α-1-phosphate) side chains. Macrophage mannose receptor (MMR) and complement receptor 3 (CR3) play a substantial role in the recognition of these specific mannoproteins and the subsequent induction of cytokines. (C)Aspergillus fumigatus galactosaminogalactan (GAG) promotes host immune protection. GAG present at the surface of the germinating conidia directly triggers activation of the NLRP3 inflammasome inside the immune cells. (D) Macrophage metabolism is rewired in response to Aspergillus fumigatus melanin. Fully mature particles of melanin at the surface of the conidia are probably removed during the swelling process, and this inhibits calcium/calmodulin (CaM) signaling in macrophages. Melanin modulation of calcium signaling induces the mTOR pathway, leading to activation of HIF-1α. HIF-1α participates in the transcriptional upregulation of a set of genes for immunometabolic responses. These include not only genes encoding enzymes involved in the glycolysis pathway but also those encoding some pro-inflammatory cytokines.
Candida auris mannan subset drives uncontrolled cytokines burst
Mannoproteins constitute the cell wall outer layer of Candida. sp yeast and are therefore the most accessible PAMP available to the PRRs of the immune system (Vendele et al., 2020, Hall et al., 2013). Most cell wall mannoproteins are glycosylphosphatidylinositol (GPI) anchored proteins, post-translationally modified by a series of glycosyltransferases that elaborate N- and O-linked mannan assembly. Evidence shows that the structure of oligomannoside side chains of these glycoproteins is Candida species dependent, and that the structure of the mannans is highly influenced by local growth conditions (Yan et al., 2020). Therefore, mannan assembly represents an attractive target for the development of diagnostic biomarkers and potentially new therapeutic antifungal drugs. In this context, a recent study has described the innate host immune response against the emerging multidrug resistant species Candida auris (Bruno et al., 2020). Since its discovery ten years ago, this yeast has dramatically spread worldwide, causing hospital outbreaks with infections in long-term care residents and high levels of mortality (Du et al., 2020). In their striking new report, Bruno and colleagues identified a new mannan oligosaccharide structure that is specific to C. auris and occurs within the acid-labile component of the Fig. 1 N-linked mannan (Bruno et al., 2020). This has two α-1,2-mannose-phosphate residues in the side chains that are elaborated on the α-1,6N-mannan backbone (Bruno et al., 2020). The mannan from C. auris was the dominant eliciting stimulus responsible for the cytokine stimulation. Investigators later revealed that the macrophage mannose receptor and CR3 were the PRRs mediating the recognition of these specific mannoproteins by innate immune cells and the potential role in the induction of strong cytokine response during C. auris infection (Fig. 1B). Interestingly, the inhibition of the Dectin-1 pathway during the infection with C. auris mediated strong IL-6 and TNF release, while during C. albicans infection, it is inhibited. Similarly, C. auris purified β-glucan was impotent in stimulating inflammatory cytokines than purified C. albicans glucan (Bruno et al., 2020). Do C. auris β-glucans are an antagonist of Dectin-1 receptor? The structure and function of C. auris glucans would merit to be elucidated. And, the interplay between mannan and β-glucan as well as potential signaling crosstalk between signals induced by these two polysaccharides remain to be fully investigated. Candida species have more or less extended families of mannosyltranferases, but the relationship between the gene set repertoire and the fine structure of the N-linked mannans is not fully understood (Hall and Gow, 2013). Since rapid adaptive changes in the yeast cell wall composition happen during fungal infection, the fungal cell wall stands out as a moving target for immunological surveillance (Pradhan et al., 2019). Such specific mannan/β-glucan balance may also occur for other Candida pathogens and it would be relevant to explore this issue in other Candida species. In addition, different cells of the innate immune system carry different repertoires of PRRs and can induce distinct immune responses to the same palette of β-glucan and mannan structures (Yadav et al., 2020).
Galactosaminogalactan calls for a strike by transcription machinery to mediate inflammasome
Aspergillus infections also represent a major problem in human health, resulting in 100,000 deaths a year worldwide due to invasive disease and high morbidity due to allergy and induced lung inflammation (Brown et al., 2012). Galactosaminogalactan (GAG) is a polysaccharide produced by Aspergillus fumigatus and is not present in the wall of Candida species. GAG is an α-1,4-linked linear heteroglycan composed of a variable combination of galactose, galactosamine and N-acetyl-galactosamine (Briard et al., 2016, Fontaine et al., 2011, Sheppard, 2011). A. fumigatus GAG has a primary function of adhesin for the fungal binding on biotic and abiotic surface (Gravelat et al., 2013). GAG exerts immunomodulatory effects with IL-1Ra release and interleukine-1 receptor (IL-1R) blockage (Gresnigt et al., 2014). Recognition of A. fumigatus by the host immune system leads to inflammasome activation, which protects against infection (Karki et al., 2015, Saïd-Sadier et al., 2010). Previous studies have shown that A. fumigatus is sensed by a variety of membrane bound innate immune receptors, including TLRs and CLRs, and cytosolic NOD-like receptors (NLRs) and absent in melanoma 2 (AIM2)-like receptors. The receptors NLRP3 and AIM2 are well documented for assembling the inflammasome and can form a single cytosolic inflammasome complex along with caspase-1 and caspase-8 to provide protection against A. fumigatus infection (Karki et al., 2015). However, the A. fumigatus specific PAMPs that regulate and activate the inflammasome have yet to be fully characterized. Recently, Briard and colleagues demonstrated for the first time that GAG acts as a PAMP by directly changing the transcription of cells leading to NLRP3 inflammasome activation (Fig. 1C) (Briard et al., 2020). Importantly, GAG-induced activation of the inflammasome provides host protection against aspergillosis as well as inflammatory models such as dextran sulfate sodium-induced colitis (Gresnigt et al., 2014, Briard et al., 2020). Mechanistically, the galactosamine subunit of GAG is necessary to interact with ribosomes and polysomes, leading to the blocking of transcription. The infected cells accumulate unfolded proteins, which drive endoplasmic reticulum stress, and proteasome overactivation, which mediates NLRP3 inflammasome activation and pyroptosis cell death (Briard et al., 2020). Interestingly, the GAG was previously shown to mediate the apoptosis of human neutrophils in contact with NK cells (Robinet et al., 2014). Do the mechanism of cell death in human neutrophils is dependent on the inflammasome? Further investigations are needed. The deacetylation of GAG is a crucial mechanism for the polysaccharide's bioactivity as an adhesin (Lee et al., 2016). The enzymatic pathway was intensively investigated to elucidate the fungal mechanism of remodeling the GAG (Lee et al., 2016, Bamford et al., 2015, Bamford et al., 2020, Lee et al., 2015). In like manner, the GAG deacetylation is required for A. fumigatus mediate innflammasome activation (Briard et al., 2020).
The interferon GTPase Irgb10 was recently shown to target the fungal cell wall and release fungal PAMPs to mediate the inflammasome activation. In addition, IRF1 upregulates the expression of Irgb10 in response to TLR and CLR signaling (Briard et al., 2019). Therefore, it will be interesting to explore how Irgb10 regulates the GAG to mediate transcription inhibition and inflammasome activation. Similarly, β-glucan is known to mediate NLRP3 inflammasome activation, although this occurs through a distinct mechanism that does not involve interaction with ribosomes (Briard et al., 2020). Future studies should address whether inflammasome activation is specific to GAG and β-glucan or represents a property that is shared by a wider range of polysaccharides. Moreover, GAG mediates IL-1Ra release and has an anti-inflammatory effect in colitis mouse model (Gresnigt et al., 2014, Gressler et al., 2019), whereas GAG also induces inflammasome activation and inflammation (Briard et al., 2020). Is therefore IL-1Ra production dependent on the inflammasome activation by negative feedback control in DSS-induced colitis? Further investigations on the relationship between inflammasome and IL-1Ra are required.
Melanin pigment sequesters cellular calcium to reprogram the immune cell fate
Melanin is a common component of conidia of A. fumigatus and the cell walls of a range of darkly pigmented fungi (van de Veerdonk et al., 2017). The two predominant types of fungal melanins are dihydroxyphenylalanine (DOPA)-melanin and dihydroxynaphthalene (DHN)-melanin (Scharf et al., 2014). Cell wall DHN-melanin has been previously shown to play a central role in A. fumigatus survival and virulence during host invasion (Scharf et al., 2014). Recently, DHN-melanin detection was shown to involve specific recognition by the C-type lectin receptor MelLeC that is present on the surface of endothelial cells (Stappers et al., 2018). Interestingly, in mice, MelLeC receptors are present only on endothelial cells, whereas in humans, MelLeC is also present on cells of the myeloid lineage (Stappers et al., 2018). Phagocytosis digestion of A. fumigatus conidia occurs after the shielding effect melanin and rodlet layers are removed or fragmented to induce the activation of the autophagy pathway termed LC3-associated phagocytosis (LAP) and successfully eradicate the conidia (Akoumianaki et al., 2016, Carrion et al., 2013). In a striking new study, Gonçalves and colleagues showed that fungal melanin is necessary to mediate macrophage metabolic reprogramming leading to increasing glycolysis during the induced host defense (Gonçalves et al., 2020). They also revealed that melanin modulates intracellular calcium availability independently to MelLeC, which in turn mediated hypoxia and mTOR pathways activation (Fig. 1D). Interestingly, addition of melanin to non-melanized conidia was not able to restore the stimulation of macrophage glycolysis. The authors concluded that melanin layer removal was crucial to the metabolic reprogramming of the immune cells (Gonçalves et al., 2020). Moreover, β-glucan mediates LAP (Kyrmizi et al., 1950), and melanin inhibits LAP (Akoumianaki et al., 2016). Does therefore the absence of melanin enhance β-glucan exposure and LAP activation to inhibit the metabolomic reprogramming of innate immune cells? This is in accordance with recent studies indicating that β-glucans may cooperate with other cell wall components to mediate immune recognition and regulation (Bruno et al., 2015).
Future directions
Recent insights provide evidence for a pivotal role of various fungal cell wall components during fungus-host interactions. These involve an array of polysaccharides that includes β-glucans, mannans, and GAG and other wall components (melanin) that represent fungal specific signatures. These components influence multiple innate and adaptive immune mechanisms, including trained immunity, inflammasome activation, immunomodulation, or LAP. In the past, a considerable amount of studies have been initiated with purified polysaccharides or dead pathogens to evaluate the immune response. However, more evidence shows that the fungal cell wall is a moving target evolving during the developement of the pathogen and that the presence of a multitude PAMPs may induce a fine-tuning of immune response compared to an isolated PAMP. Focusing research on fungal cell wall biosynthesis and remodeling during infection will likely provide new opportunities to develop innovative therapeutic approaches to fight deadly fungal infections and pro-inflammatory and autoimmune diseases. This will require the development of integrative studies that include a multitude of fungal components that articulate with a complex set of multimodal immune mechanisms. It will also be important to consolidate immunometabolism investigations by integrating data from multi-omics approaches obtained from fungal and immune cells as well as a detailed understanding of the immunomodulatory features of fungal cell walls.
Acknowledgement
NG acknowledges welcome support of a Senior Investigator (101873/Z/13/Z), Collaborative (200208/A/15/Z, 215599/Z/19/Z) and Strategic Awards (097377/Z11/Z) and the MRC Centre for Medical Mycology [MR/N006364/2].
Footnotes
Given his role as Editor in Chief, Neil Gow had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Catarina Gadelha.
References
- Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi Basel Switz. 2017;3:E57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papon N., Courdavault V., Clastre M., Bennett R.J., Heitman J. Emerging and emerged pathogenic candida species: beyond the candida albicans paradigm. PLoS Pathog. 2013;9(9):e1003550. doi: 10.1371/journal.ppat.100355010.1371/journal.ppat.1003550.g00110.1371/journal.ppat.1003550.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokas A., Mead M.E., Steenwyk J.L., Oberlies N.H., Goldman G.H., Sheppard D.C. Evolving moldy murderers: aspergillus section fumigati as a model for studying the repeated evolution of fungal pathogenicity. PLoS Pathog. 2020;16(2):e1008315. doi: 10.1371/journal.ppat.100831510.1371/journal.ppat.1008315.g00110.1371/journal.ppat.1008315.g002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rubio R., de Oliveira H.C., Rivera J., Trevijano-Contador N. The Fungal cell wall: Candida, cryptococcus, and aspergillus species. Front. Microbiol. 2019;10:2993. doi: 10.3389/fmicb.2019.02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N.A.R., Latge J.-P., Munro C.A., Heitman J. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017;5(3) doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé J.-P., Beauvais A., Chamilos G. The Cell Wall of the Human Fungal Pathogen Aspergillus Fumigatus: Biosynthesis, Organization, Immune Response, and Virulence. Annu. Rev. Microbiol. 2017;71(1):99–116. doi: 10.1146/micro.2017.71.issue-110.1146/annurev-micro-030117-020406. [DOI] [PubMed] [Google Scholar]
- Fuchs K., Cardona Gloria Y., Wolz O.-O., Herster F., Sharma L., Dillen C.A., Täumer C., Dickhöfer S., Bittner Z., Dang T.-M., et al. The Fungal Ligand Chitin Directly Binds TLR2 and Triggers Inflammation Dependent on Oligomer Size. EMBO Rep. 2018;19 doi: 10.15252/embr.201846065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller J.B., Leonardi I., Schlosser A., Flamar A.-L., Bessman N.J., Putzel G.G., Thomsen T., Hammond M., Jepsen C.S., Skjødt K., et al. Modulation of the fungal mycobiome is regulated by the chitin-binding receptor FIBCD1. J. Exp. Med. 2019;216:2689–2700. doi: 10.1084/jem.20182244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendele I., Willment J.A., Silva L.M., Palma A.S., Chai W., Liu Y., Feizi T., Spyrou M., Stappers M.H.T., Brown G.D., Gow N.A.R., Hohl T.M. Mannan Detecting C-Type Lectin Receptor Probes Recognise Immune Epitopes with Diverse Chemical, Spatial and Phylogenetic Heterogeneity in Fungal Cell Walls. PLOS Pathog. 2020;16(1):e1007927. doi: 10.1371/journal.ppat.1007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener, J., MacCallum, D.M., Brown, G.D., Gow, N.A.R. Candida Albicans Chitin Increases Arginase-1 Activity in Human Macrophages, with an Impact on Macrophage Antimicrobial Functions. mBio 8, e01820-16, doi:10.1128/mBio.01820-16. [DOI] [PMC free article] [PubMed]
- Campion E.W., Kullberg B.J., Arendrup M.C. Invasive Candidiasis. N. Engl. J. Med. 2015;373(15):1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- Erwig L.P., Gow N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016;14(3):163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M., Riksen N.P., Schlitzer A., Schultze J.L., Stabell Benn C., Sun J.C., Xavier R.J., Latz E. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli G., Bohm M., Piffer A.C., Lavenir R., Williams D.L., Neven B., Grateau G., Georgin-Lavialle S., Quintin J. β-Glucan–Induced Reprogramming of Human Macrophages Inhibits NLRP3 Inflammasome Activation in Cryopyrinopathies. J. Clin. Invest. 2020;130:4561–4573. doi: 10.1172/JCI134778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Enosi Tuipulotu D., Tan W.H., Kay C., Man S.M. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40(11):1035–1052. doi: 10.1016/j.it.2019.09.005. [DOI] [PubMed] [Google Scholar]
- Banoth B., Tuladhar S., Karki R., Sharma B.R., Briard B., Kesavardhana S., Burton A., Kanneganti T.-D. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis) J. Biol. Chem. 2020;295(52):18276–18283. doi: 10.1074/jbc.RA120.015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeln A.-C., Degenhardt K., Kaurani L., Gertig M., Ulas T., Jain G., Wagner J., Häsler L.M., Wild K., Skodras A., Blank T., Staszewski O., Datta M., Centeno T.P., Capece V., Islam M.R., Kerimoglu C., Staufenbiel M., Schultze J.L., Beyer M., Prinz M., Jucker M., Fischer A., Neher J.J. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556(7701):332–338. doi: 10.1038/s41586-018-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briard B., Karki R., Malireddi R.K.S., Bhattacharya A., Place D.E., Mavuluri J., Peters J.L., Vogel P., Yamamoto M., Kanneganti T.-D. Fungal ligands released by innate immune effectors promote inflammasome activation during aspergillus fumigatus infection. Nat. Microbiol. 2019;4(2):316–327. doi: 10.1038/s41564-018-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M., Malireddi R.K.S., Kanneganti T.-D. Fungal zymosan and mannan activate the cryopyrin inflammasome. J. Biol. Chem. 2009;284(31):20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R.A., Bates S., Lenardon M.D., MacCallum D.M., Wagener J., Lowman D.W., Kruppa M.D., Williams D.L., Odds F.C., Brown A.J.P., Gow N.A.R., Feldmesser M. The Mnn2 Mannosyltransferase Family Modulates Mannoprotein Fibril Length, Immune Recognition and Virulence of Candida Albicans. PLoS Pathog. 2013;9(4):e1003276. doi: 10.1371/journal.ppat.1003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Xia K.e., Yu Y., Miliakos A., Chaturvedi S., Zhang F., Chen S., Chaturvedi V., Linhardt R.J. Unique cell surface mannan of yeast pathogen candida auris with selective binding to IgG. ACS Infect. Dis. 2020;6(5):1018–1031. doi: 10.1021/acsinfecdis.9b0045010.1021/acsinfecdis.9b00450.s001. [DOI] [PubMed] [Google Scholar]
- Bruno M., Kersten S., Bain J.M., Jaeger M., Rosati D., Kruppa M.D., Lowman D.W., Rice P.J., Graves B., Ma Z., Jiao Y.N., Chowdhary A., Renieris G., van de Veerdonk F.L., Kullberg B.-J., Giamarellos-Bourboulis E.J., Hoischen A., Gow N.A.R., Brown A.J.P., Meis J.F., Williams D.L., Netea M.G. Transcriptional and functional insights into the host immune response against the emerging fungal pathogen Candida auris. Nat. Microbiol. 2020;5(12):1516–1531. doi: 10.1038/s41564-020-0780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Bing J., Hu T., Ennis C.L., Nobile C.J., Huang G., Xue C. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10):e1008921. doi: 10.1371/journal.ppat.1008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R.A., Gow N.A.R. Mannosylation in candida albicans: role in cell wall function and immune recognition. Mol. Microbiol. 2013;90:1147–1161. doi: 10.1111/mmi.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A., Avelar G.M., Bain J.M., Childers D., Pelletier C., Larcombe D.E., Shekhova E., Netea M.G., Brown G.D., Erwig L., Gow N.A.R., Brown A.J.P. Non-Canonical Signalling Mediates Changes in Fungal Cell Wall PAMPs That Drive Immune Evasion. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-13298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav B., Mora-Montes H.M., Wagener J., Cunningham I., West L., Haynes K., Brown A.J.P., Gow N.A.R. Differences in Fungal Immune Recognition by Monocytes and Macrophages: N-Mannan Can Be a Shield or Activator of Immune Recognition. Cell Surf. Amst. Neth. 2020;6:100042. doi: 10.1016/j.tcsw.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, G.D., Denning, D.W., Gow, N.A.R., Levitz, S.M., Netea, M.G., White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med.2012, 4, 165rv13-165rv13, doi:10.1126/scitranslmed.3004404. [DOI] [PubMed]
- Briard B., Muszkieta L., Latgé J.-P., Fontaine T. Galactosaminogalactan of Aspergillus Fumigatus, a Bioactive Fungal Polymer. Mycologia. 2016;108(3):572–580. doi: 10.3852/15-312. [DOI] [PubMed] [Google Scholar]
- Fontaine T., Delangle A., Simenel C., Coddeville B., van Vliet S.J., van Kooyk Y., Bozza S., Moretti S., Schwarz F., Trichot C., Aebi M., Delepierre M., Elbim C., Romani L., Latgé J.-P., Feldmesser M. Galactosaminogalactan, a New Immunosuppressive Polysaccharide of Aspergillus Fumigatus. PLOS Pathog. 2011;7(11):e1002372. doi: 10.1371/journal.ppat.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D.C. Molecular mechanism of aspergillus fumigatus adherence to host constituents. Curr. Opin. Microbiol. 2011;14(4):375–379. doi: 10.1016/j.mib.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravelat F.N., Beauvais A., Liu H., Lee M.J., Snarr B.D., Chen D., Xu W., Kravtsov I., Hoareau C.M.Q., Vanier G., Urb M., Campoli P., Al Abdallah Q., Lehoux M., Chabot Josée.C., Ouimet M.-C., Baptista S.D., Fritz Jörg.H., Nierman W.C., Latgé J.P., Mitchell A.P., Filler S.G., Fontaine T., Sheppard D.C., Doering T.L. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog. 2013;9(8):e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresnigt M.S., Bozza S., Becker K.L., Joosten L.A.B., Abdollahi-Roodsaz S., van der Berg W.B., Dinarello C.A., Netea M.G., Fontaine T., De Luca A., Moretti S., Romani L., Latge J.-P., van de Veerdonk F.L., Doering T.L. A polysaccharide virulence factor from aspergillus fumigatus elicits anti-inflammatory effects through induction of interleukin-1 receptor antagonist. PLoS Pathog. 2014;10(3):e1003936. doi: 10.1371/journal.ppat.1003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Man S.M., Malireddi R.K.S., Gurung P., Vogel P., Lamkanfi M., Kanneganti T.-D. Concerted Activation of the AIM2 and NLRP3 Inflammasomes Orchestrates Host Protection against Aspergillus Infection. Cell Host Microbe. 2015;17(3):357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saïd-Sadier N., Padilla E., Langsley G., Ojcius D.M., Unutmaz D. Aspergillus Fumigatus Stimulates the NLRP3 Inflammasome through a Pathway Requiring ROS Production and the Syk Tyrosine Kinase. PLoS ONE. 2010;5(4):e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briard B., Fontaine T., Samir P., Place D.E., Muszkieta L., Malireddi R.K.S., Karki R., Christgen S., Bomme P., Vogel P., Beau Rémi, Mellado E., Ibrahim-Granet O., Henrissat B., Kalathur R.C., Robinson C., Latgé J.-P., Kanneganti T.-D. Galactosaminogalactan activates the inflammasome to provide host protection. Nature. 2020;588(7839):688–692. doi: 10.1038/s41586-020-2996-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinet P., Baychelier F., Fontaine T., Picard C., Debré P., Vieillard V., Latgé J.-P., Elbim C. A Polysaccharide virulence factor of a human fungal pathogen induces neutrophil apoptosis via NK cells. J. Immunol. 2014;192(11):5332–5342. doi: 10.4049/jimmunol.1303180. [DOI] [PubMed] [Google Scholar]
- Lee M.J., Geller A.M., Bamford N.C., Liu H., Gravelat F.N., Snarr B.D., Le Mauff F., Chabot J., Ralph B., Ostapska H., Lehoux M., Cerone R.P., Baptista S.D., Vinogradov E., Stajich J.E., Filler S.G., Howell P.L., Sheppard D.C., Heitman J. Deacetylation of Fungal Exopolysaccharide Mediates Adhesion and Biofilm Formation. mBio. 2016;7(2) doi: 10.1128/mBio.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford N.C., Snarr B.D., Gravelat F.N., Little D.J., Lee M.J., Zacharias C.A., Chabot J.C., Geller A.M., Baptista S.D., Baker P., Robinson H., Howell P.L., Sheppard D.C. Sph3 Is a glycoside hydrolase required for the biosynthesis of galactosaminogalactan in aspergillus fumigatus. J. Biol. Chem. 2015;290(46):27438–27450. doi: 10.1074/jbc.M115.679050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford N.C., Le Mauff F., Van Loon J.C., Ostapska H., Snarr B.D., Zhang Y., Kitova E.N., Klassen J.S., Codée J.D.C., Sheppard D.C., Howell P.L. Structural and biochemical characterization of the exopolysaccharide deacetylase agd3 required for aspergillus fumigatus biofilm formation. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-16144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.J., Liu H., Barker B.M., Snarr B.D., Gravelat F.N., Al Abdallah Q., Gavino C., Baistrocchi S.R., Ostapska H., Xiao T., Ralph B., Solis N.V., Lehoux M., Baptista S.D., Thammahong A., Cerone R.P., Kaminskyj S.G.W., Guiot M.-C., Latgé J.-P., Fontaine T., Vinh D.C., Filler S.G., Sheppard D.C., May R.C. The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps. PLOS Pathog. 2015;11(10):e1005187. doi: 10.1371/journal.ppat.1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressler M., Heddergott C., N'Go I.C., Renga G., Oikonomou V., Moretti S., Coddeville B., Gaifem J., Silvestre R., Romani L., Latgé J.-P., Fontaine T. Definition of the Anti-Inflammatory Oligosaccharides Derived From the Galactosaminogalactan (GAG) From Aspergillus Fumigatus. Front. Cell. Infect. Microbiol. 2019;9 doi: 10.3389/fcimb.2019.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk F.L., Gresnigt M.S., Romani L., Netea M.G., Latgé J.-P. Aspergillus Fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017;15(11):661–674. doi: 10.1038/nrmicro.2017.90. [DOI] [PubMed] [Google Scholar]
- Scharf D.H., Heinekamp T., Brakhage A.A., Heitman J. Human and plant fungal pathogens: the role of secondary metabolites. PLoS Pathog. 2014;10(1):e1003859. doi: 10.1371/journal.ppat.1003859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappers M.H.T., Clark A.E., Aimanianda V., Bidula S., Reid D.M., Asamaphan P., Hardison S.E., Dambuza I.M., Valsecchi I., Kerscher B., Plato A., Wallace C.A., Yuecel R., Hebecker B., da Glória Teixeira Sousa M., Cunha C., Liu Y., Feizi T., Brakhage A.A., Kwon-Chung K.J., Gow N.A.R., Zanda M., Piras M., Zanato C., Jaeger M., Netea M.G., van de Veerdonk F.L., Lacerda João.F., Campos A., Carvalho A., Willment J.A., Latgé J.-P., Brown G.D. Recognition of DHN-Melanin by a C-Type Lectin Receptor Is Required for Immunity to Aspergillus. Nature. 2018;555(7696):382–386. doi: 10.1038/nature25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoumianaki, T., Kyrmizi, I., Valsecchi, I., Gresnigt, M.S., Samonis, G., Drakos, E., Boumpas, D., Muszkieta, L., Prevost, M.-C., Kontoyiannis, D.P., et al. Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity. Cell Host Microbe 0, doi:10.1016/j.chom.2015.12.002. [DOI] [PubMed]
- Carrion S.de.J., Leal S.M., Ghannoum M.A., Aimanianda V., Latgé J.-P., Pearlman E. The roda hydrophobin on aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J. Immunol. Baltim. Md. 2013;191(5):2581–2588. doi: 10.4049/jimmunol.1300748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves S.M., Duarte-Oliveira C., Campos C.F., Aimanianda V., ter Horst R., Leite L., Mercier T., Pereira P., Fernández-García M., Antunes D., Rodrigues C.S., Barbosa-Matos C., Gaifem J., Mesquita I., Marques A., Osório N.S., Torrado E., Rodrigues F., Costa S., Joosten L.AB., Lagrou K., Maertens J., Lacerda J.F., Campos A., Brown G.D., Brakhage A.A., Barbas C., Silvestre R., van de Veerdonk F.L., Chamilos G., Netea M.G., Latgé J.-P., Cunha C., Carvalho A. Phagosomal removal of fungal melanin reprograms macrophage metabolism to promote antifungal immunity. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-16120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrmizi I., Gresnigt M.S., Akoumianaki T., Samonis G., Sidiropoulos P., Boumpas D., Netea M.G., van de Veerdonk F.L., Kontoyiannis D.P., Chamilos G. Corticosteroids block autophagy protein recruitment in aspergillus fumigatus phagosomes via targeting dectin-1/Syk Kinase signaling. J. Immunol. Baltim. Md. 1950;2013(191):1287–1299. doi: 10.4049/jimmunol.1300132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno V.M., Shetty A.C., Yano J., Fidel P.L., Noverr M.C., Peters B.M., Krysan D., Berman J. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the nlrp3 inflammasome. mBio. 2015;6(2) doi: 10.1128/mBio.00182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]