Summary
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation technique. Many substance use disorders lack effective treatments, and TMS is expected to reduce cravings and risk of relapse by regulating brain function. Here, we introduce three alternative TMS settings and specific operations to interfere with methamphetamine use disorders. Theoretically, this protocol can also be applied to diseases with similar brain damage characteristics.
For complete details on the use and execution of this protocol, please refer to Chen et al. (2020).
Subject areas: Health Sciences, Clinical Protocol, Neuroscience
Graphical abstract

Highlights
-
•
Protocol for applying repetitive TMS to decrease the craving and relapse
-
•
Accurate positioning is one of the crucial steps of repetitive TMS treatment
-
•
The treatment process requires continuous attention to the patient’s response
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation technique. Many substance use disorders lack effective treatments, and TMS is expected to reduce cravings and risk of relapse by regulating brain function. Here, we introduce three alternative TMS settings and specific operations to interfere with methamphetamine use disorders. Theoretically, this protocol can also be applied to diseases with similar brain damage characteristics.
Before you begin
The institutional review board and the ethics committee of Shanghai Mental Health Center approved this study protocol.
Understand the basic knowledge of transcranial magnetic stimulation intervention
The mechanism of transcranial magnetic stimulation (TMS) is that through the principle of electromagnetic induction, magnetic field signals can pass through the skull and be converted into electrical signals to act on the cortex (George et al., 1999). TMS does not require invasive operations and has the advantages of high safety and reliability. Unlike single-pulse TMS stimulation, repetitive transcranial magnetic stimulation (rTMS) regulates the brain functional state by continuously stimulating specific brain regions (Diana et al., 2017). Based on the existing research, it is generally believed that the intervention effect of rTMS mainly depends on at least three mechanisms (Peng et al., 2018; Speer et al., 2000; Ziemann and Siebner, 2008): (1) Functional changes in stimulated brain regions and changes in the strength of functional connections between different brain regions.; (2) Induction of long-term potentiation or depression of the brain; (3) Induction of synchronized activities of neuronal populations.
At present, TMS is used in the examination and treatment of several neuropsychiatric diseases. For instance, the FDA approved high frequency rTMS targeting the left dorsolateral prefrontal cortex (DLPFC) for the treatment of major depression (FDA approval K061053). The clinical guidelines issued by the International Federation of Clinical Neurophysiology also suggest that rTMS targeting the DLPFC area can reduce the relapse risk of nicotine dependence. The clinical recommendation level is C (“possibly effective”) (Lefaucheur et al., 2020). In overall, TMS provides a potential mean to regulate the functional state of the brain and improve clinical symptoms.
Understand the different stimulation parameters
Before starting the intervention, the parameters that need to be determined include at least the following: the brain area of the intervention, the coil used for the intervention, and the stimulation parameters (frequency, intensity, total number of pulses, and duration of treatment).
-
1.
The brain area of the intervention
RTMS mainly affects the targeted brain region and the brain area that is functionally connected with the targeted brain area. Therefore, it is necessary to choose different brain regions for intervention according to the pathological mechanisms of different neuropsychiatric diseases.
For people with substance use disorders, it is generally believed that when the limbic circuit is stimulated by external drug-related cues, the reward signals is projected to the ventral striatum by the dopaminergic neurons in ventral tegmental area (Wolf, 2016). This process is also mediated by executive control network (Diana, 2011; Schultz, 2002; Volkow and Morales, 2015). Hence, the disruption of the executive control networks and limbic neural circuits may induce the addictive behaviors and repeated relapse (Li et al., 2018; Van Dam et al., 2014). Specifically, the cue-induced craving and drug relapse may be related to two situations. One is that the activity of the executive control network is weakened, and cognitive control becomes worse; the other is that the activity of the limbic neural circuit increases excessively when under the cue exposed (Moeller et al., 2010). Therefore, the present protocol mainly focuses on the brain regions of these two brain network, DLPFC (the core brain area of the executive control network) and ventromedial prefrontal cortex (VMPFC, the core brain area of the limbic neural circuit).
-
2.
The coil used for the intervention
There are several coils could be used in TMS stimulation: circular shape, butterfly shape (also known as figure of eight), elliptic shape, and D-shape. The coil with different shape has different stimulation feature. For instance, the butterfly coil can produce a concentrated stimulating effect on the center of the coil (e.g., MCF-B70 coil); the circular coil and the elliptical coil can produce a more average stimulation effect on the larger area of the brain under the coil; and the stimulation center of the D-shaped coil is located at the coil edge. Within the figure-of-eight coil, the angled butterfly coil (e.g., Cool D-B80 coil) (Figures 1A and 1B) can stimulate deeper than the flat one (Figures 1C and 1D). When the operation needs to stimulate a more precise position, we could choose the figure-of-eight coil; and when our goal is to stimulate a broader area of brain region, we could choose the circular coil, which have a wide range of stimulation area and average stimulation intensity.
-
3.
The stimulation parameters
Figure 1.
The outlook of the flat butterfly coil and angled butterfly coil
(A and B) The outlook of the flat butterfly coil.
(C and D) The outlook of the angled butterfly coil.
After determining the location of targeted brain region and selecting the coil, specific stimulation parameters need to be determined.
In terms of frequency, high-frequency (>5 Hz) and low-frequency (<1 Hz) modes are currently used in most studies (Lefaucheur et al., 2020). Based on the findings in the MEP measurement study of healthy subjects, some researchers believed that low-frequency stimulation is regarded as “inhibitory” and high frequency stimulation is regarded as “excitatory”. Although this is not a definite conclusion, we can set parameters based on this principle when we carry out the research and adjust the parameters according to the actual treatment effect. It should also be noted that the choice of specific parameters is also related to the disease. In the same brain area, the damage characteristics may be various in different disease, so the frequency setting needs to be changed. For example, in depression studies, it was found that high-frequency stimulation of the left DLPFC can improve depression (Gershon et al., 2003), but in patients with sleep disorders, low-frequency stimulation of the left DLPFC can improve sleep quality (Nardone et al., 2020).
Besides, theta burst stimulation (TBS) is another frequently used type of stimulation mode. TBS refers to a stimulation setting in which a fixed-frequency pulse is nested in another pulse mode with fixed-frequency. Theta burst pulse stimulation is a commonly used TBS mode in psychiatric diseases, which refers to embedding three continuous 50 Hz pulses stimulation into a 5 Hz pulse stimulation (Huang et al., 2005). It is suggested that intermittent theta burst pulse stimulation (iTBS) plays an excitatory role, while continuous theta burst pulse stimulation (cTBS) have an inhibitory effect.
For intensity, most studies are between 80 and 120% of rest motor threshold (rMT) (Lefaucheur et al., 2020). The higher the stimulus intensity and the more pulse numbers, the effect of stimulus is also higher (McDonald et al., 2011). However, high intensity of rTMS may bring more risks of adverse effects (Rossi et al., 2009). Regarding the treatment course, studies that reported significant treatment effect usually set a course for at least one week. The stimulation of multiple sessions can enhance long-term effects.
The design of the current stimulation protocol
Four stimulation settings will be introduced in this protocol. The first one uses the excitatory stimulation mode iTBS to stimulate the dorsolateral prefrontal region; the second uses the inhibitory stimulation mode cTBS to target the ventromedial prefrontal cortex; the third combines the first and second setting; the fourth is sham stimulation, which the parameter is consistent with the first or second setting. The coil used in the sham stimulation setting (i.e., MCF-P-B70 coil) is a placebo coil and no magnetic field effect is generated. The TBS parameters used in this protocol are 3-pulse 50-Hz bursts given every 200 ms (Figure 2). During the cTBS setting, a 60-s train treatment (900 pulses in total) is applied to the left VMPFC with D-B80 coil. During the iTBS setting, a 5-min train treatment (2 s on and 8 s off, 900 pulses in total) is applied to the left DLPFC with MCF-B70 coil (Lefaucheur et al., 2020; Su et al., 2020).
Figure 2.
The theta burst stimulation protocol.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Models: Organisms/Strains | ||
| Human subjects (met the DSM-5 criteria for severe methamphetamine use disorder; male; aged 18–49 years old who use methamphetamine in the past 3 months; no serious neuropsychiatric diseases) | Chen et al. (2020) | N/A |
| Other | ||
| MagPro X100 stimulator | MagVenture | N/A |
| Cool D-B80 coil | MagVenture | N/A |
| MCF-B70 coil | MagVenture | N/A |
| MCF-P-B70 coil | MagVenture | N/A |
| Keypoint EMG recorder | Dantech | N/A |
Step-by-step method details
Recruit and screen patients
Timing: 0.5 days for each potential participant
Inform the study-related information to the potential participants, and if they are willing to participate, prepare the written consent and ask the participant to sign it.
-
1.
Contact the interested subjects by telephone to initially screen the potential patients. At this stage, the following details of subjects will be obtained: the type of drugs they used, their age and gender, and whether they suffer from neuropsychiatric diseases.
-
2.
For patients who meet the requirements of the initial screening (i.e., use methamphetamine in the past 3 months; male; aged 18–49 years old; no serious neuropsychiatric diseases), invite them to conduct the face-to-face interviews, provide them information about the research, and answer potential questions.
-
3.
If patients who meet the research eligibility criteria are willing to participate (Seventy-four patients in this study, the sample size calculation methods please refer to Chen et al. (2020)), provide the written consent form to the patients, and create their individual records after they have signed it.
CRITICAL: The inclusion criteria are: (1) met the DSM-5 criteria for severe methamphetamine use disorder; (2) aged 18–49 years old; (3) normal vision and audition; (4) received no medications during treatment; (5) used methamphetamine in the past 3 months before being recruited in this study. The exclusion criteria are: (1) serious physical or neurological illness, a diagnosis of any other psychiatric disorder under DSM-5 criteria (except for nicotine use disorder); (2) any contraindications to rTMS.
Create individual record
Timing: 10 min for each potential participant
Create a treatment record sheet for each patient and record the treatment process and adverse effects during the treatment.
-
4.
Before starts treatment, a treatment document for each subject will be created. The contents of the document mainly include information such as the intensity of the intervention, the mode of the intervention, the date of the intervention. This can be recorded by the operator before and after treatment.
-
5.
Individual adverse effect record sheets should also be prepared, which are used to record the type, intensity as well as time of adverse effects that occurred during the intervention.
CRITICAL: The possible adverse effects including headache, dizzy, tinnitus, facial discomfort, jaw tremble, insomnia, somnolence, and epilepsy.
CRITICAL: Most adverse effects disappear gradually after stopping the stimulation. In the adverse reaction record, we also ask the patients to score their discomfort feeling (0–9). "0" means no adverse effect, and "9" means the adverse effect is severe, and the stimulation needs to be stopped. When the score reaches 5–8 points, it is recommended to consider reducing the current stimulation intensity by about 5% per time until the subject can tolerate the stimulation. When the score reaches 9 points, it is recommended to stop the treatment.
Assessment of craving level (baseline)
Timing: approximately 5 min (for 1 participant)
Assess the participant’s cue-induced craving scores at baseline using visual analog scale.
-
6.
Instruct the subjects to watch the methamphetamine-related figures for 5 min and let the patients to recall the last time they used methamphetamine.
-
7.
Patients report their craving level. The scores are ranging from 0 (i.e., no craving) to 100 (i.e., highest craving intensity ever experienced for methamphetamine).
Measurement of resting motor threshold
Timing: 20 min for each participant
Evaluating the resting motor threshold of each subject is one of the prerequisites for setting treatment parameters.
-
8.Settings for the instrument used for evaluation.
-
a.Transcranial magnetic stimulator: choose the single pulse stimulation mode and the coil is MCF-B70 coil.
-
b.EMG recorder: The EMG recorder should connect to the EMG recording line, which has three electrode patches (two for EMG recording and one for grounding), the EMG signals are analog bandpass filtered between 5 and 500 Hz. The sampling rate is 1000 Hz.
-
a.
CRITICAL: The coil used for the determination of the resting motor threshold should be consistent with the coil used in the later intervention. As mentioned above, since different coils have different effects when target to same brain area, the use of the same coil can ensure that the intensity of the subsequent intervention is closer to the expected.
-
9.
The coil targets the motor cortex and moves weakly during stimulation to find the correct location (Figure 3A). The electrode patches are placed on the right abductor pollicis brevis muscles and the thenar eminence position (Figure 3B). Troubleshooting 1
Note: To identify the areas of motor cortex, mark the vertex of the brain firstly by measuring the mid-point intersection between the nasion-inion and inter-aural lines. For the right abductor pollicis brevis, position the coil 1–2 cm laterally to the right area of the vertex and 1–2 cm posterior initially. The stimulation coil position should be adjusted for each subject to the area that induced motor-evoked potentials with maximal amplitude.
-
10.
According to existing studies, the resting motor threshold of most people is range between 30 and 50% of the output power of the TMS machine. Therefore, the stimulation starts from 40%, and gradually increases or decreases the intensity of the stimulation. Finally, the resting motor threshold is determined.
Note: In order to improve the efficiency of the operation, the intensity is suggested to increase by 2% each time when increasing. When the approximate range of the target intensity is determined, the intensity is reduced by 1% each time for fine-tuning.
Note: The minimum stimulation intensity that can produce five motor-evoked potentials responses of at least 50 microvolts (μV) in ten stimulation is the resting motor threshold of the subject (Figure 3C).
Figure 3.
The schematic diagram of resting motor threshold measurement
(A) Demonstration diagram of the relative position of the head and the coil.
(B) The electrode patches are placed on the right abductor pollicis brevis muscles and the thenar eminence position.
(C) Electromyogram after tie stimulation of the transcranial magnetic stimulation. The minimum stimulation intensity that can produce 5 motor-evoked potentials responses of at least 50μV in 10 stimulation is the resting motor threshold of the subject.
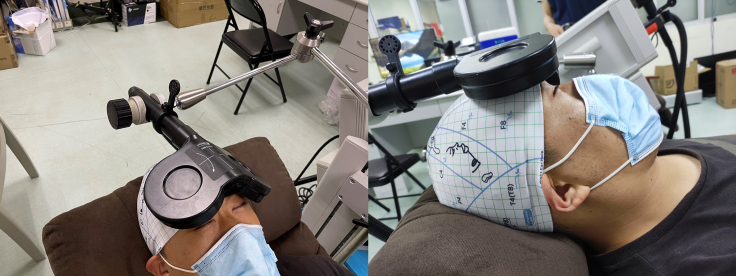
Positioning and coil placement
Timing: 10 min for each participant
-
11.
Before the intervention, it is necessary to locate the stimulation target based on the 10–20 EEG system and position the coil on the head.
-
12.
Two treatment parameters are stored in the stimulator for use in subsequent interventions. The first one is the stimulation parameters targeting DLPFC, and the second one is the stimulation parameters targeting VMPFC.
-
13.
Identify the position of the stimulation site: Before each stimulation session, the subjects take a sitting position and wear an EEG cap. The EEG international 10–20 system will be used to identify the stimulation position (Fp1 for left VMPFC and F3 for left DLPFC) (Figure 4). Troubleshooting 2
CRITICAL: It is necessary to select an EEG cap with a suitable size for each subject and wear it accurately. Generally, there are no very strict criteria for this, but at least two points should be met: (1) the subjects have no discomfort; (2) the cap is not too loose to cause the cap to shift during operation. This can ensure the accuracy of the stimulation position as much as possible.
-
14.
Placement of the stimulation coil: After determining the position of the stimulation, the stimulation coil needs to be placed correctly and the position of the coil must be fixed.
Note: For left VMPFC, the coil is rather placed on the nasal bridge so that the imaginary straight lower margin of the coil is centrally positioned on FP1 (Figure 5). The distance between the upper edge of the coil and the upper forehead is about 2 cm. The handle of the coil is turned upward to be tangent to the head and point towards the top of the head.
Note: For left DLPFC, the coil is held tangential to the participant's head and the coil is centrally positioned on F3 (Figure 6). When performing sham stimulation intervention, the stimulation coil is randomly target on the DLPFC stimulation position or VMPFC stimulation position.
Figure 4.
The targeting area based on EEG International 10–20 system positioning
(A) F3 electrode for left dorsolateral prefrontal cortex;
(B) Fp1 electrode for left ventromedial prefrontal cortex. DLPFC = dorsolateral prefrontal cortex, VMPFC = ventromedial prefrontal cortex.
Figure 5.
Schematic diagram of the positioning of the coil targeting the ventromedial prefrontal cortex
The coil is centered over Fp1, a location approximating the left ventromedial prefrontal cortex.
Figure 6.
Schematic diagram of the positioning of the coil targeting the dorsolateral prefrontal cortex
The coil is centered over F3, a location approximating the left dorsolateral prefrontal cortex.
Treatment process
Timing: 20–30 min for each participant
After setting the parameters and positioning, each subject will be stimulated separately. In addition, the operator needs to observe the patient's feelings during the intervention.
-
15.
All the subjects will be allocated to one of the four treatment groups in a 1:1:1:1 ratio according to the random number table by an independent study researcher. All subjects will receive ten sessions of treatment in two weeks (one session/day and five days/week).
-
16.
Ask the subject to lie on the chair (shown as the Figures 5 and 6) in a separate room and prepare to intervene. Troubleshooting 3
CRITICAL: The main purpose of intervention in a separate room is to ensure the implementation of blinding.
-
17.
Before starting the formal stimulation, place a thin PE foam sheet (thickness = 0.5 mm) between the coil and the stimulated brain position to reduce the potential discomfort caused by the stimulation.
-
18.
When everything is ready, start to stimulate. The stimulation intensity is first set to 80% of the rMT, and it is gradually increased to the specified stimulation intensity during the stimulation process (100% rMT when stimulating DLPFC, and 110% rMT when stimulating VMPFC). In doing so, the patient's tolerance to the stimulation can be improved. Troubleshooting 4 and 5
-
19.
For the combined stimulation group, after completing the intervention of one targeting site, it takes about 5 min to complete the replacement of the stimulation coil and reset the stimulation parameters, and then start the stimulation of another targeting site. Two treatment protocols are randomly assigned to the subjects. The stimulation process is the same as above.
CRITICAL: During the stimulation process, the subject’s head needs to be kept still to ensure that the stimulation position is accurate. It is recommended that an operator could watch by the side, if there is obvious head movement, the operator could help to adjust the head position.
CRITICAL: When performing sham stimulation intervention, the stimulation parameter is randomly set to DLPFC stimulation parameters or VMPFC stimulation parameter, but the stimulation coil uses P-B-70 coil.
Record the treatment process
Timing: 10 min for each participant
-
20.
During the treatment, the operator records the details of the treatment process and asks the subjects about the adverse effects. If it is needed, make timely adjustments to treatment arrangements, including parameter adjustments or discontinuing the treatment.
-
21.
After the treatment is completed, record the stimulation intensity of the patient's current treatment and the time to complete the treatment.
-
22.
Record the patient's adverse effect, the time point of the adverse effect, and the severity of the adverse effect.
CRITICAL: The adverse effects (e.g., headache, dizzy, facial discomfort, and insomnia) may be that the patient has not yet fully adapted to the intensity of the stimulation. In this case, by reducing 5%–10% of the stimulation intensity, the adverse effect could usually be solved. For those who still have obvious adverse effect after reducing the stimulation intensity, it is recommended to suspend the intervention. The solution could be to stop the intervention for 1–2 days and then restart the stimulation. Our research (unpublished data) has found that some subjects no longer have adverse effect in the follow-up treatment. Another solution is to ask the patient whether to withdraw from the study and provide other help to them.
CRITICAL: For the subject with serious adverse events (e.g., epilepsy), it is recommended to stop the stimulation immediately and contact the doctor for further treatment. However, it should be clarified that, according to the current studies, the probability of serious adverse events caused by TMS intervention is low.
Assessment of craving level (post-treatment)
Timing: approximately 5 min each time (for 1 participant)
Evaluate the participants’ cue-induced craving scores during and after the treatment using visual analog scale. It is assessed at post 1/2 week of intervention, post 1 week of intervention, post 3/2 weeks of intervention, and post 2 weeks of intervention.
-
23.
Instruct the subjects to watch the methamphetamine-related figures for 5 min and let the patients to recall the last time they used methamphetamine.
-
24.
Patients report their craving level. The scores are ranging from 0 (i.e., no craving) to 100 (i.e., highest craving intensity ever experienced for methamphetamine).
Expected outcomes
The outcomes of this protocol are the safety and feasibility of four different rTMS intervention protocol. The specific results depend on the evaluation content before and after the intervention. Figure 7 depicts an example of the protocol applied to patients with methamphetamine use disorder (Chen et al., 2020). The study observed that all three real stimulation protocols can significantly reduce the craving for patients with methamphetamine use disorder (Figure 7A). In addition, the average time from baseline to become treatment responder of the three real stimulation protocols is shorter than that of the sham stimulation group (Figure 7B). The patients that accepted the combined protocol had a shorter time of becoming treatment responder compared with those accepted the protocol targeting the DLPFC site. No serious adverse events are reported in the present study. All reported side effects are tolerable and mild and ameliorated gradually during the treatment duration, and no significant differences are identified in four groups.
Figure 7.
The intervention effect of four treatment group
(A) Changes of the cue-induced craving after treatment of four groups.
(B) Kaplan–Meier and Breslow analysis for response of four treatment groups. Figure reprinted with permission from Chen et al. (2020). T0 = baseline, T1/2 = post 1/2 week of intervention, T1 = post 1 week of intervention, T3/2 = post 3/2 weeks of intervention, T2 = post 2 weeks of intervention. Group A = DLPFC iTBS group, Group B = VMPFC cTBS group, Group C = iTBS + cTBS group, Group D = Sham group.
Quantification and statistical analysis
In this study, the primary outcome is the change in craving scores. The treatment response rate (≥60% reduction in craving scores) is the secondary efficacy outcome. Therefore, repeated measure ANOVA is performed to figure out the potential effect on outcomes, with time as intragroup factors and treatment groups (DLPFC iTBS vs VMPFC cTBS vs iTBS+cTBS vs Sham) as intergroup factors. Kaplan–Meier survival analysis (Breslow test) is conducted to explore the relationship between treatment group and the timepoint of patients becoming the responder. The post-hoc pairwise comparison is performed subsequently.
Limitations
Although the three real treatment setting provided by this protocol have certain evidence in terms of effectiveness, safety, and feasibility (Chen et al., 2020), there are still some limitations and can be taken as an optimization direction. First, this protocol uses the EEG international 10–20 system for identify the stimulation position. Previous study has suggested the feasibility of this method in determining the stimulation target (Noh et al., 2017). However, rTMS treatment with magnetic resonance imaging-based navigation and positioning method fully takes the diversity in the shape and size of the individual’s head into account and may bring better treatment effect. Therefore, the navigation and positioning based on magnetic resonance imaging should also be considered when designing the treatment protocol. Similarly, due to the limited of the resources, we did not use the fMRI localizer scan to detect the motor cortex and evaluate the rMT value. When conditions permit, the fMRI localizer scan should be recommended, which may be able to achieve higher accuracy. Secondly, the current treatment settings of the sham stimulation group (control group) need to be improved. We have taken several measures to improve the current blinding setting, but this is a common challenge in the rTMS clinical trials, that is, the slight pain and discomfort during treatment may weaken the blinding. Mostly, the sham stimulation setting does not cause discomfort. Therefore, if a sham stimulation group is set, multiple aspects need to be optimized to enhance the blind setting.
Troubleshooting
Problem 1
Difficult to determine the rMT (step 9).
Potential solution
In actual operation, it is difficult to determine the rMT of a small number of subjects. Many reasons may cause this phenomenon, including the width of the skull, which is the subject's innate physiological condition; another possibility is that the intensity of patient's rMT is higher than the average subjects. The usual practice is to fix the position of the coil after determining the location of the motor cortex. By increasing the stimulation intensity, when the electromyographic signal exceeding 50 uV is observed, fix the relative position of the head and the coil by hand, and gradually reduce the stimulation intensity (at a rate of 1% intensity each time), and finally determine the subject's rMT.
Problem 2
Difficult to fix the daily positioning and stimulation position (step 12 and 13).
Potential solution
Since treatment is a long-term process, there may be several operators involved in completing the intervention. Sometimes this will lead to a certain difference in the site of daily stimulation and cause the deviations in the therapeutic effect. Therefore, when encountering this situation, a skin marker can be used to mark the intervention position to keep the daily stimulation position consistent.
Problem 3
Significant head movement during the intervention (step 16).
Potential solution
In this protocol, the subjects will lie on a chair for treatment. In general, obvious head movements are rare. However, the therapeutic effect of rTMS is closely related to the distance between the coil and the brain skull. Therefore, when the subject has obvious head movement, it is recommended to record the time of the patient's head movement at the same time, and to make up for this period of time that should be intervened at the target point.
Problem 4
The subjects present with the muscle tremor on the head and face (step 18 and 19).
Potential solution
During the stimulation process, tremor of the head and face muscles is a common phenomenon. This is because TMS not only stimulates the brain, but also has a certain stimulating effect on the muscles of the head and face. This performance usually disappears immediately after stopping the stimulation. The reason for muscle tremor is that the magnetic field acts on the target brain area and also acts on the head and facial muscles near the stimulation location. There is no research showing that this phenomenon will affect the treatment effect, but this phenomenon may affect the subject’s acceptance of the treatment. Therefore, it is necessary to inform the patient that this phenomenon may exist, give a sufficient explanation, and reassure the subject before treatment.
Problem 5
The coil becomes hot during the stimulation and cannot be applied anymore (step 18 and 19).
Potential solution
When the coil is applied continuously, it will cause the coil to heat up; when the temperature exceeds the threshold, the coil will be automatically locked. After the coil has cooled down, it can be applied again.
In order to deal with this situation, there are at least two methods. One is to understand the output parameters of the transcranial magnetic stimulator used by your institution, and to reasonably arrange the number of subjects treated in the same time period; the other is to choose a coil with the dynamic cooling function. The heat dissipation performance of this type of coil is better than that of the static liquid-cooled coil in continuous use.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead contact, Min Zhao (drminzhao@smhc.org.cn).
Materials availability
This protocol did not generate any unique reagents.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFC1310400), National Natural Science Foundation of China (81771436, 81801319, 81601164), Shanghai Municipal Health and Family Planning Commission (2017ZZ02021), Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ013), Qihang Project of Shanghai Mental Health Center (2019-QH-05), Shanghai Sailing Program (19YF1442100), Shanghai Key Laboratory of Psychotic Disorders (13DZ2260500), Program of Shanghai Academic Research Leader (17XD1403300), Shanghai Municipal Science and Technology Major Project (2018SHZDZX05), and Shanghai Clinical Research Center for Mental Health (19MC1911100). The authors thank Zhenying Qian for providing the technical support.
Author contributions
M.Z., T.Z.C., and H.S. conceived the protocol for the study. T.Z.C., H.S., H.F.J., X.T.L., Q.Y.W., H.Y.T., J.Y.Z., and H.J.G. performed research. T.Z.C., H.S., N.Z., J.D., and H.F.J. analyzed data. T.Z.C. drafted the manuscript. All authors have approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Tianzhen Chen, Email: vomchan@hotmail.com.
Hang Su, Email: sh3168876@sjtu.edu.cn.
Min Zhao, Email: drminzhao@smhc.org.cn.
Data and code availability
Further information and requests for the raw datasets generated by this protocol should be directed to and will be fulfilled by the lead contact, Min Zhao (drminzhao@smhc.org.cn). There was no new code developed as part of this study.
References
- Chen T., Su H., Li R., Jiang H., Li X., Wu Q., Tan H., Zhang J., Zhong N., Du J., et al. The exploration of optimized protocol for repetitive transcranial magnetic stimulation in the treatment of methamphetamine use disorder: a randomized sham-controlled study. EBioMedicine. 2020;60:103027. doi: 10.1016/j.ebiom.2020.103027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psychiatry. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M., Raij T., Melis M., Nummenmaa A., Leggio L., Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat. Rev. Neurosci. 2017;18:685–693. doi: 10.1038/nrn.2017.113. [DOI] [PubMed] [Google Scholar]
- George M.S., Lisanby S.H., Sackeim H.A. Transcranial magnetic stimulation: applications in neuropsychiatry. Arch. Gen. Psychiatry. 1999;56:300–311. doi: 10.1001/archpsyc.56.4.300. [DOI] [PubMed] [Google Scholar]
- Gershon A.A., Dannon P.N., Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am. J. Psychiatry. 2003;160:835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- Huang Y.Z., Edwards M.J., Rounis E., Bhatia K.P., Rothwell J.C. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J.P., Aleman A., Baeken C., Benninger D.H., Brunelin J., Di Lazzaro V., Filipović S.R., Grefkes C., Hasan A., Hummel F.C., et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018) Clin. Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- Li Q., Liu J., Wang W., Wang Y., Li W., Chen J., Zhu J., Yan X., Li Y., Li Z., et al. Disrupted coupling of large-scale networks is associated with relapse behaviour in heroin-dependent men. J. Psychiatry Neurosci. 2018;43:48–57. doi: 10.1503/jpn.170011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W.M., Durkalski V., Ball E.R., Holtzheimer P.E., Pavlicova M., Lisanby S.H., Avery D., Anderson B.S., Nahas Z., Zarkowski P., et al. Improving the antidepressant efficacy of transcranial magnetic stimulation: maximizing the number of stimulations and treatment location in treatment-resistant depression. Depress. Anxiety. 2011;28:973–980. doi: 10.1002/da.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F.G., Steinberg J.L., Schmitz J.M., Ma L., Liu S., Kjome K.L., Rathnayaka N., Kramer L.A., Narayana P.A. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R., Sebastianelli L., Versace V., Brigo F., Golaszewski S., Pucks-Faes E., Saltuari L., Trinka E. Effects of repetitive transcranial magnetic stimulation in subjects with sleep disorders. Sleep Med. 2020;71:113–121. doi: 10.1016/j.sleep.2020.01.028. [DOI] [PubMed] [Google Scholar]
- Noh T.S., Rah Y.C., Kyong J.S., Kim J.S., Park M.K., Lee J.H., Oh S.H., Chung C.K., Suh M.W. Comparison of treatment outcomes between 10 and 20 EEG electrode location system-guided and neuronavigation-guided repetitive transcranial magnetic stimulation in chronic tinnitus patients and target localization in the Asian brain. Acta Otolaryngol. 2017;137:945–951. doi: 10.1080/00016489.2017.1316870. [DOI] [PubMed] [Google Scholar]
- Peng Z., Zhou C., Xue S., Bai J., Yu S., Li X., Wang H., Tan Q. Mechanism of repetitive transcranial magnetic stimulation for depression. Shanghai Arch. Psychiatry. 2018;30:84–92. doi: 10.11919/j.issn.1002-0829.217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Speer A.M., Kimbrell T.A., Wassermann E.M., J D.R., Willis M.W., Herscovitch P., Post R.M. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol. Psychiatry. 2000;48:1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- Su H., Liu Y., Yin D., Chen T., Li X., Zhong N., Jiang H., Wang J., Du J., Xiao K., et al. Neuroplastic changes in resting-state functional connectivity after rTMS intervention for methamphetamine craving. Neuropharmacology. 2020;175:108177. doi: 10.1016/j.neuropharm.2020.108177. [DOI] [PubMed] [Google Scholar]
- Van Dam N.T., Rando K., Potenza M.N., Tuit K., Sinha R. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry. 2014;71:917–925. doi: 10.1001/jamapsychiatry.2014.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Wolf M.E. Synaptic mechanisms underlying persistent cocaine craving. Nat. Rev. Neurosci. 2016;17:351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U., Siebner H.R. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul. 2008;1:60–66. doi: 10.1016/j.brs.2007.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further information and requests for the raw datasets generated by this protocol should be directed to and will be fulfilled by the lead contact, Min Zhao (drminzhao@smhc.org.cn). There was no new code developed as part of this study.