Abstract
Objective
To test efficacy, safety and tolerability of Umifenovir in non-severe COVID-19 adult patients.
Methods
We carried out randomized, double-blind, placebo-controlled, multicenter, phase III trials involving adult (18-75 years), non-severe COVID19 patients, randomized 1:1 on placebo or Umifenovir (800 mg BID, maximum 14 days) respectively along with standard-of-care. The primary endpoint for Asymptotic-mild patients was time to nasopharyngeal swab RT-PCR test negativity. For Moderate patients, the average change in the ordinal scale from the baseline scores on the eight-point WHO ordinal scale was assessed.
Results
132 patients were recruited between 3rd October to 28th April 2021, of which 9 discontinued due to various reasons. In Mild-asymptomatic patients (n=82), we found that 73% patients in the Umifenovir arm were RT-PCR negative, while 40% patients in the placebo arm were negative (P=0.004) on day 5. However, in the moderate group (n=41), the WHO scores for the Umifenovir arm was not statistically significant (P=0.125 on day 3), while it was statistically significant in the Mild-asymptomatic group (P=0.019 on day 5).
Conclusion
Umifenovir meets the primary and secondary endpoint criteria and exhibits statistically significant efficacy for Mild-asymptomatic patients. It is efficacious, safe and well-tolerated at the tested dosage of 800mg BID, maximum 14 days.
Introduction
The COVID-19 pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-Cov2) has ravaged almost every nation across the globe (World Health Organization, 2021). In India alone, over 30 million persons have been infected by the virus and about 0.4 million people have been officially declared dead due to the disease and its complications (https://www.mygov.in/covid-19). Vaccination strategies are obviously vital to control the pandemic (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice) and at the same time it is critical to have evidence-based therapeutics that can mitigate the disease that can occur in, both vaccinated, and unvaccinated persons.
Umifenovir (Arbidol) is known to have broad spectrum anti-viral activity and has earlier been approved in China and Russia for treating influenza, SARS, and Lassa viruses (Blaising et al, 2014; Cheng & Shan, 2019; Boriskin et al., 2008; Chen et al., 2020; 6. Pécheur E-I et al., 2016). It has been suggested and tested in multiple studies as a candidate for use as an anti-COVID19 therapeutic and has been suggested to act at the entry stage and at the post-entry stages by preventing viral attachment and inhibiting the release of virus particles from intracellular vesicles respectively (Xi Wang et al., 2020, Zheng et al, 2020; Blaising et al., 2013). Earlier clinical trials have reported mixed results about its efficacy (Nojomi et al., 2020; Darazam et al., 2021; Yethindra et al., 2020; Xu et al., 2020; Deng et al., 2020; Gao et al., 2020; Huang et al., 2020; Zhu et al., 2020; Lian et al., 2020). The EC50, 50% maximal effective concentration has been reported to be 4.11 µM while the 50% cytotoxic concentration, CC50, has been reported to be 31.79 (7,19). Our hypothesis, based on the evaluation of multiple in vitro and clinical studies, was that Umifenovir is a drug with a good safety profile (LD50 ∼4g/kg), and with the capacity of achieving the required EC50 with a dose of 800mg. Earlier relevant human studies had identified a Cmax ∼4.1 µM upon administration of 800 mg of Umifenovir and a half-life of about 16 hrs. (Sun et al., 2013). On the other hand, other reported clinical trials involving Umifenovir have all used a maximum of 600 mg/day as the dosage.
We therefore aimed to evaluate the Efficacy, Safety and Tolerability of Umifenovir vs Standard care of therapy through a randomized Phase III double-blinded placebo controlled trial in non-severe COVID-19 adult patients in the age group of 18-75 yrs using a dosage of 800mg BID administered orally. An entry inhibitor is expected to have more efficacy in the earlier stages of the COVID19 disease, while moderate/severe disease is supported by other host-directed clinical measures for alleviation of symptoms. Accordingly, separate endpoints were devised for Mild-asymptomatic and moderate patients respectively based on the known disease progress and nationally adopted standard-of-care treatment strategies. To our knowledge, this report is the first for a double-blind placebo controlled Phase III trial for Umifenovir against COVID-19 and furthermore no other trial has involved the dosage of 800 mg BID that has been used here.
Methods
Study design, randomization, and inclusion/exclusion of participants
A double-blind placebo controlled Phase III trial was designed to be carried out in three clinical trial centres based in Lucknow, India, viz. King George's Medical University, Ram Manohar Lohia Institute of Higher Medical Sciences and Era's Lucknow Medical College and Hospital for a total of 132 patients. All National regulatory and respective ethical committees’ permissions/ approvals were secured before the commencement of the trial. Patients were referred to the respective hospitals by a central command center under the Directorate of Medical & Health Services, State government of Uttar Pradesh (http://dgmhup.gov.in/en/default) based on positive RT-PCR results of persons with symptoms or through contact tracing of already identified COVID-19 positive patients (https://lucknow.nic.in/noval-corona-virus-covid-19/). Dosage of Umifenovir used in the study was 800mg (2 tablets, 400mg each) administered orally twice daily for 14 days plus standard care of therapy. The adherence in admitted patients was done under direct observation. For those who were isolated at home, the adherence was ensured by pill counting every 3 days. Each patient enrolled in the study gave written consent and was observed for a total of 28 days normally. Case categories according to severity was defined as per Ministry of Health & Family Welfare, Govt of India guidelines. As per the earlier reported pharmacokinetic studies, a dosage of 800mg achieves sufficient concentration to inhibit the pathogen. The drug has a half-life of about 16 hours and it was therefore decided to be administered twice daily. The standard care of therapy used was as per the Ministry of Health, Govt. of India COVID-19 treatment guidelines (https://www.mohfw.gov.in/pdf/UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated-24052021.pdf). Patients were randomized using Computerised randomization (Sequentially numbered opaque, sealed envelopes –SNOSE).
The inclusion criteria involved chiefly the following: Asymptomatic persons: aged 18-75 years, at the time of signing the Informed Consent Form (ICF), with Nasopharyngeal swab positivity in RT-PCR tests for SARS-Cov-2 antigens detected during screening of contacts or sentinel surveillance. Mild patients were those with uncomplicated upper respiratory tract viral infection and who may have non-specific symptoms such as fever, cough, expectoration, shortness of breath, myalgia, fatigue, sore throat, nasal congestion, diarrhea, loss of taste with Nasopharyngeal swab positivity in RT-PCR tests for SARS-Cov-2 antigens. Moderate disease was considered as Pneumonia with no signs of severe disease. Adults with presence of clinical features of dyspnea and or hypoxia, fever, cough, including SpO2 <94% (range 90-94%) on room air, respiratory rate more or equal to 24 per minute were included in the moderate patient category.
The main exclusion criteria were: patients with severe covid and with respiratory rate >30 breaths/min, severe respiratory distress, SpO2 <90% on room air, Cases of Acute respiratory distress syndrome (ARDS), sepsis/ septic shock, pregnant/ lactating women, patients with severe lever disease, severe renal impairment, or other comorbidities like asthma, diabetes with second and third line medicines as defined in the WHO guidance document (World Health Organization, 2020a). The clinical trial protocol is attached as Supplementary information.
Randomization and masking
Patients who were eligible as per the inclusion criteria were asked to give their consent to participate in the trial. Randomization and recruitment was administered by an independent clinical trial coordinator for true double-blinding. Patients were almost equally stratified into the Mild-asymptomatic and Moderate arms. All laboratory staff and doctors were also masked to treatment allocation and samples were identified by serial numbers.
Study population and criteria
Calculation of sample size for the overall study
The patients were assigned to the three hospitals by a Central COVID-19 command center of the State government of Uttar Pradesh, India. A total of 132 patients were to be recruited with 66 patients in each arm of the trial. The sample size of the present study was chosen based on formal statistical power calculation for the primary outcome measure i.e. nasopharyngeal swab negativity by RT-PCR test. Sample size estimation was based on assumption that the average time (duration) of discharge of patient in Standard-of-care (SOC) group is 13± 2.5 days. For any patient to be discharged in lesser time than 11.7 days we require the sample size to be calculated as:
Ƞ = 2(Z α/2 + Z β)2 σ2 / (x1 - x2)2
Where Z α/2 = 1.96 level of significance, Z β = 0.842 power of test= 80%, x1 = 11.7 days, x2 = 13 days, (x1 - x2) = 1.3, σ = 2.5 days, x1 - x2 the minimum time difference which can be significant.
Ƞ = 2 × (1.96 + 0.842)2 × 2.52 /1.32 = 58
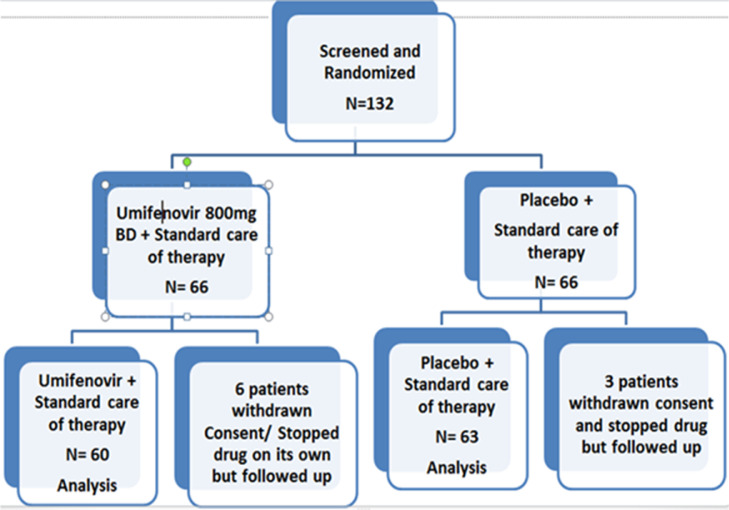
With 10% margin of dropouts and also taking into account randomization block size of 6, the required sample size was calculated to be 66 in each arm. Ultimately, 9 patients withdrew from the trial by not appearing for subsequent tests or stopped taking the medication (either Umifenovir/ placebo) leading to a total of 123 patients divided into placebo (n=63) and Umifenovir (n=60) arms respectively.
Outcomes and safety assessments
The primary endpoints for the Mild-asymptomatic patients was different from Moderate patients. For the Mild-asymptomatic patients, the primary endpoint was Time from randomization to nasopharyngeal swab negativity by two RT-PCR tests, for SARS-Cov-2 antigens, taken 24 hours apart. For moderate patients, the end point was time to improvement by one category from randomisation on the eight-category ordinal scale defined by World Health Organisation, 2020b (Table S1) & average change in the ordinal scale from baseline. The secondary outcome was Time from randomization to clinical recovery or deterioration, assessed at 0, 7, 14, 21 and 28 days, on the WHO eight-category ordinal scale. Also assessed was the proportion of patients to clinical recovery or deterioration, at 0, 7, 14, 21 and 28 days respectively, on the WHO defined eight-category ordinal scale consisting of the following categories: (a) Proportion of patients hospitalized with Severe Covid-19 pneumonia (with respiratory rate ≥30/minute and/or SpO2 < 90% in room air) or ARDS or Septic shock as per Government of India guidelines. (b) Adverse events in the two groups.
Statistical analysis:
Discrete (categorical) nasopharyngeal swab/RTPCR output (negative/positive) of two groups (placebo, n=63 and umifenovir, n=60) over the periods (day 5, 7, 9, 11, 13, 15, 17, 19, 21 and 28) were summarised in number (n) and percentage (%) and compared by chi-square (χ2) test. The WHO score of two groups over the periods (day 3, 5, 7, 14, 21 and 28) were summarised in Mean ± SE (standard error of the mean) and compared by repeated measures two factor (groups and periods) analysis of variance (ANOVA) and the significance of mean difference within (intra) and between (inter) the groups was done by Newman-Keuls post hoc test. A two-tailed (α=2) P < 0.05 was considered statistically significant.
This study is registered with the Clinical Trial Registry of India (CTRI) with Number: CTRI/2020/09/027535 and was conducted between 3rd October 2020 – 28th April 2021.
Role of the funding source
The funder had no role in the study design, conduct of the trial or the writing of the report
Results
Patients were recruited into the trial and randomized into the Umifenovir arm + standard of care or Placebo + standard of care respectively. They were stratified into Asymptomatic, Mild and Moderate categories almost uniformly. Out of 132 patients who were recruited, 9 withdrew consent or stopped taking medication on their own and were discontinued from the trial. The remaining 123 patients were found to be divided as placebo group (n=63) and Umifenovir group, (n=60) respectively (Figure 1 ). The baseline characteristics of recruited participants was assessed and is quite similar in both groups of patients and also within stratified Mild-asymptomatic and moderate patients (Table 1 ). When we examined the symptom category of patients, we found that the recruited patients were similarly distributed with Asymptomatic (35%), Mild (32%) and Moderate (33%) respectively.
Figure 1.
Patient randomization and distribution shown as a CONSORT diagram. The Umifenovir and placebo groups contained 60 and 63 patients respectively in the analysis.
Table 1.
Comparison of baseline demographic characteristics of all recruited patients between two drug groups. Age, height and weight of two groups were summarised in Mean ± SE and compared by Student's t test whereas sex were summarised in number (n) and percentage (%) and compared by χ2 test
| (A) Overall patients (n=123) | ||||
| Variable | Placebo (n=63) (%) | Umifenovir (n=60) (%) | t/χ2 value | P value |
| Age (yrs) | 47.35 ± 1.96 | 46.08 ± 1.93 | 0.46 | 0.646 |
| Sex: | ||||
| Female | 19 (30.2) | 12 (20.0) | 1.68 | 0.195 |
| Male | 44 (69.8) | 48 (80.0) | ||
| Height (cm) | 164.86 ± 0.88 | 165.60 ± 0.81 | 0.62 | 0.537 |
| Weight (kg) | 69.51 ± 1.02 | 69.03 ± 1.09 | 0.32 | 0.751 |
| (B) Mild-asymptomatic patients (n=82) | ||||
| Variable | Placebo (n=42) (%) | Umifenovir (n=40) (%) | t/χ2 value | P value |
| Age (yrs) | 45.50 ± 2.45 | 42.35 ± 2.38 | 0.92 | 0.360 |
| Sex: | ||||
| Female | 14 (33) | 9 (23) | 1.19 | 0.275 |
| Male | 28 (67) | 31 (78) | ||
| Height (cm) | 164.50 ± 1.06 | 164.25 ± 1.05 | 0.17 | 0.867 |
| Weight (kg) | 69.19 ± 1.43 | 68.40 ± 1.43 | 0.39 | 0.697 |
| (C) Moderate patients (n=41) | ||||
|---|---|---|---|---|
| Variable | Placebo (n=21) (%) | Umifenovir (n=20) (%) | t/χ2 value | P value |
| Age (yrs) | 51.05 ± 3.17 | 53.55 ± 2.61 | 0.61 | 0.548 |
| Sex: | ||||
| Female | 5 (24) | 3 (15) | 0.51 | 0.477 |
| Male | 16 (76) | 17 (85) | ||
| Height (cm) | 165.57 ± 1.61 | 168.30 ± 1.00 | 1.42 | 0.163 |
| Weight (kg) | 70.14 ± 1.14 | 70.30 ± 1.58 | 0.08 | 0.936 |
Primary endpoint analysis for Mild-asymptomatic patients
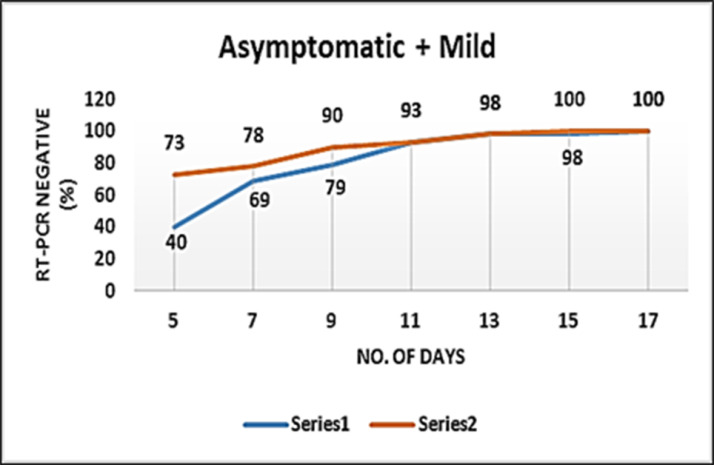
As mentioned earlier, the primary endpoint for this category of patients was time to RT-PCR nasopharyngeal swab negativity by two RT-PCR tests for SARS COV2 antigens taken 24 hrs apart from the date of randomization. In the Mild-asymptomatic group (n=82), we found that: 73% patients on the Umifenovir arm were RT-PCR negative on the 5th day (P=0.004) as compared to only 40% patients on the placebo arm (Figure 2 , Table 2 ).
Figure 2.
Time to RT-PCR-negativity in the two groups of Mild-asymptomatic patients. Orange line corresponds to Umifenovir arm while the blue curve corresponds to the placebo arm.
Table 2.
Statistical and RT-PCR negativity summary of Mild-Asymptomatic patients recruited in the clinical trial (n=82)
| RT-PCR test Day (negative) | Placebo (n=42) (%) | Umifenovir (n=40) (%) | Diff (%) | P value |
|---|---|---|---|---|
| 5 | 17 (40) | 29 (73) | 32 | 0.002 |
| 7 | 29 (69) | 31 (78) | 8 | 0.194 |
| 9 | 33 (79) | 36 (90) | 11 | 0.078 |
| 11 | 39 (93) | 37 (93) | 0 | 0.475 |
| 13 | 41 (98) | 39 (98) | 0 | 0.486 |
| 15 | 41 (98) | 40 (100) | 2 | 0.163 |
| 17 | 41 (98) | 40 (100) | 2 | 0.163 |
| 19 | 42 (100) | 40 (100) | 0 | - |
Secondary endpoint analysis for the Mild-asymptomatic patients’ category
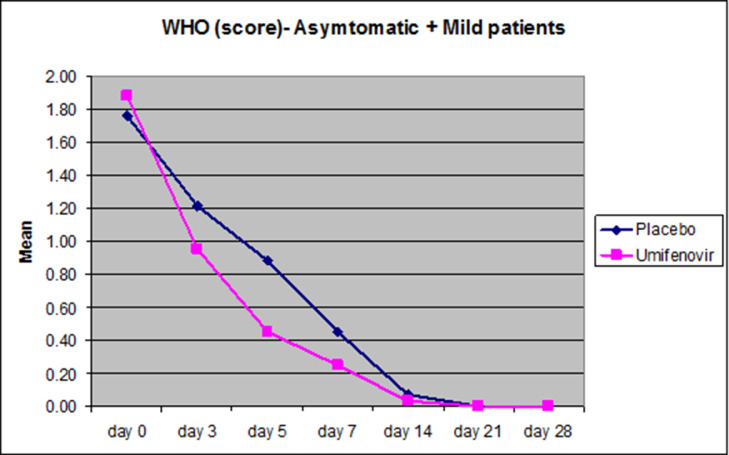
The secondary endpoint was the average change in the ordinal scale by at least one category from the baseline scores from randomization on the eight-point ordinal scale as defined by WHO. This would assess the clinical recovery of the patients on both arms of the trial in the Mild-asymptomatic patients. In this analysis we found that the WHO score on day 5 was 48.9% lower in the Umifenovir group (P=0.019) compared to the placebo group (Figure 3 , Table 3 ).
Figure 3.
Reduction in the mean WHO scores plotted in Asymptomatic and Mild patients (n=82). Pink curves represent the reduction in the mean WHO scores on days 0,3,5,7,14,21 and 28 respectively while blue curves depict the reduction in the average WHO scores on the respective days plotted on the X-axis. Significant difference in the reduction in the mean WHO score was observed on day 5 in the Mild-Asymptomatic patients (P=0.019).
Table 3.
Average WHO scores tabulated for the Mild-asymptomatic group.
| Time (days) | Mild-asymptomatic (n=82) | ||
|---|---|---|---|
| Placebo (n=42) | Umifenovir (n=40) | P value | |
| day 0 | 1.76 ± 0.14 | 1.88 ± 0.15 | 0.479 |
| day 3 | 1.21 ± 0.13 | 0.95 ± 0.12 | 0.098 |
| day 5 | 0.88 ± 0.13 | 0.45 ± 0.11 | 0.019 |
| day 7 | 0.45 ± 0.12 | 0.25 ± 0.09 | 0.414 |
| day 14 | 0.07 ± 0.05 | 0.03 ± 0.02 | 0.771 |
Overall, the primary and secondary endpoints are met for the Mild-asymptomatic category of patients.
Calculation of sample size and power of test for Mild-asymptomatic patient category.
We carried out calculations to determine the post hoc power of the above results.
Assuming a difference of 20% to be significant between Placebo and Umifenovir arms in the Mild-asymptomatic category and with α level of significance and with 80% power of the test the sample size per group is: n = {2*(Zα/2 + Zβ)2 *P*Q}/Δ2 where; Zα/2 =1.96, Zβ=0.842, P=0.9, Q=0.1 and Δ=0.2.
This gives n=35.3, i.e n=36.
Hence the minimum sample size per group in this study was determined to be n=36.
[P = Pooled rate of response; Q = 1-P; Zα/2 = Desired level of significance (0.05)
Zβ = Value of Z when power is 80%; Δ = minimum difference in rate of response of placebo and treatment group to be significant].
Based on this, the post hoc power of the results was estimated to be 84.5%. Since the estimated power is more than the expected power of test, it can be concluded that the sample size studied is sufficient to justify the significant effect of the Umifenovir group over the placebo group in the Mild-asymptomatic patients too.
Analysis of trial endpoints for Moderate category patients.
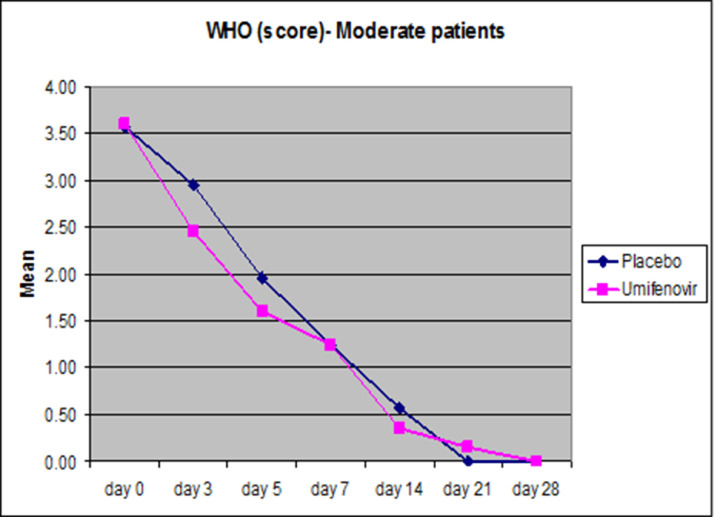
As mentioned earlier, for Moderate patients, the average change in the ordinal scale from the baseline scores from randomization on the eight-point ordinal scale as defined by WHO was calculated as the primary endpoint. The distribution of WHO score, Mean ± SE, of the two treatment groups in Moderate patients (n=41) is given in Figure 4 , Table 4 .
Figure 4.
Pink lines corresponds to Umifenovir patients in the Moderate category, while blue represents the placebo category. Both sets of patients received the standard-of-care.
Table 4.
Average WHO scores (Mean ± SE) tabulated for the Moderate group (n=41)
| Time (days) | Moderate (n=41) | ||
|---|---|---|---|
| Placebo (n=21) | Umifenovir (n=20) | P value | |
| day 0 | 3.57 ± 0.11 | 3.60 ± 0.11 | 0.930 |
| day 3 | 2.95 ± 0.19 | 2.45 ± 0.22 | 0.125 |
| day 5 | 1.95 ± 0.32 | 1.60 ± 0.32 | 0.281 |
| day 7 | 1.24 ± 0.32 | 1.25 ± 0.32 | 0.971 |
| day 14 | 0.57 ± 0.24 | 0.35 ± 0.20 | 0.497 |
| day 21 | 0.00 ± 0.00 | 0.15 ± 0.15 | 0.646 |
We found that in the Moderate patients group the reduction in the mean WHO score was not statistically significant (P=0.125 & 0.281 on days 3 and 5 respectively).
Adverse Events (AE)
We found that Umifenovir was well tolerated. No serious adverse events were noted in the patients and additionally no deaths were seen in any of the groups. A total of 14 patients with minor adverse events were noted (Table 5 ) with symptoms ranging from headache, stomach ache, nausea and vomiting. The patients who exhibited minor AEs were almost equally divided between the Umifenovir and Placebo groups respectively. Further our assessment of all patients on 0,7,14,21 and 28 days on eight-category ordinal scale defined by WHO supported no deterioration of the clinical status. Additionally, the analysis of laboratory parameters also showed that clinically significant changes were not found in both patient groups. This is as expected, as Umifenovir has been safely used for over 25 years as an over the counter medicine and is in line with other reported trials.
Table 5.
Tabulation of adverse events.
| Category | Symptom | Number of patients | Resolved (Y/N) |
|---|---|---|---|
| Umifenovir group | |||
| Asymptomatic | Stomach ache | 1 | Y |
| Mild | Nausea | 2 | Y |
| Mild | Headache | 1 | Y |
| Asymptomatic | Nausea with Vomiting | 2 | Y |
| Asymptomatic | Headache/ Nausea | 1 | Y |
| Placebo group | |||
| Asymptomatic | Stomach ache | 1 | Y |
| Mild | Nausea | 1 | Y |
| Asymptomatic | Vomiting | 1 | Y |
| Moderate | Nausea with Vomiting | 1 | Y |
| Moderate | Headache/ Nausea | 1 | Y |
| Asymptomatic | Stomach ache/ headache | 1 | Y |
| Mild | Stomach ache / Nausea/ Vomiting | 1 | Y |
Discussion
Umifenovir is a safe drug used for over 25 years in Russia and China against Influenza. It has been approved for use in children and pregnant women from the second trimester onwards in these countries. It was used as a standard of care/ trialled in the latter countries in the earlier stages of the COVID19 pandemic and the earlier trials suggested better benefits as compared to drugs like Lopinavir/Ritonavir. However, retrospective studies involving hospitalization or severe cases were not clear in their conclusion and the reports suggested that additional studies are needed.
Our own hypothesis, based on earlier reports, suggested that early administration of the drug should be useful for COVID-19 patients and also that the dosage of Umifenovir was much less than that needed to achieve the Cmax suggested for use against SARS-Cov2. This was also suggested by other studies (Wang et al., 2020). We therefore designed separate primary endpoints for Mild-asymptomatic and moderate patients respectively.
To the best of our knowledge, the present trial is the first one involving Umifenovir against SARS-Cov2 that is double-blinded, placebo controlled one. The earlier clinical trials involving Umifenovir against SARS-Cov2 did not involve placebo control. Further, the dosage in the earlier reported trials did not take into account the earlier suggested Cmax of 4.1 µM needed for efficacy of Umifenovir against SARS-Cov2. A single dose of 800 mg of Umifenovir in healthy patients were reported to have a Cmax of about 4.1 µM and this corresponds to the IC50 of ∼4.1 µM reported against SARS-Cov2 for Umifenovir. The reported half-life of ∼14-16 hrs and the good safety profile of the drug led us to rationally propose a dosage of 800mg twice a day for the repurposing strategy involving Umifenovir against SARS-Cov2.
In the trials, we found that Umifenovir was safe and well tolerated and only few minor events like headache, stomach ache and nausea were reported and this also was distributed almost equally between the Umifenovir and standard of care arms respectively. No negative disease progression was noted in both arms and the patients steadily improved. No deaths were also reported in either arm. This is similar to the reports of minor adverse events in other trials involving Umifenovir.
In the present trial the primary endpoint involving asymptotic and mild patients was time to nasopharyngeal swab negativity by two RT-PCR tests for SARS COV2 antigens taken 24 hrs apart from the date of randomization. While the secondary endpoint was the average change in the ordinal scale from the baseline scores from randomization on the eight-point ordinal scale as defined by WHO.
In the Mild-asymptomatic patients group (n=82), we found that 73% patients on the Umifenovir arm were RT-PCR negative on the 5th day as compared to only 40% patients on the placebo arm (P=0.004). Hence the trial meets the primary endpoint criteria for this patient category. Our confidence in the result for the Mild-asymptomatic patients is further bolstered by the post hoc statistical analysis that was estimated to be 84.5% as compared to the originally calculated 80%. Statistically significant clinical recovery (P = 0.002) was also observed for the Mild-asymptomatic patients on the 5th day as assessed by the WHO score analysis (secondary endpoint) for Umifenovir vs Placebo groups. The WHO score is a measure of how the patients in the cohort are becoming clinically better and was captured on days 0 (date of randomization), 3, 5,7,14, 21, and 28 respectively.
For Moderate patients, the average change in the ordinal scale from the baseline scores from randomization on the eight-point ordinal scale as defined by WHO was the primary endpoint. The baseline scores were similar between the respective placebo and Umifenovir arms on day 0. We found that the WHO scores for the Umifenovir arm suggested faster improvement as compared to the Placebo arm (P=0.125 on day3) in the moderate patients, but was not statistically significant. However, a limitation of the trial was the smaller number of patients in the moderate patients group, and we therefore suggest a larger trial for moderate patients to take these results further.
In view of the safety profile we suggest studies to evaluate efficacy in children and pregnant/ breast-feeding women too, especially as no other therapeutic is available for this population segments. We also recommend future studies for evaluation of Umifenovir as a prophylactic as this would be useful for high-risk contacts. Both the latter suggestions are supported by the fact that Umifenovir is used as a prophylactic against influenza and also approved for use in children and pregnant women.
Overall, there is an urgent need for effective and safe treatments for COVID-19 patients and our results demonstrate the efficacy and use of Umifenovir in Mild-asymptomatic adult COVID-19 patients in the dosage tested here.
Contributors
TKK, VB, RR, SS, SKR and HR contributed to the study concept and protocol design. HR, VA, MMAF, ZAK, HK, JF, VaS and VS contributed to protocol implementation and verified the clinical data integrity. AS, CBT, NG and NM contributed to the chemistry inputs for the study. TKK, RR, VB, SS, PRM, SKR and RKT coordinated the collation of the data. Integrity of the data were independently audited by a third party and all the authors had access to the data. MPSN and MS conducted statistical analysis coordinated by RR and VB. Study was supervised by RR, VB, SS, PRM, SKR and TKK.
Declaration of conflict of interests
The authors declare no competing or conflict of interests
Data sharing
The study protocol is attached as a supplementary file.
Individual patient data used in the study is not available.
Acknowledgements
M/s Medizest Pharmaceutical Pvt Ltd, Goa, India, is acknowledged for providing the Umifenovir and placebo tablets used in the study. The human resources of Product Development Centre (PDC) of ICMR, New Delhi were used for the study. We acknowledge Council of Scientific and Research, Ministry of Science and Technology, Government of India for funding the study. This manuscript is communicated by CSIR-Central Drug Research Institute with number 10317.
Funding source Council of Scientific and Industrial Research, Ministry of Science and Technology, Government of India, funded and sponsored the study via grant number MLP2038.
Ethical approval
All required ethical and regulatory approvals were taken before the start of the clinical trial. The protocol and informed consent forms were approved by the Drugs Controller General of India and the Institutional ethics committees. All patients gave their written informed consent. The trial was conducted as per the guidelines of the Central Drugs Standard Control Organization, the National regulatory authority in India.
References
Blaising, J. et al. Arbidol inhibits viral entry by interfering with clathrin dependent trafficking. Antivir. Res. 2013; 100:215–219.
Blaising, J., Polyak, S. J. & Pecheur, E. I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 2014;107: 84–94.
Boriskin YS, Leneva IA, Pécheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008; 15:997–1005.
Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 2020; 92:418–23
Cheng ZJ, Shan J. 2019 Novel coronavirus: where we are and what we know. Infection 2020 Available from: https://doi:10.1007/s15010-15020-01401-y
Darazam IA, Shervin Shokouhi, Masoud Mardani, Mohamad Amin Pourhoseingholi, Mohammad Mahdi Rabiei, Firouze Hatami, Minoosh Shabani, Omid Moradi, Farid Javandoust Gharehbagh, Seyed Sina Naghibi Irvani, Mahdi Amirdosara, Mohammadreza Hajiesmaeili, Omidvar Rezaei, Ali Khoshkar, Legha Lotfollahi, Latif Gachkar, Hadiseh Shabanpour Dehbsneh, Negar Khalili, Azam Soleymaninia, Akram Hoseyni Kusha, Maryam Taleb Shoushtari, Parham Torabinavid. Umifenovir in hospitalized moderate to severe COVID-19 patients: A randomized clinical trial Int Immunopharmacol. 2021;99:107969. doi: 10.1016/j.intimp.2021.107969.
Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, Hong Z, Xia J, Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. 2020 Mar 11. pii: S0163-4453(20)30113-4. doi: 10.1016/j.jinf.2020.03.002.
Huang D, He Yu, Ting Wang, Huan Yang, Rong Yao, and Zongan Liang Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis J Med Virol. 2020: 10.1002/jmv.26256.
Lian N, Xie H, Lin S, et al. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26:917-921.
Nojomi M, Yassin Z, Hossein Keyvani, Mahin Jamshidi Makiani, Maryam Roham, Azadeh Laali, Nasir Dehghan, Mehrnaz Navaei, Mitra Ranjbar. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial BMC Infect Dis. 2020; 20:954. doi: 10.1186/s12879-020-05698-w.
Pécheur E-I, Borisevich V, Halfmann P, Morrey JD, Smee DF, Prichard M, et al. The synthetic antiviral drug Arbidol inhibits globally prevalent pathogenic viruses. J Virol. 2016;90:3086–92.
Sun, Y. et al. Pharmacokinetics of single and multiple oral doses of arbidol in healthy Chinese volunteers. Int. J. Clin. Pharmacol. Ther. 51, 423–432 (2013).
Wang X, Ruiyuan Cao, Huanyu Zhang, Jia Liu, Mingyue Xu, Hengrui Hu, Yufeng Li, Lei Zhao, Wei Li, The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discovery (2020) 6:28. https://doi.org/10.1038/s41421-020-0169-8
Wei Gao, Si Chen, Kun Wang, Rongzhang Chen, Qian Guo, Jingjing Lu, Xiaodong Wu, Yanan He, Qiaoyun Yan, Shengyun Wang, Feilong Wang, Li Jin, Jing Hua and Qiang Li Clinical features and efficacy of antiviral drug, Arbidol in 220 nonemergency COVID-19 patients from East-West-Lake Shelter Hospital in Wuhan: a retrospective case series Virol J. 2020; 17:162
World Health Organization Guidelines on second- and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus. Available at https://www.who.int/publications-detail/guidelines-on-second–and-third-line-medicines-and-type-of-insulin-for-the-control-of-blood-glucose-levels-in-non-pregnant-adults-with-diabetes-mellitus, 2020a
World Health Organization R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis Accessed on 3.6.2020 available at https://www.who.int/blueprint/priority-diseases/key-action/COVID19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf, 2020b
World Health Organization. Corona virus overview. WHO. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019, Accessed on 9/7/2021, 2021
Xu K, Hongliu CAI, Yihong S, et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J. Zhejiang Univ (Med Sci) 2020;49:147-157
Yethindra V, Tagaev T, Uulu, M S, Parihar Y.Efficacy of umifenovir in the treatment of mild and moderate covid-19 patients International Journal of Research in Pharmaceutical Sciences 11(Special Issue 1):506-509, 2020.
Zheng LU, Zhang L, Huang J, Nandakumar KS, Liu S, Cheng K. Potential treatment methods targeting 2019-nCoV infection. Eur J Med Chem. 2020;205:112687. https://doi.org/10.1016/j.ejmech.2020.112687
Zhu Z, Z. Lu and T. Xu et al., Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19, Journal of Infection, https://doi.org/10.1016/j.jinf.2020.03.060
Footnotes
This study is registered with the Clinical Trial Registry of India (CTRI) with Number: CTRI/2020/09/027535.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.11.025.
Appendix. Supplementary materials
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.