Abstract
Objective:
Beta-adrenergic receptor signaling, a critical mediator of sympathetic nervous system influences on physiology and behavior, has long been proposed as one contributor to subjective stress. Yet prior findings are surprisingly mixed about whether beta-blockade (e.g., propranolol) blunts subjective stress, with many studies reporting no effects. We re-evaluated this question in the context of an acute psychosocial stressor with more comprehensive measures and a larger-than-typical sample. We also examined the effects of beta-blockade on psychophysiological indicators of sympathetic and parasympathetic nervous system reactivity, given that beta-blockade effects for these measures specifically under acute psychosocial stress are not yet well-established.
Methods:
In a double-blind, randomized, placebo-controlled study, 90 healthy young adults received 40 mg of the beta-blocker propranolol or placebo. Participants then completed the Trier Social Stress Test, which involved completing an impromptu speech and difficult arithmetic in front of evaluative judges. Self-reported emotions and appraisals as well as psychophysiology were assessed throughout.
Results:
Propranolol blunted TSST pre-ejection period reactivity (b=9.68, p=.003), a marker of sympathetic nervous system activity, as well as salivary alpha amylase reactivity (b=−.50, p=.006). Critically, propranolol also blunted negative, high arousal emotions in response to the stressor (b=−.22, p=.026), but cognitive appraisals remained intact (bs<−.17, ps>.10).
Conclusions:
These results provide updated experimental evidence that beta-adrenergic blockade attenuates negative, high arousal emotions in response to a psychosocial stressor while also blunting sympathetic nervous system reactivity. Together, these findings shed light on the neurophysiological mechanisms by which stressors transform into the subjective experience we call “stress.”
Keywords: Emotion, Appraisals, Stress, Beta-Blockade, Propranolol, Psychophysiology
Psychological stressors have long been appreciated as determinants of physical health, emotional well-being, and social behavior (1–5). Importantly, subjective stress—the affective feelings and appraisals that individuals experience in the face of a stressor—is sometimes more predictive of health and wellbeing than “objective” measures such as cardiovascular or neuroendocrine markers (5–8). Despite the predictive utility of subjective stress, we know surprisingly little about how subjective stress is generated in the first place. Some work has tested potential neurophysiological contributions to subjective stress in humans by administering beta-blockers such as propranolol, which block beta-adrenergic receptors, a critical signaling pathway for epinephrine and norepinephrine (9,10). This study aimed to provide a more complete understanding of the effects of beta-blockade on the acute stress experience while also shedding light on longstanding questions about the nature of human emotion.
Current neuroscientific perspectives argue that the brain’s core function is allostasis, the process of monitoring, managing, and coordinating physiology to support an organism’s movement, growth, reproduction, and behavior (11,12). Two closely interworking systems by which the brain may in part enact allostasis are the sympathetic nervous system (SNS) and adrenergic/noradrenergic systems. These systems are known to support arousal and the mobilization of neurophysiological resources underpinning alertness, saliency, and behavioral coping (13–15). In particular, the catecholamines epinephrine and norepinephrine are released by the medulla in the adrenal glands and by the ends of sympathetic nerve fibers, serving as the primary neurotransmitters that convey SNS signaling to peripheral organs (16). Epinephrine and norepinephrine subsequently act by binding to alpha- and beta-adrenergic receptors, found widely across the body and brain (17,18).
Beta-adrenergic receptor signaling has long been implicated in the generation of affect (e.g., feeling tense, stressed, anxious), given its role in conveying epinephrine- and norepinephrine-mediated SNS signals to peripheral organs. The idea that peripheral signals contribute to affect is consistent with early theories of emotion (19,20) as well as current theories arguing that both the body’s physiological states and interoception of those states help generate affect, or feelings of valence (pleasure vs. displeasure) and arousal (activation vs. quiescence; (21,22)). To test these ideas, past research has examined the effects of beta-blocker administration on affect, acute stress, and/or mood disorder symptoms, with propranolol being the most widely used. Propranolol is a highly lipophilic, non-selective beta-blocker, meaning that it can cross the blood-brain barrier easily and blocks the binding of epinephrine and norepinephrine across all types of beta-adrenergic receptors. In treatment, it has been mostly used to reduce hypertension, tachycardia, and muscle tremors but is sometimes prescribed off-label to reduce anxiety in acutely stressful situations such as musical performances or public speaking (9). Despite this off-label use, a long history of experimental evidence remains equivocal about the effects of propranolol (and other types of beta-blockers) on subjective ratings of anxiety, stress, and affect (24–51).
Mixed findings may be due to several limitations of prior research. First, most studies are likely underpowered. Specifically, the effect size for propranolol on affect is probably small, yet propranolol groups in most studies are n<20 (Table S1, Supplemental Digital Content [SDC]). Furthermore, emotion, affect, or subjective stress are inconsistently measured. Studies tend to focus on a narrow subset of feelings (e.g., state anxiety, single-item stress ratings), suggesting that null effects could be driven by impoverished measurement. Indeed, people tend to report a range of feelings during stressors in addition to anxiety and fear, including anger, embarrassment, and shame (51), yet these other emotions have remained largely ignored in past beta-blockade work. Effects are further complicated by some studies examining drug effects on affect only at rest and other studies examining drug effects only in reaction to a stimulus (e.g., stressor). To address these ambiguities, we assessed the effects of propranolol on a variety of emotional states, ranging in valence and arousal, both at rest (pre/post drug) and with respect to acute stressor reactivity (pre/post stressor). Lastly, although appraisals are another oft-measured dimension of subjective stress (52), to our knowledge, there are no published findings on the effects of propranolol on stress appraisals. Thus, we aimed to provide initial evidence clarifying the effects of beta-blockade on appraisals.
Given that stressors also impact physiology and health, it is critical to examine both subjective and physiological changes in parallel. Consequently, we assessed the extent to which beta-blockade impacts autonomic and neuroendocrine markers of the SNS, parasympathetic nervous system (PNS), and hypothalamic-pituitary-adrenal (HPA)-axis, which are known to shift during acute stressors. This allowed us to disambiguate specific effects of beta-blockade on the reactivity of several physiological systems implicated in stress. As our primary SNS indicator, we measured pre-ejection period (PEP), a cardiovascular measure of sympathetic influence on the cardiac cycle. We also measured salivary alpha amylase (sAA), given that it may in part reflect SNS activity (53,54). Although classic work shows that beta-blockade lengthens PEP at rest, during physical exercise, and under cognitive load (55–58), there is little work examining the effects of beta-blockade on PEP under psychosocial stress (i.e., the Trier Social Stress Test or TSST), with most work instead focusing on blood pressure (BP) and heart rate (HR) or neuroendocrine measures such as sAA and cortisol (17,19,20,44–46). We additionally tested the specificity of beta-blockade on SNS vs. PNS reactivity (62) by assessing respiratory sinus arrhythmia (RSA), a marker of parasympathetic cardiac influence. Finally, we built on prior work examining effects of beta-blockade on HPA-axis markers such as cortisol (39,60,63,64), in order to clarify whether past null effects are replicable while further confirming that the effects of beta-blockade are SNS-specific.
To test the above hypotheses and gaps in the literature, we used a preregistered, double-blind, randomized, placebo-controlled design and manipulated beta-adrenergic signaling via administration of a single 40 mg dose of propranolol (n=43) vs. placebo (n=47) prior to the TSST (65). Drawing on diverse tools from psychopharmacology, psychophysiology, and affective science, we used comprehensive, repeated measures of emotions, appraisals, autonomic psychophysiology, and salivary markers in a sample size that more than doubles that of most prior studies. We hypothesized that TSST exposure would result in increased unpleasant, high arousal emotions (e.g., anxiety, anger), and that pre-treatment with propranolol would blunt the intensity of these feelings. To determine specificity, we also examined negative, low arousal emotions (e.g., boredom), positive, high arousal emotions (e.g., excitement) and positive, low arousal emotions (e.g., contentment). We further explored the effects of beta-blockade on TSST appraisals, clarifying whether beta-adrenergic signaling contributes to affect only or if it also influences how people evaluate stressors. Although we hypothesized that beta-blockade should alter affect, it was less clear whether beta-blockade would alter appraisals given the lack of prior research in this area. One possibility is that appraisals may be less sensitive to in-the-moment neurophysiological fluctuations relative to affect, as appraisals may draw more upon schemas about the situational features of stressors (52,66). Finally, we predicted that propranolol would blunt SNS reactivity but sought to contrast this specificity against PNS and HPA reactivity.
Method
Participants
Ninety healthy young adults (44% female; 56.7% White; Mage: 20.29 ± 1.46 years, 18-25 years; MBMI: 22.78 ± 2.47 kg/m2, 18.5-28.9 kg/m2; Table 1) were recruited from the University of North Carolina at Chapel Hill and its surrounding community via flyers, class announcements, and listservs. Eligibility was assessed via telephone interviews. Individuals were excluded if they reported prior use of beta-blockers, a history of mental or physical health problems, regular nicotine or recreational drug use, prescription medication use, pacemaker or cardiac irregularities, BMI over 33 kg/m2, or resting HR/BP below propranolol safety guidelines (< 60bpm, 80mm/Hg). Participants were instructed to come to the lab well-hydrated, having eaten a normal meal, and refraining from caffeine, high sugar, or exercise that day. On the session day, participants had to report good health, no use of over-the-counter medications, and must exhibit a resting HR/BP within the safety cutoff range. Below we describe procedures and measures but see SDC for further details and CONSORT diagram.
Table 1.
Sample characteristics compared by condition.
| Demographics | Placebo | Propranolol | Total | p-value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 21 (23.3%) | 19 (21.1%) | 40 (44.4%) | .962 a |
| Male | 26 (28.9%) | 24 (26.7%) | 50 (55.6%) | |
| Race n (%) | ||||
| Asian descent | 12 (13.3%) | 11 (12.2%) | 23 (25.6%) | .876 a |
| African descent | 5 (5.6%) | 3 (3.3%) | 8 (8.8%) | |
| European descent | 27 (30.0%) | 24 (26.7%) | 51 (56.7%) | |
| Bi- or multi-racial | 2 (2.2%) | 4 (4.4%) | 6 (6.7%) | |
| Other | 1 (1.1%) | 1 (1.1%) | 2 (2.2%) | |
| Age, mean (years) | 20.49 ± 1.56 | 20.07 ± 1.28 | 20.29 ± 1.46 | .173 b |
| BMI (kg/m2), mean ± SD | 23.09 ± 2.43 | 22.44 ± 2.50 | 22.78 ± 2.47 | .220 b |
| Objective SES, mean ± SD | 16.52 ± 1.93 | 16.19 ± 1.89 | 16.36 ± 1.91 | .408 b |
Note: Frequency counts show percentages of total sample. Objective SES was operationalized as the mean years of education that both parents completed.
Difference tested with Pearson’s chi-square.
Difference tested with independent samples t-tests.
Procedure
The study was pre-registered with ClinicalTrials.gov (Trial ID: NCT02972554) and approved by the university’s institutional review board. After informed written consent, all participants completed the study from 12-5 PM, with procedures time-matched to control for diurnal effects (e.g., cortisol). See Figure 1 for timeline. Each participant was randomly assigned to receive either a single 40 mg dose of propranolol or placebo, self-administered orally under supervision. We chose a 40 mg dose given that this is both a common dosage used in prior studies with healthy adults (28,37,40) and given that this is a common dosage for one-time performance anxiety situations. Drug randomization was completed and provided in identical capsules by the university’s Investigational Drug Services Pharmacy. Staff and participants were blind to condition, except the study physician (SMB), who remained on call for participant safety but did not interact with participants or researchers. Importantly, all participants remained in their originally assigned conditions, and there were no changes to study design, selection and exclusion criteria, or procedures.
Figure 1.
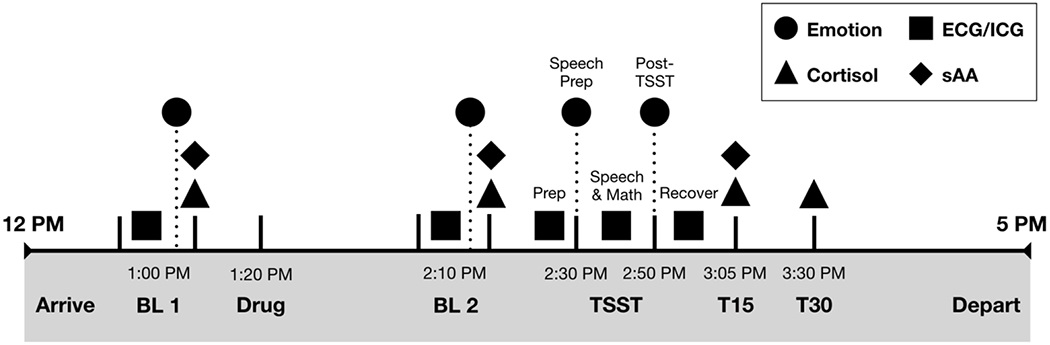
Study timeline illustrating repeated measure timing of self-reported emotions, continuous electrocardiogram (ECG) and impedance cardiography (ICG), and salivary cortisol and alpha-amylase measures. Note that, although not depicted here, appraisals were measured alongside emotion, but only at the TSST Prep and Post-TSST timepoints.
Participants completed the TSST during the 1-2 hours following oral administration of propranolol, when propranolol effects are strongest (67). Participants had 2-min to prepare a speech about their dream job, then gave that speech for 10-min in front of a panel of neutral evaluative judges, whereafter they completed 5-min of impromptu verbal arithmetic (serial subtraction). Participants rated their emotions at baseline before drug administration (pre-drug baseline or BL1), 60-min after drug administration (post-drug baseline or BL2), immediately after TSST speech preparation, and immediately after the full TSST ended. Appraisals were assessed at TSST prep and post-TSST. We measured autonomic changes continuously across six epochs: 5-min BL1, 5-min BL2, 2-min TSST prep, 10-min TSST speech, 5-min TSST arithmetic task, and 7-min recovery post-TSST. Finally, participants provided passive drool saliva samples at BL1, BL2, plus 15-min and 30-min following TSST completion (T15 and T30). Blood samples for inflammatory markers were also collected, but results are published elsewhere (68). See also other work examining separate, secondary questions with this data (69). All participants were debriefed, paid (US$100), and discharged once physiological vitals returned to baseline.
Measures
Self-Reported Emotions.
We used an expanded 40-item version of the Positive & Negative Affect Schedule (PANAS; (70)). Participants rated how intensely they were experiencing each emotion on a Likert scale from 1 (not at all) to 5 (extremely). Following prior standardizations (71,72), mean scores covered the four quadrants of negative, high arousal (e.g., stressed), negative, low arousal (e.g., bored), positive, high arousal (e.g., excited), and positive, low arousal (e.g., relaxed). See SDC for all items.
Self-Reported Appraisals.
We focused on challenge and threat appraisals, thought to occur when an individual perceives a situation to be challenging but manageable vs. threatening without sufficient coping resources (73,74). Challenge-threat appraisals were collected immediately after TSST prep and post-TSST, with 6 items for challenge appraisals (e.g., “I have the abilities to perform the upcoming task successfully”) and 6 items for threat appraisals (e.g., “The previous task was very demanding”) on a Likert scale from 1 (strongly disagree) to 7 (strongly agree). As a third, more diverse appraisal measure, we assessed participants’ negative evaluations of the self and the stressful situation. This negative appraisal measure presented 25 negative descriptors capturing evaluations of personal responsibility for performance (internal attributions or self-evaluations, e.g., blame, incompetence, failure) vs. appraisals about the situation’s controllability and unexpectedness (external attributions or evaluations of the experimenters and situation, e.g., unfair, wronged), on a Likert scale from 1 (not at all) to 6 (extremely). Finally, as a more direct measure of participants’ evaluations of the TSST itself, participants rated on 6-items how difficult, stressful, and enjoyable they found the speech and math tasks (e.g., “The math task was difficult”) on a Likert scale from 1 (not at all) to 6 (extremely). As the negative appraisals and TSST task ratings queried how participants perceived how the TSST went, these were only administered post-TSST. See SDC for further details.
Autonomic Psychophysiology.
To assess sympathetic and parasympathetic activity, we collected continuous electrocardiography (ECG) and impedance cardiography (ICG) at a sampling rate of 1000 Hz using Mindware Technologies (Gahanna, OH, USA). Data for analyses were drawn from the last minute of each baseline, the first minute from each stress phase (preparation, speech, arithmetic), and the last minute of recovery. PEP, a marker of SNS-specific influence on the heart (75), captures the length of time (ms) between the onset of depolarization and the start of left ventricular contraction. Shorter (smaller) PEP values suggest faster periods of cardiac contractility via SNS signaling. RSA is characterized as heart rate variability (HRV) synchronized with the respiratory cycle, wherein the R-to-R interval (the length of time between heartbeats) is shorter (faster) during inhalation and longer (slower) during exhalation. Prior studies suggest that RSA reflects parasympathetic influence of the vagus nerve on the heart (76). Higher RSA values suggest less withdrawal of the PNS. In addition to PEP and RSA, we extracted mean HR (beats per minute or bpm) given its prevalence in past research on beta-blockade and stress. However, HR is a general measure that incorporates both SNS and PNS contributions; as such, we do not focus on HR in the main text (see SDC). HR was used as a covariate in models with RSA, given recent recommendations (77). Finally, respiration was estimated from ICG to parse apart respiration from RSA but was not otherwise analyzed. See SDC for further discussion of ECG/ICG measurement, scoring, and reliability.
Salivary Measures.
Saliva samples were frozen and stored at −80°C until analysis. Salivary concentrations were assessed using commercially available chemiluminescence immunoassay with high sensitivity (IBL International, Hamburg, Germany) following manufacturer instructions. Cortisol was analyzed in duplicate, sAA in singlet. The inter- and intra-assay coefficients of variation for cortisol were both <8%; the inter-assay coefficient of variation for sAA was <6%.
Covariates.
Participants self-reported weight and height, from which BMI was calculated and included in all models (78). Additional covariates were sex and socioeconomic status (SES; operationalized as mean years of parental education), given work showing that both alter stress reactivity (79). For salivary models, we adjusted for the menstrual cycle (menstrual, follicular, ovulation, or luteal phases estimated from participants’ reported first day of their last period and cycle length), given work showing that stage of menstrual cycle alters HPA reactivity (80).
Statistical Analyses
Cortisol and sAA were log-transformed, given their right-skewed distribution. We also examined and excluded outliers that were ± 3 SDs from the mean on any measure within each timepoint within each condition; there were only a few such outliers with most timepoints across measures having no outliers. All data were analyzed in R using the lme4 package (81). As timepoints are nested within individuals, we used multilevel modelling with the inclusion of a random intercept to model individual differences in each outcome. For analyses, drug was coded 0=Placebo, 1=Propranolol and, consistent with other psychology studies, sex was coded 0=Female, 1=Male. Additionally, we aggregated across the TSST speech and math tasks for our index of reactivity during the active stressor but examined TSST prep as its own timepoint, as it likely reflects anticipatory stress. We conducted analyses both with respect to the pre-drug baseline (BL1) and post-drug baseline (BL2) as these test different questions. Analyses with respect to BL1 serve as a manipulation check while also testing the effects of propranolol on our outcomes of interest during a neutral resting state (from pre-drug to post-drug baselines). Analyses with respect to BL2 provide a purer test of how propranolol, once in effect, alters reactivity to the stressor. Throughout the main text, we report most results with respect to BL2, given that this is the strongest test of drug effects on reactivity. In a few cases, we also report manipulation checks comparing BL1 to BL2 but are here careful to specify which baseline is being discussed. See SDC for BL1 results.
Results
Multilevel models were used to assess the main effects of timepoint (i.e., baseline and task effects), main effects of drug, and timepoint x drug interactions on emotion and appraisal reports, sympathetic reactivity (i.e., PEP), parasympathetic reactivity (i.e., RSA), sAA reactivity, and HPA-axis reactivity (i.e., cortisol). Conditions were matched on sex, age, and race/ethnicity (Table 1) and did not differ on depressive or anxiety symptoms, recent perceived life stress, fear of evaluation (SDC Table 2), BMI, or SES. Unstandardized coefficients are presented throughout the Results, but confidence intervals and standardized betas (β) are presented in tables, with βs serving as effect size estimates.
Table 2.
Multilevel fixed effects for emotion reports across drug, timepoint, and drug x timepoint.
| Predictors | b | β | S.E. | p | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|
| Mean negative, high arousal emotions | ||||||
|
| ||||||
| Intercept | 1.19 | .01 | .283 | <.001 | .64 | 1.75 |
| Drug | −.03 | −.17 | .084 | .742 | −.19 | .14 |
| TSST Prep | .38 | .28 | .068 | <.001 | .24 | .51 |
| Post-TSST | .75 | .63 | .068 | <.001 | .61 | .88 |
| Drug x TSST Prep | −.18 | −.09 | .099 | .068 | −.38 | .01 |
| Drug x Post-TSST | −.22 | −.11 | .099 | .026 | −.42 | −.03 |
| Sex | .10 | .11 | .063 | .104 | −.02 | .23 |
| BMI | −.01 | −.00 | .023 | .975 | −.05 | .04 |
| SES | −.01 | −.04 | .017 | .530 | −.04 | .02 |
|
| ||||||
| Mean negative, low arousal emotions | ||||||
|
| ||||||
| Intercept | .94 | .01 | .187 | <.001 | .57 | 1.31 |
| Drug | .08 | .13 | .053 | .129 | −.02 | .19 |
| TSST Prep | −.06 | −.12 | .040 | .166 | −.14 | .02 |
| Post-TSST | .01 | −.00 | .040 | .777 | −.07 | .09 |
| Drug x TSST Prep | −.02 | −.02 | .058 | .718 | −.14 | .09 |
| Drug x Post-TSST | −.03 | −.03 | .058 | .653 | −.14 | .09 |
| Sex | .02 | .04 | .042 | .642 | −.06 | .10 |
| BMI | .01 | .04 | .015 | .666 | −.02 | .04 |
| SES | .01 | .09 | .011 | .266 | −.01 | .03 |
|
| ||||||
| Mean positive, high arousal emotions | ||||||
|
| ||||||
| Intercept | 1.81 | .00 | .584 | .003 | .66 | 2.96 |
| Drug | .06 | .01 | .151 | .708 | −.24 | .35 |
| TSST Prep | −.03 | −.03 | .091 | .710 | −.21 | .15 |
| Post-TSST | .07 | .00 | .091 | .465 | −.11 | .25 |
| Drug x TSST Prep | −.02 | −.01 | .132 | .891 | −.28 | .24 |
| Drug x Post-TSST | −.13 | −.04 | .132 | .332 | −.39 | .13 |
| Sex | .27 | .19 | .131 | .045 | .01 | .53 |
| BMI | −.02 | −.04 | .047 | .681 | −.11 | .07 |
| SES | −.01 | −.03 | .035 | .721 | −.08 | .06 |
|
| ||||||
| Mean positive, low arousal emotions | ||||||
|
| ||||||
| Intercept | 2.48 | .00 | .582 | <.001 | 1.33 | 3.63 |
| Drug | −.18 | −.05 | .155 | .242 | −.49 | .12 |
| TSST Prep | −.56 | −.27 | .102 | <.001 | −.76 | −.36 |
| Post-TSST | −.61 | −.35 | .102 | <.001 | −.81 | −.41 |
| Drug x TSST Prep | .25 | .08 | .148 | .096 | −.04 | .54 |
| Drug x Post-TSST | .09 | .03 | .148 | .550 | −.20 | .38 |
| Sex | .14 | .09 | .131 | .304 | −.12 | .39 |
| BMI | −.06 | −.10 | .047 | .232 | −.15 | .04 |
| SES | .01 | .03 | .034 | .740 | −.06 | .08 |
Note: Significant effects (p<.05) are bolded. SEs are with respect to the unstandardized coefficients.
Effects on Subjective Stress
Affect.
As predicted, we found a main effect of timepoint on negative, high arousal emotions (Figure 2, Table 2), such that these emotions were more intense following both the speech prep (anticipatory stress), b=.38, SE=.07, p<.0001, and immediately after the TSST, b=.75, SE=.07, p<.0001 relative to the post-drug baseline. During speech prep, the interaction between drug x timepoint was nonsignificant (b=−.18, SE=.10, p=.068), but immediately after the TSST, those on propranolol reported lower negative, high arousal emotions relative to those on placebo, b=−.22, SE=.10, p=.026. Interestingly, there was no main effect of drug on negative, high arousal emotions from the pre-drug to post-drug baseline when participants were at rest. Specifically, there was no difference in negative, high arousal emotions between propranolol vs. placebo at the post-drug baseline (b=−.03, SE=.08, p=.742 in Table 2) nor was there a significant interaction of drug x post-drug baseline relative to the pre-drug baseline (b=.02, SE=.09, p=.837 in SDC Table S2), suggesting that propranolol administration did not alter negative, high arousal emotions during a neutral, resting state. As a secondary question, we examined whether propranolol impacted other emotions besides negative, high arousal states. There were significant TSST timepoint main effects on other affective quadrants (e.g., decreased positive, low arousal emotions), but drug x TSST effects were specific to negative, high arousal emotions (Table 2).
Figure 2. Placebo vs. propranolol effects across time on (a) negative, high arousal emotions, (b) PEP, and (c) sAA.
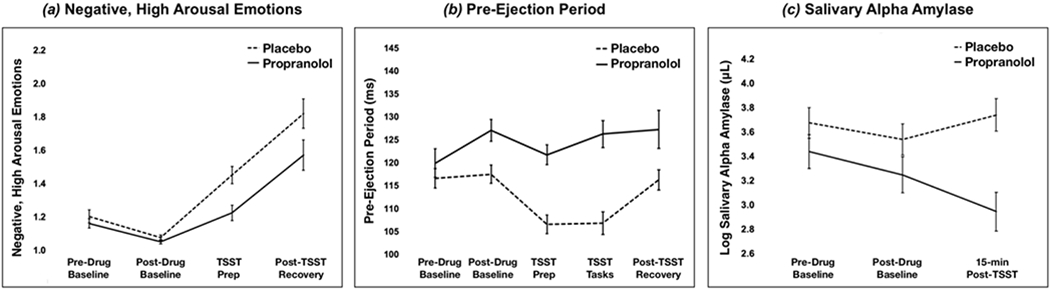
Marginal means and standard errors depicted but see Tables 2 and 4 for significant effects. Note that lower PEP represents shorter (faster) periods of cardiac contractility, consistent with greater SNS activity.
Appraisals.
As would be expected with the TSST, challenge appraisals decreased from speech prep to post-TSST, b=−.55, SE=.12, p<.0001; negative appraisals also increased over time, b=.38, SE=.07, p<.0001 (Table 3). There was no significant change in threat appraisals from speech prep to post-TSST (b=.04, SE=.12, p=.754). Interestingly, beta-blockade did not alter appraisals on any measure nor were any drug x timepoint interactions significant (all bs <−.30-.17, ps>.10). In addition to challenge/threat and negative appraisals, an independent t-test revealed no differences in how individuals on propranolol (M=3.90, SD=.87) vs. placebo (M=3.91, SD=.79) judged the TSST as being difficult, stressful, or unenjoyable, t(88)=.07, p=.943.
Table 3.
Multilevel fixed effects for appraisals across drug, timepoint, and drug x timepoint.
| Predictors | b | β | S.E. | p | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|
| Mean challenge appraisals | ||||||
|
| ||||||
| Intercept | 3.81 | .00 | .791 | <.001 | 2.25 | 5.37 |
| Drug | .08 | .02 | .198 | .674 | −.31 | .48 |
| Post-TSST | −.55 | −.31 | .120 | <.001 | −.79 | −.32 |
| Drug x Post-TSST | −.10 | −.03 | .176 | .574 | −.45 | .25 |
| Sex | .17 | .09 | .179 | .351 | −.19 | .52 |
| BMI | −.13 | −.19 | .064 | .039 | −.26 | −.01 |
| SES | .05 | .11 | .047 | .254 | −.04 | .15 |
|
| ||||||
| Mean threat appraisals | ||||||
|
| ||||||
| Intercept | 4.99 | .00 | .887 | <.001 | 3.24 | 6.74 |
| Drug | −.30 | −.12 | .217 | .172 | −.73 | .13 |
| Post-TSST | .04 | .05 | .117 | .754 | −.20 | .27 |
| Drug x Post-TSST | .11 | .03 | .172 | .510 | −.23 | .45 |
| Sex | −.03 | −.01 | .201 | .895 | −.42 | .37 |
| BMI | .09 | .12 | .072 | .236 | −.06 | .23 |
| SES | −.06 | −.11 | .053 | .275 | −.16 | .05 |
|
| ||||||
| Mean negative appraisals | ||||||
|
| ||||||
| Intercept | 2.14 | .03 | .451 | <.001 | 1.25 | 3.03 |
| Drug | −.06 | −.14 | .112 | .592 | −.28 | .16 |
| Post-TSST | .38 | .28 | .070 | <.001 | .24 | .52 |
| Drug x Post-TSST | −.17 | −.08 | .102 | .102 | −.37 | .03 |
| Sex | .05 | .05 | .100 | .627 | −.15 | .25 |
| BMI | −.00 | −.01 | .036 | .935 | −.07 | .07 |
| SES | −.04 | −.15 | .027 | .116 | −.10 | .01 |
Note: Significant effects (p<.05) are bolded. Reference category was TSST Prep. SEs are with respect to the unstandardized coefficients.
Effects on Physiology
As expected, PEP was shorter (faster) during both the anticipatory stress (prep) and social evaluative (speech, math) TSST phases relative to the post-drug baseline, bs=−10.75, −10.69, SEs=2.23, 2.21, ps<.0001 (Figure 2, Table 4). Critically, propranolol altered PEP both at the post-drug baseline, b=9.37, SE=3.14, p=.003, and throughout the TSST speech and math tasks, b=9.68, SE=3.24, p=.003. Individuals on propranolol showed significantly longer PEP both at rest post-drug (BL2) and during the TSST, relative to placebo, indicating less SNS reactivity among those on propranolol. Beta-blockade did not significantly alter PEP during TSST prep nor post-stressor recovery relative to the post-drug baseline (respectively, bs= 5.21, 2.16, ps>.10). We also examined drug and timepoint effects on sAA, a salivary measure under both SNS and PNS control. There were no effects of drug nor timepoint (respectively, bs=−.25, .20, ps>.10), but there was an interaction of drug x timepoint, b=−.50, SE=.18, p=.006, such that those on propranolol showed blunted sAA reactivity at 15-min post-TSST compared to the post-drug baseline, relative to placebo. Interestingly, there were no effects of drug nor interaction of drug x timepoint on RSA (when adjusting for HR; see SDC for unadjusted effects). Similarly, there were no drug nor drug x timepoint effects on cortisol reactivity, although we replicated the well-established TSST elicitation of increased cortisol. See Table 4 and SDC for more on RSA and cortisol.
Table 4.
Multilevel fixed effects for physiological measures across drug, timepoint, and drug x timepoint.
| Predictors | b | β | S.E. | p | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|
| Mean pre-ejection period | ||||||
|
| ||||||
| Intercept | 128.38 | .00 | 10.714 | <.001 | 107.30 | 149.46 |
| Drug | 9.37 | .42 | 3.143 | .003 | 3.19 | 15.56 |
| TSST Prep | −10.75 | −.22 | 2.230 | <.001 | −15.14 | −6.36 |
| TSST Tasks | −10.69 | −.16 | 2.214 | <.001 | −15.05 | −6.34 |
| TSST Recovery | −1.62 | −.02 | 2.247 | .471 | −6.04 | 2.80 |
| Drug x Prep | 5.21 | .07 | 3.277 | .113 | −1.23 | 11.66 |
| Drug x Tasks | 9.68 | .13 | 3.237 | .003 | 3.31 | 16.05 |
| Drug x Recovery | 2.16 | .03 | 3.273 | .510 | −4.28 | 8.60 |
| Sex | 5.64 | .17 | 2.477 | .025 | .770 | 10.52 |
| BMI | .67 | .06 | .905 | .465 | −1.12 | 2.45 |
| SES | −.89 | −.11 | .630 | .164 | −2.13 | .36 |
|
| ||||||
| Log-transformed salivary alpha-amylase | ||||||
|
| ||||||
| Intercept | 1.96 | −.00 | .833 | .021 | .32 | 3.61 |
| Drug | −.25 | −.25 | .203 | .220 | −.65 | .15 |
| Post-TSST T15 (15-min) | .20 | −.02 | .125 | .120 | −.05 | .44 |
| Drug x T15 | −.50 | −.13 | .176 | .006 | −.85 | −.15 |
| Sex | .06 | .03 | .230 | .788 | −.39 | .52 |
| Menses Cycle | .03 | .04 | .077 | .713 | −.12 | .18 |
| BMI | −.08 | −.11 | .066 | .258 | −.21 | .06 |
| SES | .10 | .19 | .048 | .049 | .00 | .19 |
|
| ||||||
| Mean respiratory sinus arrhythmia | ||||||
|
| ||||||
| Intercept | 11.39 | .00 | .680 | <.001 | 10.05 | 12.73 |
| Drug | −.24 | −.12 | .194 | .210 | −.63 | .14 |
| TSST Prep | .19 | .09 | .171 | .258 | −.14 | .53 |
| TSST Tasks | .30 | .07 | .201 | .131 | −.09 | .70 |
| TSST Recovery | −.18 | −.04 | .160 | .275 | −.49 | .14 |
| Drug x Prep | .06 | .01 | .231 | .792 | −.39 | .52 |
| Drug x Tasks | −.25 | −.05 | .242 | .305 | −.72 | .23 |
| Drug x Recovery | .14 | .03 | .228 | .548 | −.31 | .59 |
| Heart rate | −.05 | −.64 | .005 | <.001 | −.06 | −.04 |
| Sex | −.26 | −.11 | .140 | .072 | −.53 | .02 |
| BMI | .01 | .02 | .050 | .797 | −.09 | .11 |
| SES | −.05 | −.10 | .036 | .142 | −.13 | .02 |
|
| ||||||
| Log-transformed salivary cortisol | ||||||
|
| ||||||
| Intercept | .66 | .00 | .647 | .311 | −.61 | 1.93 |
| Drug | .45 | .21 | .178 | .013 | .10 | .80 |
| Post-TSST T15 (15-min) | .87 | .43 | .132 | <.001 | .61 | 1.13 |
| Post-TSST T30 (30-min) | .60 | .28 | .132 | <.001 | .34 | .86 |
| Drug x T15 | −.08 | −.02 | .190 | .693 | −.45 | .30 |
| Drug x T30 | −.10 | −.03 | .190 | .611 | −.47 | .28 |
| Sex | .33 | .18 | .178 | .070 | −.02 | .68 |
| Menses Cycle | .00 | −.00 | .059 | .999 | −.12 | .12 |
| BMI | .06 | .10 | .051 | .216 | −.04 | .16 |
| SES | −.01 | −.01 | .037 | .893 | −.08 | .07 |
Note: Significant effects (p<.05) are bolded. SEs are with respect to the unstandardized coefficients.
Discussion
We demonstrated that pre-treatment with propranolol altered affective experiences but not appraisals during an acute psychosocial stressor. Specifically, individuals on beta-blockade reported lower negative, high arousal emotions while also exhibiting lower SNS reactivity in response to the stressor, relative to those on placebo. Although consistent with some prior work wherein propranolol blunted anxiety (23,28,32,39,43,44,47,49), the present findings contrast with several studies that did not find blunting of subjective stress (26,27,37,38,41,42,45,46,48,82). These inconsistencies in prior work may be due in part to small sample sizes and narrow measures of subjective stress—issues we sought to address herein. Moreover, the present findings reveal both psychological and physiological specificity in the effects of beta-blockade. Beta-blockade blunted negative, high arousal emotions, PEP, and sAA, but not low arousal emotions, positive emotions, appraisals, nor measures of the PNS or HPA-axis (RSA, cortisol). Together, these findings affirm that beta-adrenergic signaling supports SNS-specific physiological responses while also helping transform a potentially stressful situation into the subjective experience we call “stress.”
The experimental design and specificity of findings yield intriguing insights about the nature of emotion and stress. First, these findings may provide tentative evidence for the Jamesian and constructionist hypothesis that the peripheral body can contribute to affect (19–22). Although propranolol crosses the blood-brain barrier and acts on both the peripheral and central nervous systems, ongoing work with beta-blockers that have peripheral-predominant effects are informative. For example, atenolol is a hydrophilic beta-blocker that cannot easily cross the blood-brain barrier and is selective to β1-receptors which predominate in the heart (83). Both older and recent studies suggest that atenolol can exert anxiolytic and arousal-blunting effects (47,83,84), indicating that SNS and related signaling via peripheral beta-adrenergic receptors may influence affect. As such, one possibility of the present findings is that propranolol blunted affect in part via peripheral beta-adrenergic receptors. However, as we did not design this study to adjudicate between peripheral and central pathways, future work is needed to test the degree to which effects on affect are mediated via peripheral vs. central beta-adrenergic receptors.
Another insight from the present findings is that the effects of beta-blockade on affect were context-dependent: propranolol did not alter emotions (of any type) from pre- to post-drug resting baselines, and only mattered in the stressful context. Yet propranolol was physiologically active after administration, modulating SNS activity during the same post-drug baseline, as demonstrated by significantly slower PEP in the propranolol group. These results are consistent with “affect-as-information” and constructionist models in affective science (21,85), which hypothesize that physiological changes can influence psychological states particularly when those changes have relevance for the immediate situation. For instance, recent work showed that another physiological state, hunger, intensified affective perceptions and experiences, but only when individuals were in negative but not neutral or positive affective contexts (86). These findings provide converging evidence that allostatic changes across the body and brain, when made meaningful in a relevant situation, can influence the nature and intensity of affective states.
Although we found that beta-blockade altered affect, it did not alter appraisals of the stressor. Longstanding work finds that appraisals and affect are often correlated (87); this was true herein (see SDC). Indeed, individuals who reported greater negative, high arousal emotions in response to the TSST were more likely to appraise the TSST as a negative event (i.e., they made more negative internal and external evaluations: r=.79, p<.001) and to interpret the TSST as less of a positive challenge (r=−.50, p<.001) and more as a threat (r=.59, p<.001). Despite these associations, propranolol only blunted negative, high arousal emotions while appraisals remained intact. All participants reported appraising the TSST similarly as a stressor, but only those on placebo experienced it as emotionally unpleasant and highly arousing. This may suggest that beta-adrenergic signaling either selectively or more robustly impacts the generation of affective states without necessarily altering cognitive evaluations. Thus, although affect and appraisals are both dimensions of subjective stress, they likely reflect different underlying processes (e.g., affect may draw more upon ongoing physiology and interoception whereas appraisals may draw more upon stable, a priori knowledge or schemas about situational features). Alternative possibilities are that beta-adrenergic signaling (whether central or peripheral) may influence other appraisal dimensions than those measured herein, or there may be other neurophysiological pathways (e.g., HPA-axis) not impaired by propranolol that are still influencing appraisals.
As hypothesized, we also found that propranolol blunted the SNS indicator PEP after drug administration and throughout the stressor. We replicated a similar pattern of results with sAA. Although the extent to which sAA can be used as an index of SNS activity vs. a more general autonomic index remains debated (53,54), the present finding that propranolol blunted sAA reactivity replicates prior work (59) and aligns with existing interpretations that sAA is (at least in part) under SNS control. Interestingly, effects of propranolol were specific to PEP and sAA reactivity and did not extend to PNS (RSA) or HPA-axis (cortisol) markers. This specificity is consistent with evidence that beta-adrenergic signaling mediates post-synaptic SNS effects, but not PNS cardiac effects (76). Although past literature has found mixed effects of beta-blockade on RSA (88), recent work argues that it is important to account for HR in RSA analyses to parse out confounding SNS effects (77). Consistent with this possibility, as reported in the SDC, we found a drug x timepoint effect on RSA in unadjusted models, but this effect was nonsignificant after adjusting for HR. Finally, although cortisol significantly increased in response to the TSST, pre-treatment with propranolol did not alter these effects. To date, prior studies have been equivocal about the effects of beta-blockade on HPA reactivity (39,60,63,64). The present findings are in line with interpretations that cortisol, as an end-product of the HPA-axis, may be less sensitive to SNS signaling, at least in the context of acute psychosocial stress in healthy young adults.
This study has several limitations. First, we administered a single 40 mg dose of propranolol to mimic what is typically prescribed for the treatment of performance-related anxiety, but results may not generalize to chronic propranolol use or different dosages. For example, the effects herein might differ at another dosage amount (e.g., 60 or 80 mg) or frequency (e.g., across several days). Relatedly, prior null effects of beta-blockade on emotion could be due in part to using other dosages, but this remains unclear given that some prior studies with null emotion effects also used a single 40 mg dose (30,37,40,46). However, one consideration is that stronger dosages (e.g., 80 mg) of beta-blockade may exert more overt physiological effects which could lead to unblinding (89), altering the ways in which participants attribute and report their emotions.
Other limitations include the fact that we only assessed effects in healthy young adults, so results should be replicated in other populations (people with mood disorders; older adults). Because we used more comprehensive measures that took longer to complete than a few items, another limitation is that participants may have shifted to a different state between responding to the first and final item in each self-report period. Future studies could reduce this possibility by focusing on negative, high arousal emotions, given our findings. Another unanswered question is the extent to which beta-blockade alters cross-system inter-connections during conditions of acute stress (e.g., correlations between SNS and PNS indicators). Finally, it should be noted that the pharmacological effects of propranolol on emotion cannot be isolated to the peripheral body, brain, or both. Although propranolol is a non-selective beta-blockade, acting upon all types of beta-adrenergic receptors (e.g., β1, β2), it appears to have slightly greater affinity for β2-receptors (90). Given that atenolol is peripherally predominant and selective to β1-receptors, future extensions could contrast propranolol and atenolol or other beta-blockers (e.g., nadolol) to triangulate central vs. peripheral effects and the role of beta-adrenergic receptor classes in subjective stress and affect.
In sum, the present study leveraged comprehensive methods and measures from psychopharmacology, affective science, and psychophysiology to clarify the murky literature on beta-blockers, emotion, and stress. We found evidence that beta-adrenergic signaling does indeed causally contribute to affective experiences during an acute psychosocial stressor. Although everyone experiences challenging or difficult life events and daily stressors, growing work emphasizes that it is often subjective stress that is more predictive of downstream health and well-being (6–8). As such, understanding how different neurophysiological systems exacerbate or dampen the stress experience may help reveal why some people have more intense emotional responses to negative life events than others. The present findings affirm that the SNS and related adrenergic/noradrenergic systems help instantiate human affective experiences, while also expanding our mechanistic knowledge about the pathways linking stress and health.
Supplementary Material
Conflicts of Interest and Source of Funding:
JKM received support from a Ruth L. Kirschstein National Research Service Award predoctoral fellowship from the National Institute on Aging (1F31AG055265-01A1) as well as a T32 postdoctoral fellowship from the National Heart, Lung, and Blood Institute (5T32HL007560-37) via the University of Pittsburgh Department of Psychiatry. EKS received support from the Australian National Health and Medical Research Council APP1147498. The North Carolina Translational and Clinical Sciences (NC TraCS) Institute provided further assistance, via support from the National Center for Advancing Translational Sciences (NCATS; UL1TR002489). The authors have no financial disclosures or conflicts of interest to report.
Acronyms:
- BMI
body mass index
- HPA axis
hypothalamic-pituitary-adrenal axis
- HR
heart rate
- PEP
pre-ejection period
- PNS
parasympathetic nervous system
- RSA
respiratory sinus arrhythmia
- sAA
salivary alpha-amylase
- SNS
sympathetic nervous system
- TSST
Trier Social Stress Test
Footnotes
Trial Registration: NCT02972554
References
- 1.Burger K, Samuel R. The role of perceived stress and self-efficacy in young people’s life satisfaction: A longitudinal study. Journal of Youth and Adolescence. 2017;46:78–90. [DOI] [PubMed] [Google Scholar]
- 2.O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Wolkowitz OM, Blackburn EH, Epel ES. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain, Behavior, and Immunity. 2012;26:573–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin. 2014;140:774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685. [DOI] [PubMed] [Google Scholar]
- 6.Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, Khazeni N. Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain, Behavior, and Immunity. 2016;54:158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore RC, Eyler LT, Mausbach BT, Zlatar ZZ, Thompson WK, Peavy G, Fazeli PL, Jeste D V. Complex interplay between health and successful aging: Role of perceived stress, resilience, and social support. The American Journal of Geriatric Psychiatry. 2015;23:622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shavitt S, Cho YI, Johnson TP, Jiang D, Holbrook A, Stavrakantonaki M. Culture moderates the relation between perceived stress, social support, and mental and physical Health. Journal of Cross-Cultural Psychology. 2016;47:956–80. [Google Scholar]
- 9.Steenen SA, van Wijk AJ, van der Heijden GJMG, van Westrhenen R, de Lange J, de Jongh A. Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. Journal of Psychopharmacology. 2016;30:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan A Propranolol: A 50-year historical perspective. Annals of Indian Academy of Neurology. 2019;22:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterling P, Laughlin S. Principles of neural design. Principles of Neural Design. Cambridge, MA.: MIT Press Books; 2015. 1–542 p. [Google Scholar]
- 12.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. [DOI] [PubMed] [Google Scholar]
- 13.Berridge CW. Noradrenergic modulation of arousal. Brain Research Reviews. 2008;58:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satpute AB, Kragel PA, Barrett LF, Wager TD, Bianciardi M. Deconstructing arousal into wakeful, autonomic and affective varieties. Neuroscience Letters. 2019;693:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga MS, editor. The cognitive neurosciences. The MIT Press; 1995. p. 703–20. [Google Scholar]
- 16.William Tank A, Lee Wong D. Peripheral and central effects of circulating catecholamines. In: Comprehensive Physiology. Hoboken, NJ: John Wiley & Sons, Inc.; 2015. p. 1–15. [DOI] [PubMed] [Google Scholar]
- 17.Stiles GL, Caron MG, Lefkowitz RJ. Beta-adrenergic receptors: Biochemical mechanisms of physiological regulation. Physiological Reviews. 1984;64:661–743. [DOI] [PubMed] [Google Scholar]
- 18.Reznikoff GA, Manaker S, Rhodes CH, Winokur A, Rainbow TC. Localization and quantification of beta-adrenergic receptors in human brain. Neurology. 1986;36:1067–1067. [DOI] [PubMed] [Google Scholar]
- 19.James W What is an emotion? Mind. 1884;34:188–205. [Google Scholar]
- 20.Schachter S, Singer J. Cognitive, social, and physiological determinants of emotional state. Psychological Review. 1962;69. [DOI] [PubMed] [Google Scholar]
- 21.Barrett LF. The theory of constructed emotion: An active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience. 2017;12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacCormack JK, Lindquist KA. Bodily contributions to emotion: Schachter’s legacy for a psychological constructionist view on emotion. Emotion Review. 2017;9:36–45. [Google Scholar]
- 23.Stone WN, Gleser GC, Gottschalk LA. Anxiety and β-adrenergic blockade. Archives of General Psychiatry. 1973;29:620–22. [DOI] [PubMed] [Google Scholar]
- 24.Tyrer PJ, Lader MH. Physiological and psychological effects of ±-propranolol, +-propranolol and diazepam in induced anxiety. British Journal of Clinical Pharmacology. 1974;1:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drew P, Barnes J, Evans S. The effect of acute beta-adrenoceptor blockade on examination performance. British Journal of Clinical Pharmacology. 1985;19:783–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.File S, Lister R. A comparison of the effects of lorazepam with those of propranolol on experimentally-induced anxiety and performance. British Journal of Clinical Pharmacology. 1985;19:445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krantz DS, Contrada RJ, LaRiccia PJ, Anderson JR, Durel LA, Dembroski TM, Weiss T. Effects of beta-adrenergic stimulation and blockade on cardiovascular reactivity, affect, and type A behavior. Psychosomatic Medicine. 1987;49:146–58. [DOI] [PubMed] [Google Scholar]
- 28.Currie D, Lewis R, McDevitt D, Nicholson A, Wright N. Central effects of beta-adrenoceptor antagonists. I-Performance and subjective assessments of mood. British Journal of Clinical Pharmacology. 1988;26:121–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyck JB, Chung F. A comparison of propranolol and diazepam for preoperative anxiolysis. Canadian Journal of Anaesthesia. 1991;38:704–9. [DOI] [PubMed] [Google Scholar]
- 30.Jakobsson J, Rane K, Ryberg G. Oral premedication one hour before minor gynaecological surgery - does it have any effect? Acta Anaesthesiologica Scandinavica. 1995;39:359–63. [DOI] [PubMed] [Google Scholar]
- 31.Head A, Kendall MJ, Ferner R, Eagles C. Acute effects of beta blockade and exercise on mood and anxiety. British Journal of Sports Medicine. 1996;30:238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mealy K, Ngeh N, Gillen P, Fitzpatrick G, Keane FB, Tanner A. Propranolol reduces the anxiety associated with day case surgery. The European Journal of Surgery. 1996;162:11–14. [PubMed] [Google Scholar]
- 33.Elman MJ, Sugar J, Fiscella R, Deutsch TA, Noth J, Nyberg M, Packo K, Anderson RJ. The effect of propranolol versus placebo on resident surgical performance. Transactions of the American Ophthalmological Society. 1998;96:283–91; discussion 291-4. [PMC free article] [PubMed] [Google Scholar]
- 34.Harmer CJ, Perrett DI, Cowen PJ, Goodwin GM. Administration of the beta-adrenoceptor blocker propranolol impairs the processing of facial expressions of sadness. Psychopharmacology. 2001;154:383–89. [DOI] [PubMed] [Google Scholar]
- 35.Tyrer PJ, Lader MH. Response to propranolol and diazepam in somatic and psychic anxiety. British Medical Journal. 1974;14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers RD, Lancaster M, Wakeley J, Bhagwagar Z. Effects of beta-adrenoceptor blockade on components of human decision-making. Psychopharmacology. 2004;172:157–64. [DOI] [PubMed] [Google Scholar]
- 37.Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. Journal of Cognitive Neuroscience. 2007;19:468–78. [DOI] [PubMed] [Google Scholar]
- 38.Andrews J, Pruessner JC. The combined propranolol/TSST paradigm – A new method for psychoneuroendocrinology. Felmingham K, editor. PLoS ONE. 2013;8:e57567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreifus L, Engler H, Kissler J. Retrieval-induced forgetting under psychosocial stress: No reduction by delayed stress and beta-adrenergic blockade. Neurobiology of Learning and Memory. 2014;110:35–46. [DOI] [PubMed] [Google Scholar]
- 40.Ernst M, Lago T, Davis A, Grillon C. The effects of methylphenidate and propranolol on the interplay between induced-anxiety and working memory. Psychopharmacology. 2016;233:3565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali N, Nitschke JP, Cooperman C, Pruessner JC. Suppressing the endocrine and autonomic stress systems does not impact the emotional stress experience after psychosocial stress. Psychoneuroendocrinology. 2017;78:125–30. [DOI] [PubMed] [Google Scholar]
- 42.Steptoe A, Ronaldson A, Kostich K, Lazzarino AI, Urbanova L, Carvalho LA. The effect of beta-adrenergic blockade on inflammatory and cardiovascular responses to acute mental stress. Brain, Behavior, and Immunity. 2018;70:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartley LR, Ungapen S, Davie I, Spencer DJ. The effect of beta adrenergic blocking drugs on speakers’ performance and memory. British Journal of Psychiatry. 1983;142:512–17. [DOI] [PubMed] [Google Scholar]
- 44.Gottschalk LA, Stone WN, Gleser GC. Peripheral versus central mechanisms accounting for antianxiety effects of propranolol. Psychosomatic Medicine. 1974;36:47–56. [DOI] [PubMed] [Google Scholar]
- 45.Ashton H, Millman JE, Telford R, Thompson JW. A comparison of some physiological and psychological effects of propranolol and diazepam in normal subjects. British Journal of Clinical Pharmacology. 1976;3:551–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakano S, Gillespie HK, Hollister LE. Propranolol in experimentally induced stress. Psychopharmacology. 1978;59:279–84. [DOI] [PubMed] [Google Scholar]
- 47.Landauer AA, Pocock DA, Prott FW. Effects of atenolol and propranolol on human performance and subjective feelings. Psychopharmacology. 1979;60:211–15. [DOI] [PubMed] [Google Scholar]
- 48.Taylor EA, Harrison J, Turner P. Propranolol in experimentally induced stress. The British Journal of Psychiatry. 1981;139:545–49. [DOI] [PubMed] [Google Scholar]
- 49.Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. The American Journal of Medicine. 1982;72:88–94. [DOI] [PubMed] [Google Scholar]
- 50.Salem S, McDevitt D. Central effects of single oral doses of propranolol in man. British Journal of Clinical Pharmacology. 1984;17:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blascovich J, Mendes WB. Social psychophysiology and embodiment. In: Fiske ST, Gilbert DT, Lindzey G, editors. The Handbook of Social Psychology. 5th ed. New York, NY: John Wiley & Sons, Inc.; 2010. p. 194–227. [Google Scholar]
- 52.Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- 53.Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34:486–96. [DOI] [PubMed] [Google Scholar]
- 54.Nagy T, van Lien R, Willemsen G, Proctor G, Efting M, Fülöp M, Bárdos G, Veerman ECI, Bosch JA. A fluid response: Alpha-amylase reactions to acute laboratory stress are related to sample timing and saliva flow rate. Biological Psychology. 2015;109:111–19. [DOI] [PubMed] [Google Scholar]
- 55.Achong MR, Piafsky KM, Ogilvie RI. Duration of cardiac effects of timolol and propranolol. Clinical Pharmacology & Therapeutics. 1976;19:148–52. [DOI] [PubMed] [Google Scholar]
- 56.Harris WS, Schoenfeld CD, Weissler AM. Effects of adrenergic receptor activation and blockade on the systolic pre-ejection period, heart rate, and arterial pressure in man. Journal of Clinical Investigation. 1967;46:1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunt D, Sloman G, Clark RM, Hoffmann G. Effects of beta-adrenergic blockade on the systolic time intervals. The American Journal of the Medical Sciences. 1970;259:97–113. [DOI] [PubMed] [Google Scholar]
- 58.Benschop RJ, Nieuwenhuis EE, Tromp EA, Godaert GL, Ballieux RE, van Doornen LJ. Effects of beta-adrenergic blockade on immunologic and cardiovascular changes induced by mental stress. Circulation. 1994;89:762–69. [DOI] [PubMed] [Google Scholar]
- 59.van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31:137–41. [DOI] [PubMed] [Google Scholar]
- 60.von Känel R, Kudielka BM, Metzenthin P, Helfricht S, Preckel D, Haeberli A, Stutz M, Fischer JE. Aspirin, but not propranolol, attenuates the acute stress-induced increase in circulating levels of interleukin-6: A randomized, double-blind, placebo-controlled study. Brain, Behavior, and Immunity. 2008;22:150–57. [DOI] [PubMed] [Google Scholar]
- 61.Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31:586–98. [DOI] [PubMed] [Google Scholar]
- 62.Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31:599–608. [DOI] [PubMed] [Google Scholar]
- 63.Benschop RJ, Jacobs R, Sommer B, Schürmeyer TH, Raab JR, Schmidt RE, Schedlowski M. Modulation of the immunologic response to acute stress in humans by beta-blockade or benzodiazepines. FASEB Journal. 1996;10:517–24. [DOI] [PubMed] [Google Scholar]
- 64.Kudielka BM, Fischer JE, Metzenthin P, Helfricht S, Preckel D, von Känel R. No effect of 5-day treatment with acetylsalicylic acid (aspirin) or the beta-blocker propranolol (inderal) on free cortisol responses to acute psychosocial stress: A randomized double-blind, placebo-controlled study. Neuropsychobiology. 2007;56:159–66. [DOI] [PubMed] [Google Scholar]
- 65.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. [DOI] [PubMed] [Google Scholar]
- 66.Moors A, Ellsworth PC, Scherer KR, Frijda NH. Appraisal theories of emotion: State of the art and future development. Emotion Review. 2013;5:119–24. [Google Scholar]
- 67.Williams FM, Leeser JE, Rawlins MD. Pharmacodynamics and pharmacokinetics of single doses of ketanserin and propranolol alone and in combination in healthy volunteers. British Journal of Clinical Pharmacology. 1986;22:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacCormack JK, Gaudier-Diaz MM, Armstrong-Carter EL, Arevalo JMG, Meltzer-Brody S, Sloan EK, Cole SW, Muscatell KA. Beta-adrenergic blockade blunts inflammatory and antiviral/antibody gene expression responses to acute social stress. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacCormack JK, Armstrong-Carter EL, Humphreys KL, Muscatell KA. Neurophysiological contributors to advantageous risk-taking: An experimental psychopharmacological investigation. Social Cognitive and Affective Neuroscience. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. Armes: University of Iowa; 1994. [Google Scholar]
- 71.Stevenson RA, Mikels JA, James TW. Characterization of the affective norms for english words by discrete emotional categories. Behavior Research Methods. 2007;39:1020–24. [DOI] [PubMed] [Google Scholar]
- 72.Warriner AB, Kuperman V, Brysbaert M. Norms of valence, arousal, and dominance for 13,915 English lemmas. Behavior Research Methods. 2013;45:1191–1207. [DOI] [PubMed] [Google Scholar]
- 73.Tomaka J, Blascovich J, Kelsey RM, Leitten CL. Subjective, physiological, and behavioral effects of threat and challenge appraisal. Journal of Personality and Social Psychology. 1993;65:248–60. [Google Scholar]
- 74.Mendes WB, Blascovich J, Major B, Seery M. Challenge and threat responses during downward and upward social comparisons. European Journal of Social Psychology. 2001;31:477–97. [Google Scholar]
- 75.Newlin DB, Levenson RW. Pre-ejection period: Measuring beta-adrenergic influences upon the heart. Psychophysiology. 1979;16:546–53. [DOI] [PubMed] [Google Scholar]
- 76.Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–96. [DOI] [PubMed] [Google Scholar]
- 77.de Geus EJC, Gianaros PJ, Brindle RC, Jennings JR, Berntson GG. Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology. 2019;56:e13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sokol-Hessner P, Lackovic SF, Tobe RH, Camerer CF, Leventhal BL, Phelps EA. Determinants of propranolol’s selective effect on loss aversion. Psychological Science. 2015;26:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dedovic K, Wadiwalla M, Engert V, Pruessner JC. The role of sex and gender socialization in stress reactivity. Developmental Psychology. 2009;45:45–55. [DOI] [PubMed] [Google Scholar]
- 80.Duchesne A, Pruessner JC. Association between subjective and cortisol stress response depends on the menstrual cycle phase. Psychoneuroendocrinology. 2013;38:3155–59. [DOI] [PubMed] [Google Scholar]
- 81.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- 82.Mazzuero G, Galdangelo F, Zotti AM, Bertolotti G, Tavazzi L. Effects of propranolol, atenolol, and chlordesmethildiazepam on response to mental stress in patients with recent myocardial infarction. Clinical Cardiology. 1987;10:293–302. [DOI] [PubMed] [Google Scholar]
- 83.Armstrong C, Kapolowicz MR. A preliminary investigation on the effects of atenolol for treating symptoms of anxiety. Military Medicine. 2020;185:e1954–60. [DOI] [PubMed] [Google Scholar]
- 84.Benjamin AB, Dooley TP. Anxiolytic benefits of compounded atenolol–scopolamine in eight patients in psychiatry. Personalized Medicine in Psychiatry. 2020;19–20:100051. [Google Scholar]
- 85.Storbeck J, Clore GL. Affective arousal as information: How affective arousal Influences judgments, learning, and memory. Social and Personality Psychology Compass. 2008;2:1824–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacCormack JK, Lindquist KA. Feeling hangry? When hunger is conceptualized as emotion. Emotion. 2018;19:301–19. [DOI] [PubMed] [Google Scholar]
- 87.Siemer M, Mauss I, Gross JJ. Same situation--different emotions: How appraisals shape our emotions. Emotion. 2007;7:592–600. [DOI] [PubMed] [Google Scholar]
- 88.Pitzalis MV, Mastropasqua F, Massari F, Passantino A, Totaro P, Forleo C, Rizzon P. β-blocker effects on respiratory sinus arrhythmia and baroreflex gain in normal subjects. Chest. 1998;114:185–91. [DOI] [PubMed] [Google Scholar]
- 89.Park J, Bang H, Cañette I. Blinding in clinical trials, time to do it better. Complementary Therapies in Medicine. 2008;16:121–23. [DOI] [PubMed] [Google Scholar]
- 90.Baker JG. The selectivity of β -adrenoceptor antagonists at the human β 1, β 2 and β 3 adrenoceptors. British Journal of Pharmacology. 2005;144:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


