Abstract
The Syrian hamster (Mesocricetus auratus) is a solitary and naturally territorial animal, with female hamsters being more aggressive than males. This behavior makes handling difficult because they are usually housed in groups, which can lead to aggressive behavior. The objective of this study was to refine the management of Syrian hamsters in order to minimize aggressiveness, reduce the animal injuries, and lessen the risk of accidents among laboratory animal technicians due to the hamster aggression during handling. The experiment was conducted at the Center for Animals Experimentation, Oswaldo Cruz Institute. Four groups of hamsters were observed by video recording: group 1 (group-housed males, 6 to 8 wk of age), group 2 (group-housed females 6 to 8 wk of age), group 3 (group-housed female, 3 to 4 wk of age), and group 4 (individually housed females, 6 to 8 wk of age). Group 1 animals were less aggressive and agitated both during housing and during handling by the animal technician as compared with groups 2 and 3. Groups 2 and 3 showed greater agitation and aggression. Marked reduction in the level of aggressiveness and agitation was observed in group 4 as compared with all other groups evaluated during handling by the animal technician. Male hamsters housed in groups of 4 and females housed individually has reduced risks of accident during handling, thereby averting distress and consequent physiologic alterations. Avoiding these risks is essential to obtaining reliable experimental results.
The Syrian hamster (Mesocricetus auratus) as a laboratory animal originated from a litter captured in Syria and sent to the United States. These animals gave origin to the current lineages of hamsters. Syrian hamsters are poorly sociable animals with nocturnal habits; they temporarily come together during reproduction and separate again after copulation. These hamsters originate from environments such as deserts and grasslands in cold or hot ecosystems but inhabit a less intense microclimate due to their underground tunnels and galleries.1 Although Syrian hamsters can be bred in captivity, they are territorial, aggressive, and solitary animals.4 This behavior makes them difficult to handle for research use because they are often housed in groups. Environmental enrichment and conditioning to handling can improve the welfare and facilitate the handling of hamsters.5,6
Several features of Mesocricetus auratus make them good model for studies of behavior and human diseases. These features include the uncomplicated reproduction, natural resistance to certain diseases, susceptibility to introduced pathogenic agents, unique anatomic and physiologic conditions, and a short reproductive cycle that facilitates the formation of colonies.2 Animal experiments have been and continue to be important for the advancements in animal and human health. Good animal welfare improves the quality of the experiment and reduces or even prevents the loss of experimental animals due to stress. In addition, an animal exhibiting a low level of stress poses fewer risks to the animal handlers.8
The objective of this study was to apply refinement methods to the handling of Syrian hamsters (Mesocricetus auratus) to minimize aggressiveness, reduce the loss of animals due to injuries caused by fighting, and attenuate the risk of accidents (bites, scratches) for research personnel due to hamster aggressiveness during handling.
Materials and Methods
Animals.
The hamsters (n = 64; 16 males and 48 females) used in this study were kept on ventilated racks (Alesco, Brazil) equipped with a controlled ventilation system (10 to 20 air changes per hour) in the laboratory animal facility of the Carlos Chagas Pavilion, Center for Animal Experimentation (CEA), Oswaldo Cruz Institute (IOC), Oswaldo Cruz Foundation (Fiocruz). The hamsters were housed in polypropylene cages (Alesco, Brazil) measuring 490 mm × 340 mm × 160 mm under a 12-h light:dark cycle at a temperature of 21 ± 2 °C, at a relative humidity of 40% to 60%, with ad libitum access to autoclaved water and standard chow (Nuvilab, Brazil) for hamsters and access to a paper towel as environmental enrichment material.
Ethics.
The procedures adopted in the study followed the Guide for the Care and Use of Laboratory Animals7 and were carried out in accordance with Brazilian law, licensed under number L-043/2016 by the Animal Use Ethics Committee of CEUA/IOC, Fiocruz.
Routine practices for managing and handling hamsters.
As part of the daily management routine, stress was managed by minimizing noise and avoiding quick and inattentive movements, rough handling of cage components, and external odors. Careful attention was given to handling of cages to avoid perturbations of the hamsters. This included avoidance of unintentional dragging or hitting the cage. The cage was handled carefully, with the grid being manipulated in such a way as to avoid strident noises.
During interaction with the animals for cage change, the hamsters were approached slowly, allowing them to interact with the handler’s hand. If asleep, the animal was awakened carefully to avoid frightening it. The thumb and index finger were then used as “tweezers” on the hamster’s back to lift it, always trying to grab a good amount of skin (Figure 1) and to transfer the hamster calmly to another cage. Then, the old nest was transferred to the new cage, and more paper was provided.
Figure 1.

Cage change of a hamster using the thumb and index finger as “tweezers” on the animal’s back to lift it, always grabbing a good amount of skin in a calm manner.
Group assignment.
The hamsters were transported from the Institute of Science and Technology breeding facility in Biomodels (ICTB/Fiocruz) to the laboratory facility of the Carlos Chagas Pavilion, CEA/IOC/Fiocruz. The hamsters were allowed to adapt to the environment and handling for 3 wk and were then divided into the following 4 groups: Group 1, 4 cages with 4 group-housed males each, 6 to 8 wk of age; Group 2, 4 cages with 4 group-housed females each, 6 to 8 wk of age; Group 3, 4 cages with 4 group-housed females each, 3 to 4 wk of age (immediately after weaning); Group 4, 16 females housed one per cage, 6 to 8 wk of age.
Each group consisted of a total of 16 animals. The groups 1, 2, and 3 were observed for 4 wk. In view of the large number of cages, group 4 was divided and monitored at different time points; however, the methodology was the same as that used for the other groups in which each cage was observed for 4 wk.
Evaluation of aggressiveness.
Natural behaviors of hamsters were observed once a week. Aggression was evaluated at 2 different time points. First, during physical restraint for transfer from the cage, the number of hamsters showing an aggressive behavior (struggling, vocalizing, and/or trying to bite the professional’s hand) was recorded. Second, after cage change, the duration of threats (aggressive postures) and aggressions (attacks and bites) among hamsters in the group was recorded at intervals of up to 20 min, up to 40 min, up to 60 min, and up to 80 min were established.
Data analysis.
We performed a cross-sectional observational study in which we analyzed the intraspecific aggression of hamsters and their behavior during physical restraint for transfer from the cage. Hamsters were grouped based on age and sex.
The Kruskall–Wallis test (non-parametric, 5% significance level) was used to determine whether there is a statistically significant difference in aggressive behavior during physical restraint for cage change and intraspecific aggressive behavior after cage transfer.
The comparison of aggressive behavior during physical restraint for cage change and intraspecific aggressive behavior after cage transfer between the groups of hamsters was performed using the Dwass–Steel–Critcholw–Fligner pairwise comparisons test (non-parametric, 5% significance level). All analyses were performed using the software jamovi (Version 1.6).
Results
In group 1 (4 cages with 4 group-housed males each, 6 to 8 wk of age), one hamster showed aggressive behavior during physical restraint for cage change during the first week. No aggressive behavior was observed in the other weeks. After cage transfer, intraspecific aggression was for up to 20 min in all 4 weeks.
In group 2 (4 cages with 4 group-housed females each, 6 to 8 wk of age), 14 hamsters showed aggressive behavior during physical restraint for cage change in the first week, 13 in the second week, and 16 in the third and fourth weeks. After cage transfer, intraspecific aggression was observed for up to 80 min in all 4 weeks.
In group 3 (4 cages with 4 group-housed females each, 3 to 4 wk of age (immediately after weaning), 11 hamsters showed aggressive behavior during physical restraint for cage change in the first week, 6 in the second and third weeks, and 7 in the fourth week. After cage transfer, intraspecific aggression was observed for up to 60 min in the first week and for up to 40 min in the second, third and fourth weeks.
In group 4 (16 females housed one per cage, 6 to 8 wk of age), one hamster showed aggressive behavior during physical restraint for cage change during the second week. Aggressive behavior was not observed in the other weeks. Intraspecific interaction was not possible for this group because the hamsters were housed individually.
The Kruskall–Wallis test (P ` 0.001) showed a statistically significant difference in aggressive behavior during physical restraint for cage change and intraspecific aggressive behavior after cage transfer of the animals in the groups studied.
The Dwass–Steel–Critcholw–Fligner was used to perform pairwise comparisons between groups. Groups 1 and 4 showed less aggression during restraint than did group 2 (P ` 0.001) (Figure 2). Groups 1 and 4 showed less aggression during restraint (P ` 0.017) than did group 3. (Figure 2). Finally, group 1 had a shorter period of intraspecific aggressive behavior after cage transfer than did groups 2 and 3 (P ` 0.001) (Figure 3).
Figure 2.
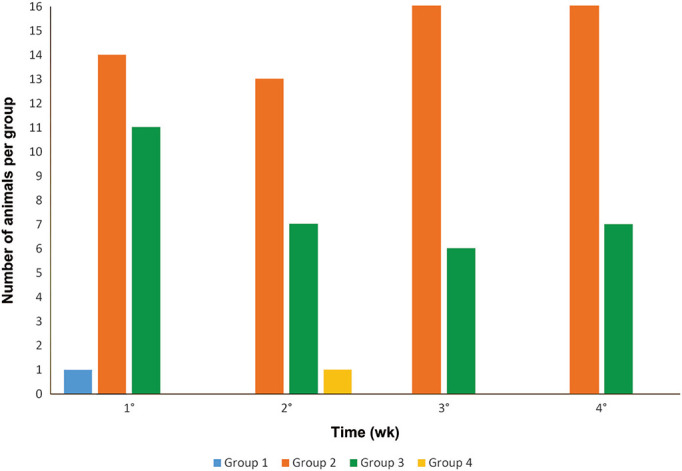
Number of aggressive hamsters during handling in groups 1, 2, 3 and 4 over a period of 4 wk.
Figure 3.
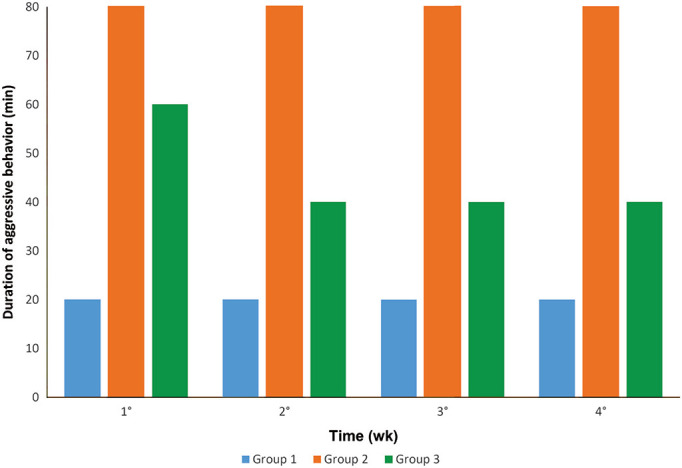
Duration of intraspecific aggression after cage change in groups 1, 2 and 3 over a period of 4 wk.
Discussion
Syrian hamsters (Mesocricetus auratus) are territorial and solitary animasl1,2 that exhibit aggressive behavior when housed with other hamsters.3,11 As observed in the present study, maintaining one female animal per cage will reduce the aggressiveness of hamsters during handling. We housed hamsters in groups with a maximum of 4 animals (80 to 120g per cage in cages measuring 490 mm × 340 mm × 160 mm) because this number is stated in Normative Resolution Number 15 of CONCEA4 for this cage size. Groups of 2 or 3 hamsters were not tested because having one or 2 fewer hamsters in the cage was did not reduce aggression.1
Previous studies have reported that male hamsters are less aggressive than female hamsters.1,2,11 In the present study, group-housed females were more aggressive (intraspecific aggression or aggression during handling) than males, regardless of age, and individually housed females were less aggressive than females housed in groups of 4 per cage. We therefore suggest individual housing of females used for experimentation, assuming this is feasible depending on available space in the animal facility.
Our data showed that group-housed males achieved a non-aggressive level of interaction, such that individual housing of males was not necessary, although it could be optimal given the natural behavior of hamsters.3 For this reason, the use of males might be more suitable for experiments in which stress could compromise the research outcomes or interpretation or if the research itself would cause substantial stress to hamsters In addition, good handling practices and separating experimental hamsters with respect to age and sex can reduce aggression among hamsters and between hamsters and the handler. The choice of the age, sex, and number of animals per cage depends on factors related to the research and space availability in the animal facility.
Maintaining best practices of animal handling will improve both research outcomes and animal welfare. As best practices, we suggest that male hamsters be housed in groups of 4 per cage and females housed individually. These conditions reduce the risk of injury during handling and avoid distress and consequent physiologic changes in the hamsters. Adapting housing conditions to mimic the natural condition of free-living animals and allow the manifestation of natural behaviors will improve both research outcomes and animal wellbeing.6,9,10
References
- 1. Andrad A, Pinto SC, Oliveira RS. [Internet]. 2002. Animais de Laboratório: criação e experimentação. Rio de Janeiro: Editora FIOCRUZ, 388 p. ISBN: 85-7541-015-6. SciELO Books. [Cited 08 November 2020]. Available at: https://static.scielo.org/scielobooks/sfwtj/pdf/andrade-9788575413869.pdf
- 2. Carissimi AS, Bernardino Merusse JL. 2009. Inter-reacao do Desenho Arquitetonico. p 73– 81. In: Lapchik VBV, Mattaraia VGM, KO GM, editors. Cuidados e manejos de animais de laboratório. São Paulo: Atheneu. [Google Scholar]
- 3. Cervantes MC, Delville Y. 2007. Individual differences in offensive aggression in golden hamsters: A model of reactive and impulsive aggression? Neuroscience 150: 511– 521. 10.1016/j.neuroscience.2007.09.034. PubMed [DOI] [PubMed] [Google Scholar]
- 4. Conselho Nacional de Controle da Experimentação Animal – CONCEA. [Internet]. 2013. Resolução Normativa n° 15 de 16 de dezembro de 2013. [Cited 17 November 2020]. Available at: https://portal.anhembi.br/wp-content/uploads/2019/10/Resolu%C3%A7%C3%A3o-Normativa-N%C2%BA-15-de-16-de-dezembro-de-2013.pdf.
- 5. Conselho Nacional de Controle da Experimentação Animal – CONCEA. [Internet]. 2016. Resolução Normativa n° 33 de 18 de novembro de 2016. [Cited 05 December 2020]. Available at: https://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/22073702/do1-2016-11-21-resolucao-normativa-n-33-de-18-de-novembro-de-2016-22073453
- 6. Fischer ML, Aguero WP, Rodrigues GS, Simao-Silva DP, Moser AM. 2016. Enriquecimento ambiental como princípio ético nas pesquisas com animais. Revista Bioetica 24: 532– 541. 10.1590/1983-80422016243153. [DOI] [Google Scholar]
- 7. Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 8. Lapchik VBV, Mattarala VGM, Ko GM. 2009. Cuidados e Manejos de Animais de Laboratório. São Paulo: Atheneu Editora. Bibliografia; ISBN 978-85-388-0075-0. [Google Scholar]
- 9. Low DD, Peng JZL, Tay YQ, Pang J. 2019. Playtime! Exploring habituation techniques in laboratory rats. Lab Animal Sci Prof Dec: 42– 45. [Google Scholar]
- 10. Paixão RL, Schramm FR. 1999. Ética e experimentação animal: o que é debatido? Caderno de Saúde Pública, Rio de Janeiro 15 Suppl 1: 99– 110. 10.1590/S0102-311X1999000500011. [DOI] [PubMed] [Google Scholar]
- 11. Taravosh-Lahn K, Delville Y. 2004. Aggressive behavior in female golden hamsters: development and the effect of repeated social stress. Horm Behav 46: 428– 435. 10.1016/j.yhbeh.2004.03.007. [DOI] [PubMed] [Google Scholar]


