Abstract
Introduction:
Scar theories propose that elevated depression and anxiety can predispose people to future decreased executive function (EF) via heightened inflammation across decades. However, more longitudinal (versus cross-sectional) research on this topic is needed.
Objective:
We thus investigated if increased major depressive disorder (MDD), generalized anxiety disorder (GAD), and panic disorder (PD) severity predicted EF decrement 18 years later via heightened inflammation.
Method:
Community-dwelling adults participated in this study. Time 1 (T1) MDD, GAD, and PD severity (Composite International Diagnostic Interview–Short Form), T2 inflammation (interleukin-6, C-reactive protein, and fibrinogen blood levels concentration), and T2 and T3 EF (Brief Test of Adult Cognition by Telephone) were measured. The waves of assessment were spaced approximately 9 years apart. Structural equation modeling was conducted.
Results:
Higher T1 MDD and GAD (but not PD) severity forecasted elevated T2 inflammation (Cohen’s d = 0.116–0.758). Greater T2 inflammation level predicted lower T3 EF following 9 years (d = −0.782–−0.636). The T1 MDD–T3 EF and T1 GAD–T3 EF negative associations were mediated by T2 inflammation, and explained 38% and 19% of the relations, respectively. Direct effects of higher T1 GAD and MDD predicting lower T3 EF were also observed (d = −0.585–−0.560). Significant effects remained after controlling for socio-demographic, lifestyle, medication use, various illness variables across time, and T2 EF.
Conclusions:
Inflammation may be a mechanism explaining the T1 MDD–T3 EF and T1 GAD–T3 EF relations. Treatments that target inflammation, worry, and/or depression may prevent future EF decline.
Keywords: executive functioning, affective neuroscience, worry, generalized anxiety disorder, panic disorder, major depressive disorder, longitudinal, mediation
Executive function (EF) is a set of complex multidimensional top-down mental control systems active in governing myriad behavioral and cognitive processes, such as learning, recalling facts, evaluating, decision-making, and risk-taking (Diamond, 2013). We thus depend on our frontoparietal cortices-mediated EF capacities to navigate the challenges and opportunities of daily life. EF deficits have been related consistently to problems with relationships, career attainment, weight management, emotion regulation, as well as physical and mental health (Schweizer et al., 2019). This is likely because global EF and its subdomains (e.g., working memory, shifting, inhibition) (Miyake & Friedman, 2012) are intrinsically linked to important social behaviors and cognitive functioning (Yan, Hong, Liu, & Su, 2020). Therefore, understanding risk factors for EF deficits is essential.
Scar theories propose that increased psychiatric disorder severity predicts future poorer EF via elevated allostatic load, referred to as buildup of stress-related wear-and-tear of hypothalamic-pituitary-adrenal (HPA) axis and related biological systems (Majd, Saunders, & Engeland, 2020). Inflammatory allostatic load can be defined as heightened bloodstream levels of markers of inflammatory activity, such as circulating C-reactive protein (CRP), fibrinogen, and interleukin-6 (IL-6) (McEwen & Gianaros, 2011). One key inflammatory marker is IL-6, a proinflammatory cytokine emitted by non-immune cells, specific white blood cells (macrophages, monocytes), and T-cells (Rose-John, 2018). Moreover, IL-6 spurs the production of two acute-phase inflammatory markers in the liver and related organs. This includes CRP, which refers to complex proteins created by bodily injury, infection, trauma, or advanced cancer (Wu, Potempa, El Kebir, & Filep, 2015). Another pro-inflammatory cytokine synthesized by IL-6 in the liver is fibrinogen, which is a glycoprotein that synthesizes fibrin in the liver and is involved in blood coagulation wherein excessive amounts can reflect vascular endothelial dysfunction (Mosesson, 2005). IL-6, CRP, and fibrinogen have been shown to form one latent construct of inflammatory allostatic load (Hostinar, Lachman, Mroczek, Seeman, & Miller, 2015). Further, scar models assert that higher depression and anxiety symptom severity increase future levels of IL-6, CRP, and fibrinogen as well as forecast future cognitive decline through months and decades of excessive glucocorticoid resistance and cortisol accumulation in the HPA (Kiecolt-Glaser, Derry, & Fagundes, 2015). Collectively, these frameworks propose that greater psychiatric symptoms predict subsequent heightened inflammation and reduced EF across protracted timescales.
Supporting scar theories, myriad data showed that increased repetitive negative thinking, anxiety, and depression predicted EF and related cognitive deficits over relatively long durations. For instance, upsurge in excessive worry and trait negative affect forecasted later decline in EF facets and processing speed in community mid-life adults across 9 to 23 years (Zainal & Newman, 2020; Zainal & Newman, 2021b). Taken together, higher depression and anxiety severity could predict subsequent impairments in EF across decades.
Twelve studies thus far have tested if heightened depression and anxiety forecasted increased inflammation across comparatively long periods. Consistent with scar models, greater subjective stress was linked to increased IL-6 level following 6 years in dementia caregivers (Kiecolt-Glaser et al., 2003). Similarly, multi-ethnic premenopausal women with higher depression severity displayed steeper 5-year rise in fibrinogen (Matthews et al., 2007). Similar results occurred in other community-dwelling populations. For example, elevated depression predicted 5- to 12-year increase in IL-6 or CRP serum levels in relatively healthy, African American and White Caucasian young, middle-aged, and older men and women (Deverts et al., 2010; Luciano et al., 2012; Stewart, Rand, Muldoon, & Kamarck, 2009). Replicating these findings, racially diverse middle-aged women with more self-reported depression showed higher CRP levels 7 years later (Matthews et al., 2010). These results also extend to anxiety disorders, wherein mid-life adults with increased generalized anxiety disorder (GAD), panic disorder (PD), and post-traumatic stress showed larger rise in high-sensitivity CRP after approximately 5 to 16 years (Copeland, Shanahan, Worthman, Angold, & Costello, 2012; Glaus et al., 2018; Sumner et al., 2017). Moreover, among children and adolescents, greater initial depressive symptoms predicted higher future IL-6 serum across 20 weeks to 3 years in three unique studies (see recent meta-analysis by Colasanto, Madigan, & Korczak, 2020). Based on this evidence, heightened common psychiatric disorder severity would likely relate to greater future inflammation.
To date, 15 studies have determined if larger inflammation levels predicted subsequent worsening of EF and related abilities over lengthy durations. Lending credence to another scar theory tenet, data from 13 studies across 7 nations in North America, Europe, and Asia showed that elevated IL-6, CRP, and fibrinogen were related to future all-cause dementia (Darweesh et al., 2018), as long as 25 years later (Schmidt et al., 2002). Likewise, high (vs. low) CRP or IL-6 serum concentrations were associated with weaker EF, general cognitive ability, executive attention, memory, or orienting following 3 to 10 years among youths and older adults (Mac Giollabhui et al., 2020; Zheng & Xie, 2018). Therefore, it is plausible that more circulating inflammatory markers (i.e., latent variable composite of IL-6, CRP, and fibrinogen levels) would precede and forecast reduced EF a decade later.
On that account, we aimed to test if higher major depressive disorder (MDD), GAD, and PD severity would predict poorer future global EF via greater inflammation. This objective is important for several reasons. Globally, many nations are increasingly facing social, financial, and personal struggles linked to growing life expectancy and rising prevalence of major neurocognitive disorders and other inflammation-related psychiatric diseases (Foreman et al., 2018). Better comprehension of the risk factors of heightened inflammation and EF decline can thus guide the development and refinement of empirically-supported treatments. Moreover, unlike most previous research, we used a latent structural equation modeling (SEM) (vs. manifest regression) approach which minimizes measurement error (Tomarken & Waller, 2005). Further, we examined markers of inflammatory activity (CRP, fibrinogen, IL-6) that have been understudied in GAD and PD relative to other HPA indices (e.g., cortisol, adrenocorticotropic hormone) (Daniels, Olsen, & Tyrka, 2020). Fibrinogen, CRP, and IL-6, were of key interest as they have been extensively theorized to coincide with elevated depression and anxiety severity over time (Kop et al., 2010; Whooley et al., 2007). Also, our prospective dataset builds on cross-sectional studies on the relations among common psychiatric symptoms, EF, and inflammation (e.g., refer to systematic reviews and meta-analyses by Colasanto et al., 2020; Smith, Au, Ollis, & Schmitz, 2018; Snyder, 2013; Snyder, Miyake, & Hankin, 2015). Therefore, it moves us closer to understanding potential cause-effect associations. Based on scar models and data, we hypothesized that higher MDD, GAD, and PD severity would uniquely predict heightened inflammation (IL-6, CRP, and fibrinogen levels latent composite) 9 years later. Moreover, we predicted that increased inflammation would lead to a subsequent reduced latent EF composite following 9 years. Further, we explored if support for these hypotheses would remain above and beyond demographic, socio-economic, medical illnesses, lifestyle, and medication use variables.
Method
Participants
This study used the publicly accessible MIDUS dataset comprising three waves: 1995 (Time 1; T1); 2004 (Time 2; T2; 9 years after T1); and 2013 (Time 3; T3; 18 years after T1 and 9 years after T2) (details about the original study can be found in Brim et al., 2019; Love, Seeman, Weinstein, & Ryff, 2010; Ryff & Lachman, 2018, 2019; Ryff, Seeman, & Weinstein, 2019; Ryff et al., 2017). At T1, participants (n = 945) averaged 45.27 years (SD = 11.41, range = 25 to 74), 55.56% were female, and 20.42% had college education. Also, they were mostly White (91.11%), while the remaining 8.89% were African American, Asian, Native American, or Pacific Islander. Table 1 presents the statistics and correlation matrix of the study variables.
Table 1.
Descriptive Statistics and Correlation Matrix of Study Variables in the Primary Models
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 1. Age | – | ||||||||||||
| 2. Female | −0.03 | – | |||||||||||
| 3. T1 MDD | −.094*** | .131*** | – | ||||||||||
| 4. T1 GAD | −.110*** | .125*** | .440*** | – | |||||||||
| 5. T1 PD | −.086** | .126*** | .315*** | .306*** | – | ||||||||
| 6. T2 IL-6 | .188*** | .039 | .070* | .037 | −.005 | ||||||||
| 7. T2 CRP | .039 | .152*** | .113*** | .071* | .019 | .493*** | – | ||||||
| 8. T2 FGN | .117*** | .143*** | .090** | .006 | .032 | .342*** | .442*** | – | |||||
| 9. T3 VF | −.282*** | −.031 | −.017 | −.052 | −.030 | −.113*** | −.099*** | −.079* | – | ||||
| 10. T3 NS | −.258*** | −.097** | −.078* | −.071* | −.087* | −.097*** | −.107*** | −.112*** | .252*** | – | |||
| 11. T3 BC | −.413*** | −.147*** | −.074* | −.071* | −.021 | −.088* | −.064 | −.100** | .385*** | .446*** | – | ||
| 12. T3 SGST | −.126*** | −.009 | −.040 | .041 | .034 | −.027 | −.058 | −.032 | .174*** | .055 | .139*** | – | |
| 13. T3 DBS | −.214*** | .018 | −.035 | −.031 | −.061 | −.027 | −.032 | −.062 | .193*** | .272*** | .302*** | .131*** | – |
|
| |||||||||||||
| M or n | 45.27 | 525 | 0.63 | 0.98 | 0.73 | 0.66 | 0.32 | 5.79 | 19.42 | 2.50 | 37.45 | 27.41 | 5.08 |
| SD or % | 11.10 | 55.56 | 1.16 | 1.74 | 1.70 | 0.43 | 0.76 | 0.26 | 5.87 | 1.64 | 11.43 | 3.69 | 1.46 |
| Min | 25.00 | – | 0.00 | 0 | 0.00 | 0.08 | −0.65 | 3.81 | 3.00 | 0.00 | 3.00 | 0.00 | 0.00 |
| Max | 74.00 | – | 7.00 | 9.00 | 8.00 | 1.23 | 1.34 | 6.60 | 4.00 | 8.00 | 83.00 | 29.00 | 8.00 |
| Skewness | 0.26 | −0.22 | 1.76 | 2.25 | 2.17 | −0.02 | .09 | −1.11 | 0.43 | 0.43 | 0.32 | −5.23 | 0.17 |
| Kurtosis | −0.63 | −1.95 | 1.65 | 5.37 | 3.48 | −1.48 | −1.51 | 5.49 | 0.34 | −0.07 | 0.59 | 33.19 | −0.16 |
Note.
p ≤ .001
p ≤ .05.
M=mean; SD=standard deviation; MDD=major depressive disorder severity; GAD=generalized anxiety disorder severity; PD=panic disorder severity; IL-6=interleukin-6; CRP=C-reactive protein; FGN=fibrinogen; VF=verbal fluency; NS=number series; BC=backward counting; SGST=stop-and-go-switch task mixed task; DBS=digit backward span. Inflammation serum levels have been log transformed to achieve univariate normal distributions of the data.
Measures
The present investigation focused on participants who consented to complete the in-person clinical interview, cognitive testing, and biomarker data collection (Love et al., 2010). Note also that GAD, MDD, and PD symptom severity were assessed at T1, T2, and T3, inflammation was measured at T2, and a performance-based EF test was administered at T2 and T3. Inflammation and EF were not measured at T1.
T1, T2, and T3 Psychiatric Disorder Severity.
The Diagnostic and Statical Manual–Third Edition–Revised (DSM-III-R)–consistent Composite International Diagnostic Interview–Short Form (CIDI-SF) (American Psychiatric Association, 1987; Kessler, Andrews, Mroczek, Ustun, & Wittchen, 1998; Wittchen, Zhao, Kessler, & Eaton, 1994) was used to measure the summed total symptom severity score for MDD, GAD, and PD separately. For MDD severity, participants reported the extent to which they experienced MDD symptoms in the past 12 months for at least two weeks (7-item; depressed mood, loss of interest or pleasure in most things, appetite changes, fatigue, sleep disturbances, suicidal ideation, worthlessness). Participants indicated ‘Yes’ (coded as ‘1’) or ‘No’ (coded as ‘0’) to presence of each symptom. For GAD severity, respondents endorsed the degree to which they experienced a series of symptoms due to their excessive worries for about half the days or most days during the past year (9-item; i.e., excessive worry, uncontrollable worry, fatigue, feeling keyed up, issues falling asleep, issues staying asleep, irritability, lassitude, muscle tension, restlessness) on a 4-point Likert scale (0 = never to 1 = sometimes or often) for each item. For PD severity, respondents disclosed past-year encounters with panic symptoms (8-item; i.e., unexpected panic attacks in situations most people would not feel anxious, shortness of breath, chest or stomach tightness, pain or discomfort, hot flashes or chills, heart palpitations, sweating, trembling or shaking) by answering ‘Yes’ (‘1’) or ‘No’ (‘0’) for each item. In addition, a minimum of three symptoms needed to occur concurrently to constitute a panic attack; a necessary yet insufficient condition of a PD diagnosis in addition to unanticipated panic attacks. For each symptom measure, a composite symptom severity score was calculated by summing responses to all items. The CIDI-SF MDD, GAD, and PD severity scales have shown good internal consistency (αs = .941–.982 herein), strong retest-reliability, and excellent sensitivity (89.6–96.6%) and specificity (93.9–99.8%) (Kessler et al., 1998).
T2 Markers of Inflammatory Activity.
After fasting overnight, participants provided inflammation assays based on a standard protocol (Love et al., 2010). The researchers froze the samples at −60° to −80°C with dry ice while transporting them to the laboratory, where they were stored at −65°C for monthly batch evaluation to guarantee uniformity across data collection sites (Ryff et al., 2019). IL-6 was assessed from blood serum via enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) (Friedman & Herd, 2010). Fibrinogen was measured from serum on a BNII nephelometer with a partially automated and adapted Claus approach (Clauss, 1957), and CRP was quantified via a particle-enhanced immunonephelometric test (Dade Behring Inc., Deerfield, IL) (Friedman & Herd, 2010). All inflammation level values were calculated in duplicate; any assays more than 10 pg/mL were rerun in diluted sera to fall within the normal distribution (Morozink, Friedman, Coe, & Ryff, 2010). The coefficients of variance between- and within-laboratories for all protein markers were within acceptable limits (4.09–12.30%) (Boylan, Cundiff, Fuller-Rowell, & Ryff, 2020). To create a latent T2 inflammation variable, we treated each of the three IL-6, CRP, and fibrinogen levels as manifest indicators in a confirmatory factor analysis (CFA). This SEM approach is statistically recommended as it minimizes measurement error and increases power (Tomarken & Waller, 2005). Table 1 shows that the biomarkers were significantly moderately correlated with each other (rs = .342–.493).
T2 and T3 Executive Functioning.
At T2 and T3, but not T1, EF was assessed with the Brief Test of Adult Cognition by Telephone (BTACT) (Lachman, Agrigoroaei, Tun, & Weaver, 2014) using five indices: (1) Backward Digit Span (tracking and recalling number sequences of increasing length); (2) Category Verbal Fluency (naming as many unique animals or food in 1 minute); (3) Number Series (identifying a pattern and completing a number sequence with the last digit); (4) 30-Seconds and Counting Task (counting as many digits backwards from 100 in 30 seconds); and (5) Stop-and-Go Switch Task (SGST; inhibition and shifting subtests comprising alternating blocks of normal and reverse conditions). Specifically, across 32 SGST trials, participants needed to respond as swiftly and accurately to random changes in normal and reverse conditions within 2 to 6 trials depending on the signs ‘NORMAL’ and ‘REVERSE’ (details can be found in Lachman et al., 2014). These BTACT EF subtests have shown good four-week retest reliability (r = .82–.83) (Lachman et al., 2014), as well as sufficient convergent validity (rs = .41–.52 with different EF measures) and discriminant validity (rs = .16–.17 with memory assessments) (Lachman et al., 2014). A latent EF construct was created by using these 5 manifest indicators as suggested by a prior psychometric validation study (Lachman et al., 2014).
Potential Covariates Measured at T1, T2, and/or T3.
Table 2 presents the descriptive data of potential covariates across all time-points. Based on the literature (Beydoun et al., 2019; Eyre & Baune, 2012; Friedman & Herd, 2010; Spyridaki, Avgoustinaki, & Margioris, 2016), we adjusted for these covariates in our mediation models: T1 age, gender (male vs. female), T1 household total income (from wage, pension, social security, and other sources), T1 tertiary education status, T2 EF, as well as T1, T2, and T3 number of medical illnesses (past-year diseases related to asthma, tuberculosis, lung, bones, backache, skin, thyroid, hay fever, stomach, bladder, constipation, gall bladder, foot, varicose veins, AIDS/HIV, lupus, gum/mouth, hypertension, alcohol/drug, migraine, chronic sleep, diabetes, neurological disorders, stroke, ulcer, hernia, piles/hemorrhoids, swallowing, itch, dry/sore skin, scaly skin, hand rash, pimples, face rash, warts, sweating, and hair loss), body mass index (BMI) (kg/m2), smoking status, exercise habits (presence of exercise at least 20 minutes 3 times/week), and prescription medication use (for anxiety, depression, arthritis, birth control, cardiovascular disease, diabetes, headaches, hormone replacement, hyperlipidemia, hypertension, lung issues, or ulcers). Also, based on the literature on pseudodementia (Brodaty & Connors, 2020; Pozzoli, De Carlo, & Madonna, 2019), T1 comorbid MDD, GAD, and PD severity and T2, and T3 MDD, GAD, and PD severity were adjusted for as covariates.
Table 2.
Descriptive Statistics of Covariates
| Continuous Variables | M | SD | Skewness | Kurtosis | Min | Max |
|
| ||||||
| T1 Body mass index | 26.43 | 4.94 | 0.69 | 1.97 | 12.26 | 51.60 |
| T2 Body mass index | 27.80 | 4.94 | 0.88 | 1.54 | 14.23 | 54.91 |
| T3 Body mass index | 28.14 | 5.93 | 1.13 | 1.94 | 16.14 | 56.82 |
| T1 Chronic illnesses | 2.16 | 2.19 | 1.34 | 1.86 | 0.00 | 12.00 |
| T2 Chronic illnesses | 2.17 | 2.14 | 1.60 | 4.11 | 0.00 | 16.00 |
| T3 Chronic illnesses | 2.89 | 3.04 | 1.51 | 2.63 | 0.00 | 20.00 |
| T2 MDD Severity | 0.50 | 1.06 | 2.05 | 2.83 | 0.00 | 4.00 |
| T3 MDD Severity | 0.48 | 1.04 | 2.15 | 3.27 | 0.00 | 4.00 |
| T2 GAD Severity | 0.76 | 1.57 | 2.57 | 6.95 | 0.00 | 9.00 |
| T3 GAD Severity | 0.75 | 1.58 | 2.63 | 7.35 | 0.00 | 9.00 |
| T2 PD Severity | 0.61 | 1.57 | 2.62 | 5.99 | 0.00 | 8.00 |
| T3 PD Severity | 0.59 | 1.53 | 2.70 | 6.71 | 0.00 | 8.00 |
| T2 EF composite | 0.24 | 0.87 | 0.10 | −0.09 | −2.41 | 2.92 |
| T1 Household income ($) | 82,356 | 60,065 | 1.35 | 1.82 | 0.00 | 300000.00 |
|
| ||||||
| Categorical Variables | n | % | Skewness | Kurtosis | Min | Max |
|
| ||||||
| T1 Tertiary education | 688 | 72.80 | −1.06 | −0.78 | 0.00 | 1.00 |
| T1 Chronic illnesses (≥ 2) | 481 | 50.90 | −0.04 | −2.00 | 0.00 | 1.00 |
| T2 Chronic illnesses (≥ 2) | 515 | 54.50 | −0.18 | −1.97 | 0.00 | 1.00 |
| T3 Chronic illnesses (≥ 2) | 56c | 60.11 | −0.41 | −1.83 | 0.00 | 1.00 |
| T1 Regular exercise | 74 c | 78.41 | −1.38 | 0.09 | 0.00 | 1.00 |
| T2 Regular exercise | 755 | 79.89 | −1.49 | 0.23 | 0.00 | 1.00 |
| T3 Regular exercise | 759 | 80.32 | −1.53 | 0.33 | 0.00 | 1.00 |
| T1 Smoking status | 416 | 44.02 | 0.24 | −1.95 | 0.00 | 1.00 |
| T2 Smoking tatus | 414 | 43.81 | 0.25 | −1.94 | 0.00 | 1.00 |
| T3 Smoking status | 400 | 42.33 | 0.31 | −1.91 | 0.00 | 1.00 |
| T1 Medication use | 367 | 38.84 | 0.45 | −1.79 | 0.00 | 1.00 |
| T2 Medication use | 574 | 60.74 | −0.45 | −1.81 | 0.00 | 1.00 |
| T3 Medication use | 605 | 64.02 | −0.59 | −1.66 | 0.00 | 1.00 |
Note. EF=executive functioning; GAD=generalized anxiety disorder symptom severity; MDD=major depressive disorder symptom severity; Max=maximum; Min=minimum; T1=time 1; T2=time 2; T3=time 3. Regular exercise referred to engagement in physical workouts for at least 20 mins, 3 times/week.
Data Analyses
We performed SEM CFA and mediation analyses with the lavaan package (Rosseel, 2012) using RStudio software (Version 1.3.959). To evaluate the fit of our models, we utilized the χ2 goodness-of-fit statistic as well as practical fit indices i.e., confirmatory fit index (CFI; Bentler, 1990) and root mean square error of approximation (RMSEA; Steiger, 1990).
Mediation analyses were conducted via a product-of-coefficients approach of the indirect effects (a × b) of the regression coefficients of T1 MDD, GAD, or PD severity forecasting T2 inflammation (a path), and T2 inflammation predicting T3 EF (b path), above and beyond the direct effect (c’ path or T1 symptom severity–T3 EF association). We presented the regression coefficients (β) and 95% confidence intervals (CI) as well as used bootstrapping with 10,000 resampling draws (Cheung & Lau, 2008). The mediation effect size is the ratio of the indirect effect (a*b) to the total effect, c = a*b + c’, expressed in percentage of variance that the T2 inflammation mediator accounted for the T1 psychiatric disorder symptom severity–T3 EF relation (Cheung & Lau, 2008; Preacher & Kelley, 2011; Wen & Fan, 2015). In all of our analyses, we removed the following items that may function as confounders to test the independent effect of T1 MDD and GAD severity on T3 EF, mediated via T2 inflammation: (a) “have a lot more trouble concentrating than usual” from the MDD composite; (b) “trouble concentrating due to worry” and “trouble remembering due to worry” from the GAD composite.
In total, there were 3.31% missing data points in the current study. Missing data were handled with full information maximum likelihood (FIML). FIML (vs. listwise deletion) was apt as it uses all available data to compute model parameters and because the data was missing at random (Graham, 2009) (Little’s MCAR test: χ2(12) = 34.91, p = .14). Cohen’s d effect size was calculated using the formula d = 2t /√(df) (Dunlap, Cortina, Vaslow, & Burke, 1996; Dunst, Hamby, & Trivette, 2004; Lakens, 2013). Thus, d values of 0.2, 0.5, and 0.8, represent small, moderate, and large effect sizes, respectively (Cohen, 1988). Also, given multiple comparisons, we applied Simes Bonferroni correction approach to guard against Type I error (Simes, 1986).
Power Analysis
We conducted an a priori Monte Carlo power analysis (Arnold, Hogan, Colford, & Hubbard, 2011) to determine if our sample was adequately powered to detect a conservative effect size estimate for d = 0.15 for the a, b, and c’ paths. Analyses revealed 98.2–100.0% power to identify significant direct and indirect effects. Thus, our sample was sufficiently powered to test the study hypotheses.
Results
Measurement Models
CFA suggested that the measurement models had excellent fit for separate models of T1 MDD, GAD, or PD as predictors (χ2(101–132) = 78.812–150.890, p = .125–.333, CFI = .995–.997, RMSEA = .008–.013). Statistically significant factor loadings (all ps < .001) were observed for the indicators of latent T1 MDD (βs = .646–.952), T1 GAD (βs = .605–.970), and T1 PD (βs = .477–.823), T2 inflammation (3-item; βs = .564–.773) and T3 EF (5-item; βs = .224–.699).
Direct Effects and Mediation Models
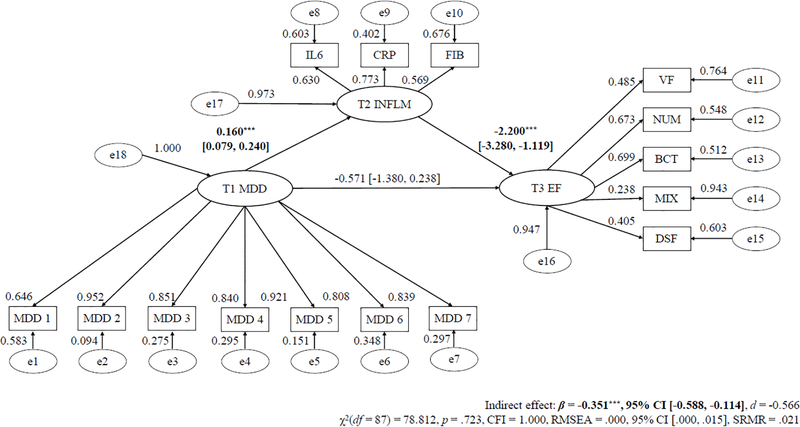
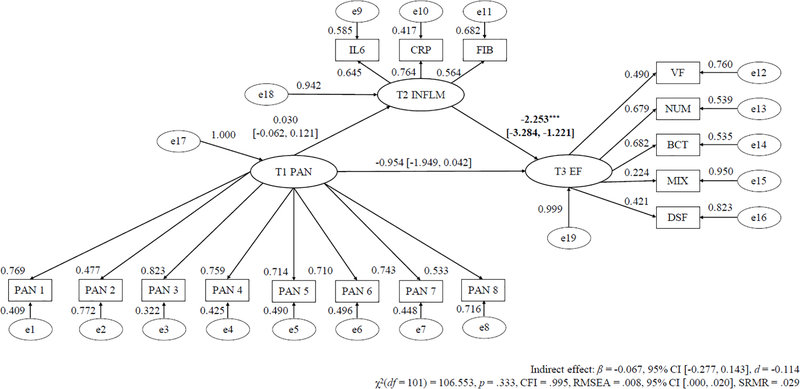
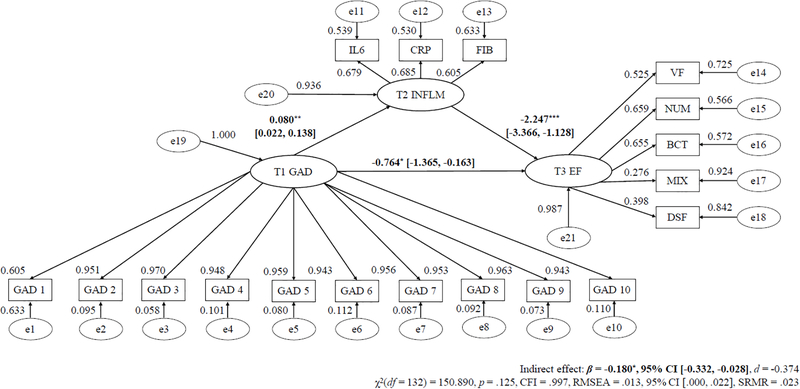
Tables 3 to 5 alongside Figures 1 to 3 display the mediation models for T1 symptom severity predicting T3 EF via T2 inflammation with the regression slope estimates for T1 MDD, GAD, and PD severity as the predictor, respectively.
Table 3.
MDD Severity Predicting T3 EF Via T2 Inflammation
| All Samples | β (SE) | p |
|---|---|---|
| Standardized Factor Loadings: Past Year MDD Severity | ||
| Depressed mood for at least 2 weeks | .646a | – |
| Lose interest in most things | .952*** (0.022) | < .001 |
| Feel more tired out or low on energy than is usual | .851*** (0.014) | < .001 |
| Lose your appetite or appetite increased | .840*** (0.028) | < .001 |
| Have more trouble falling asleep than usual | .921*** (0.026) | < .001 |
| Feel down on yourself, no good, or worthless | .808*** (0.029) | < .001 |
| Think a lot about death | .839*** (0.022) | < .001 |
|
| ||
| Standardized Factor Loadings: Inflammatory Markers | ||
| Interleukin-6 | .630a | – |
| C-Reactive Protein | .773*** (0.200) | < .001 |
| Fibrinogen | .569*** (0.051) | < .001 |
|
| ||
| Standardized Factor Loadings: EF Composite | ||
| Category Fluency | .485a | – |
| Number Series | .673*** (0.044) | < .001 |
| Backward Counting | .699*** (0.319) | < .001 |
| Stop-and-Go Signal Mixed Task | .238*** (0.053) | < .001 |
| Digit Backward Span | .405*** (0.027) | < .001 |
|
| ||
| Unstandardized Regression Slopes | ||
| T1 MDD → T2 Inflammation (a path) | 0.160*** (0.041) | < .001 |
| T2 Inflammation → T3 EF (b path) | −2.200*** (0.551) | < .001 |
| T1 MDD → T2 Inflammation → T3 EF (c path) | −0.351** (0.121) | .004 |
| Total effect | −0.922* (0.404) | .023 |
Note.
p < .001
p < .01
p < .05.
Model Fit Indices – χ2(87)=78.812, p=.723, CFI=1.000, RMSEA=.000. CFI=confirmatory fit index; β=Regression weight estimate; EF=executive functioning; MDD=major depressive disorder symptom severity; RMSEA=root mean square error of approximation; SE=standard error.
Unit loading identification was used in the confirmatory factor analysis portion of the structural equation model.
Table 5.
Mediation Model of T1 PD Severity Predicting T3 EF Via T2 Inflammation
| All Samples | β (SE) | p |
|---|---|---|
| Standardized Factor Loadings: Past Year PD Severity | ||
| Sudden panic spell or attack in unlikely places | 0.769a | – |
| Panic occurred in danger or during center of attention | 0.477*** (0.073) | < .001 |
| Heart pound during panic attack | 0.823*** (0.082) | < .001 |
| Chest or stomach pain during panic attack | 0.759*** (0.089) | < .001 |
| Sweat during panic attack | 0.714*** (0.080) | < .001 |
| Tremble during panic attack | 0.710*** (0.080) | < .001 |
| Hot flashes during panic attack | 0.743*** (0.081) | < .001 |
| Things seem unreal during panic attack | 0.533*** (0.063) | < .001 |
|
| ||
| Standardized Factor Loadings: Inflammatory Markers | ||
| Interleukin-6 | 0.645a | − |
| C-Reactive Protein | 0.764*** (0.168) | < .001 |
| Fibrinogen | 0.564*** (0.044) | < .001 |
|
| ||
| Standardized Factor Loadings: EF Componte | ||
| Category Fluency | 0.490a | − |
| Number Series | 0.679*** (0.039) | < .001 |
| Backward Counting | 0.682*** (0.293) | < .001 |
| Stop-and-Go Signal Mixed Task | 0.224*** (0.048) | < .001 |
| Digit Backward Span | 0.421*** (0.027) | < .001 |
|
| ||
| Unstandardized Regression Slopes | ||
| T1 PD → T2 Inflammation (a path) | 0.030 (0.047) | .524 |
| T2 Inflammation → T3 EF (b path) | −2.253*** (0.526) | < .001 |
| T1 PD → T2 Inflammation → T3 EF (c path) | −0.067 (0.107) | .532 |
Note.
p < .001.
Model Fit Indices – χ2(101)=106.553, p=.333, CFI=.995, RMSEA=.008. CFI=confirmatory fit index; β=Regression weight estimate; PD=panic disorder symptom severity; RMSEA=root mean square error of approximation; SE=standard error.
Unit loading identification was used in the confirmatory factor analysis portion of the structural equation model.
Figure 1. Longitudinal SEM Mediation Path of Baseline MDD Severity Predicting Future Reduced Executive Functioning Via Inflammation.
Note. * p < .05;. * p < .01; *** p < .001.
λ = standardized factor loading; ε = item residual variance; ζ = factor residual variance; MDD = major depressive disorder; IL-6 = interleukin-6; CRP = C-reactive protein; FGN = fibrinogen; VF = verbal fluency; NS = number series; BC = backward counting; SGST = stop-and-go-switch task mixed task; BDS = backward digit span; SEM = structural equation modeling. Parameters a, b, and c refers to unstandardized regression coefficients.
Figure 3. Longitudinal SEM Mediation Path of Baseline PD Severity Predicting Future Reduced Executive Functioning Via Inflammation.
Note. * p < .05;. * p < .01; *** p < .001.
λ = standardized factor loading; ε = item residual variance; ζ = factor residual variance; PD = panic disorder; IL-6 = interleukin-6; CRP = C-reactive protein; FGN = fibrinogen; VF = verbal fluency; NS = number series; BC = backward counting; SGST = stop-and-go-switch task mixed task; BDS = backward digit span; SEM = structural equation modeling. Parameters a, b, and c refers to unstandardized regression coefficients.
T1 MDD Predicting T3 EF Via T2 Inflammation.
The direct effect only model (χ2(53) = 47.848, p = .647, CFI = 1.000, RMSEA = .000) and main effect mediation model (χ2(87) = 78.812, p = .723, CFI = 1.000, RMSEA = .000) had excellent fit. Higher T1 MDD severity was significantly related to lower T3 EF (direct effect; β = −0.904, 95% CI [−1.683, −0.125], d = −0.560).2 Additionally, elevated T1 MDD severity significantly predicted heightened T2 inflammation (β = 0.160, 95% CI [0.079, 0.240], d = 0.758), and increased T2 inflammation substantially forecasted lower T3 EF (β = −2.200, 95% CI [−3.280, −1.119], d = −0.779). The path of T1 MDD severity predicting lower T3 EF via T2 inflammation level was also significant (β = −0.351, 95% CI [−0.588, −0.114], d = −0.566). T2 inflammation level explained 38.069% of the T1 MDD symptom–T3 EF relation. Moreover, the mediation effect of T1 MDD severity predicting lower T3 EF via T2 inflammation level remained significant after controlling for age, gender, education, and ethnicity, T2 EF, T1, T2, and T3 BMI, physical exercise frequency, smoking status, number of chronic medical illnesses, and use of various prescription medications, as well as T1 GAD and PD severity and T2 and T3 MDD, GAD, and PD severity (d = −0.573 to −0.242).
T1 GAD Predicting T3 EF Via T2 Inflammation.
The direct effect only model (χ2(89) = 95.257, p = .306, CFI = .999, RMSEA = .009) and main effect mediation model (χ2(132) = 150.890, p = .132, CFI = .997, RMSEA = .013) showed good fit. Higher T1 GAD severity considerably forecasted lower T3 EF (β = −0.954, 95% CI [−1.579, −0.330], d = −0.585). Larger T1 GAD severity significantly predicted more T2 inflammation 9 years later (β = 0.080, 95% CI [0.022, 0.138], d = 0.438), and higher T2 inflammation level notably forecasted lower T3 EF following 9 years (β = −2.247, 95% CI [−3.366, −1.128], d = −0.636). Moreover, the mediation path of T1 GAD severity forecasting T3 EF via T2 inflammation was significant (β = −0.180, 95% CI [−0.332, −0.028], d = −0.374), such that T2 inflammation level accounted for 19.068% of the T1 GAD–T3 EF association. Further, the mediation path of T1 GAD symptoms predicting T3 EF via T2 inflammation remained statistically significant after adjusting for age, gender, ethnicity, T2 EF, T1, T2, and T3 BMI, physical exercise frequency, smoking status, number of chronic medical illnesses, and degree of prescription medication use, as well as T1 MDD and PD severity as well as T2 and T3 MDD, GAD, and PD symptoms (d = −0.377 to −0.179).
T1 PD Predicting T3 EF Via T2 Inflammation.
The mediation model (χ2(101) = 106.553, p = .333, CFI = .995, RMSEA = .008) and direct effect only model (χ2(64) = 72.114, p = .227, CFI = .990, RMSEA = .012) showed excellent fit. Higher T1 PD significantly forecasted lower T3 EF (β = −1.032, 95% CI [−2.013, −0.052], d = −0.467). However, T1 PD severity did not considerably predict T2 inflammation (β = 0.030, 95% CI [−0.062, 0.121], d = 0.116), but higher T2 inflammation substantially forecasted reduced T3 EF (β = −2.253, 95% CI [−3.284, −1.221], d = −0.782). Further, the mediation path of T1 PD severity forecasting T3 EF via T2 inflammation was not significant (β = −0.067, 95% CI [−0.277, 0.143], d = −0.114).3 Moreover, the mediation path of T1 PD symptoms forecasting T3 EF through T2 inflammation remained statistically non-significant when controlling for age, gender, and ethnicity, T2 EF, T1, T2, and T3 BMI, physical exercise frequency, smoking status, number of chronic medical illnesses, and use of diverse prescription medications, as well as T1 MDD and GAD severity, and T2 and T3 MDD, GAD, and PD severity (d = −0.184 to −0.059).
Discussion
Partially supporting scar models, these novel findings suggested that elevated inflammation may be a mechanism by which increased MDD and GAD (but not PD) symptom severity leads to future EF decline 18 years later in community adults. Thus, for young, mid-life, and older adults, greater MDD and GAD severity made them more likely to experience increased systemic inflammation, and higher plasma IL-6, CRP, and fibrinogen levels predicted lowered EF 9 years later (accounting for 38.069% and 19.068% of the T1 MDD–T3 EF and T1 GAD–T3 EF dimensional relations, respectively, across an 18-year duration). Noteworthy was that all significant mediation and direct effects were observed after adjusting for comorbid T1 MDD, GAD, and PD symptom severity, socio-demographic, lifestyle, BMI, chronic medical illness, and T2 EF variables that could have confounded the pattern of results. To our knowledge, the present study replicated and extended a study that similarly showed higher baseline MDD and GAD severity predicted EF deficits 20 months later via increased inflammation in another community-dwelling adult sample (Zainal & Newman, in press). We offer several potential theoretical accounts for these results.
Why did greater MDD and GAD severity predict increased inflammation? This may be explained by the fact that those with depression and pathological worry may be particularly susceptible to accrue such heightened inflammation. Across long durations, elevated MDD and GAD likely induced hyperactivation of the inflammatory structure of the central nervous system (CNS; Hanisch, 2002) laden with glia (e.g., astrocytes, ependymal cells, microglia, Neuron-glial antigen 2, oligodendrocytes) (Khandaker, Zammit, Lewis, & Jones, 2016; Sild, Ruthazer, & Booij, 2017). For example, unhealthy lifestyles, mindsets, and non-constructive repetitive negative thinking in MDD and GAD may trigger long-term glial changes that result in more secretion of cytokines by astrocytes and microglia. This hypothesis has been advanced by glia theories and supported with consistent evidence (Sild et al., 2017). Relatedly, based on the neurovisceral integration (Thayer & Ruiz-Padial, 2006) and perseverative cognition (Ottaviani et al., 2016) theories and substantiating evidence (Michopoulos, Powers, Gillespie, Ressler, & Jovanovic, 2017), higher GAD and MDD severity may evoke disinhibition of autonomic CNS structures (e.g., HPA, sympathetic nervous system in the midbrain, forebrain, and hindbrain), reduce heart rate variability and vagal tone, and thereby raise inflammation over time. It is also plausible that social withdrawal, hypersomnia, and suboptimal diet and nutrition, that are more characteristic of MDD and GAD (vs. PD) may mediate the relation between increased depression and worry predicting more subsequent IL-6, CRP, fibrinogen, and related biomarkers (e.g., interleukin-1β) across prolonged periods. Abundant cross-sectional, experimental, and prospective data buttress these notions (Kiecolt-Glaser et al., 2015; Renna, O’Toole, Spaeth, Lekander, & Mennin, 2018). Future empirical prospective cross-panel or ecological momentary assessment studies can test these ideas directly to advance understanding on how MDD and GAD leads to rise in future inflammation levels.
Moreover, for all mediation models, heightened inflammation predicted reduced EF capacity over a 9-year timeframe. Such patterns parallel meta-analytic data from 7 distinct studies that adults with higher IL-6 serum were typically 1.42 times more at risk of facing decrements in general cognitive ability across as long as 7 years (Bradburn, Sarginson, & Murgatroyd, 2018). Neurophysiological theories may partially explain this replicated finding. Cytokines and related assays (e.g., IL-1β, tumor necrosis factor-α) created in excessive quantities can hinder or reduce neurogenesis, alter synaptic plasticity, or afflict brain cells by inhibiting long-term potentiation and activating the microglia across long time spans. Such notions proposed by cytokine models of cognitive functioning have gathered plentiful evidence (McAfoose & Baune, 2009). On that note, findings can also in part be accounted for by cognition-inflammation theories and myriad supporting data that inflammatory markers may cross the blood-brain-barrier and adversely impact neurons in EF- and related brain areas (e.g., caudate nucleus, prefrontal cortex) in the long-term (Nation et al., 2019). Elevated plasma viscosity (or increased inflammation) may also directly impair EF in the long run by introducing aberrations in tryptophan or kynurenine metabolism, and by lowering cerebral blood flow or brain connectivity in frontal-striatal-limbic regions (e.g., cingulate cortex, medial temporal lobe). Ample cross-sectional and longitudinal evidence supports these ideas (Warren et al., 2018). Clearly, these propositions warrant further investigation.
Direct effects were also observed, such that increased MDD, GAD, and PD severity forecasted lower EF 18 years later for the entire sample. These results fill a key knowledge gap given the paucity of longitudinal data on EF-psychopathology relations (Snyder et al., 2015, p. 328). In addition, findings may suggest that increased depression (Royall, Palmer, Chiodo, & Polk, 2012), pathological worry (Zainal & Newman, 2020) and anxiety sensitivity (or fear of fear) characteristic of PD (Otto et al., 2016) promotes poor health behaviors and precipitates a neurodegenerative process (e.g., wear-and-tear of the HPA) that could negatively affect future EF across lengthy timescales. Future research can continue to empirically test these hypotheses.
This study has several limitations. First, the associations among common psychiatric disorders, EF, and inflammation are complex and bi-directional (Zainal & Newman, 2020; Zainal & Newman, 2018), and deserve more scrutiny. We were unable to follow best practices and statistically control for baseline inflammation and EF (Maxwell & Cole, 2007) as they were not measured in the dataset. However, the findings were similar even after T2 EF was controlled for. Further, as T1 inflammation was not measured, we could not test the vulnerability hypothesis (Majd et al., 2020) which posits that inflammation predicts later psychiatric disorder severity. For instance, it is plausible that elevated inflammation can induce a set of illness behaviors, such as prolonged activity restriction and social withdrawal, that may precipitate future psychopathology. However, the pattern of findings remained similar above and beyond inclusion of BMI, a proxy of inflammation (Oddy et al., 2018), in the mediation models with MDD, GAD, or PD as unique predictors. Also, unmeasured third variables, such as genetics (e.g., presence of APOE ɛ4 allele) (Gustavson et al., 2019), may contribute to our findings and merit attention. In addition, as the psychiatric symptom measures herein were DSM-III-R-derived, future research should test our predictions with DSM-5-consistent scales. Additionally, given the mostly White sample herein, replication efforts can clarify if results extend to culturally-diverse populations, as studies have shown ethnic differences in the connections among anxiety and depression symptoms, cognition, and inflammation (Beydoun et al., 2018). Nonetheless, study strengths include the 18-year longitudinal dataset, test of moderators, latent variable approach, large sample size, and use of comprehensive EF battery.
If the pattern of findings here are reproduced in succeeding investigations, some clinical implications merit consideration. It is plausible that successfully treating MDD in younger and older adults may reduce the likelihood of undue inflammation and future long-term EF decline. This idea is indirectly supported by meta-analytic data that lifestyle- and mindset-altering cognitive-behavioral and mindfulness-based therapies can notably decrease common mental health problem symptom severity alongside inflammatory markers (Sanada et al., 2020), and enhance EF (Zainal & Newman, 2021a). Moreover, efforts to enhance comprehension of the interaction between psychotropic drugs’ efficacy and inflammation (Miller & Raison, 2016), their effectiveness (Jha & Trivedi, 2018), and the anti-depressant effects of vitamin supplementation (e.g., omega-3 fatty acids) (Kiecolt-Glaser, Belury, Andridge, Malarkey, & Glaser, 2011) have been underway. Such endeavors, particularly with gold-standard randomized controlled trials, justify continuing attention given their potential to personalize treatments for depression and anxiety disorders.
Supplementary Material
Figure 2. Longitudinal SEM Mediation Path of Baseline GAD Severity Predicting Future Reduced Executive Functioning Via Inflammation.
Note. * p < .05;. * p < .01; *** p < .001.
λ = standardized factor loading; ε = item residual variance; ζ = factor residual variance; GAD = generalized anxiety disorder; IL-6 = interleukin-6; CRP = C-reactive protein; FGN = fibrinogen; VF = verbal fluency; NS = number series; BC = backward counting; SGST = stop-and-go-switch task mixed task; BDS = backward digit span; SEM = structural equation modeling. Parameters a, b, and c refers to unstandardized regression coefficients.
Table 4.
Mediation Model of T1 GAD Severity Predicting T3 EF Via T2 Inflammation
| All Samples | β (SE) | p |
|---|---|---|
| Standardized Factor Loadings: Past Year GAD Severity | ||
| Excessive worry | .605a | – |
| Uncontrollable worry | .951*** (0.155) | < .001 |
| How often restless due to worry | .970*** (0.165) | < .001 |
| How often keyed up due to worry | .948*** (0.152) | < .001 |
| How often irritable due to worry | .959*** (0.162) | < .001 |
| Trouble falling asleep due worry | .943*** (0.179) | < .001 |
| Trouble staying asleep due worry | .956*** (0.183) | < .001 |
| Low on energy due to worry | .953*** (0.180) | < .001 |
| Tire easily due to worry | .963*** (0.177) | < .001 |
| Sore or aching due to worry | .943*** (0.184) | < .001 |
|
| ||
| Standardized Factor Loadings: Inflammatory Markers | ||
| Interleukin-6 | .679a | – |
| C-Reactive Protein | .685*** (0.191) | < .001 |
| Fibrinogen | .605*** (0.060) | < .001 |
|
| ||
| Standardized Factor Loadings: EF Composite | ||
| Category Fluency | .525a | – |
| Number Series | .659*** (0.046) | < .001 |
| Backward Counting | .655*** (0.311) | < .001 |
| Stop-and-Go Signal Mixed Task | .276*** (0.062) | < .001 |
| Digit Backward Span | .398*** (0.030) | < .001 |
|
| ||
| Unstandardized Regression Slopes | ||
| T1 GAD → T2 Inflammation (a path) | 0.080** (0.030) | .007 |
| T2 Inflammation → T3 EF (b path) | −2.247*** (0.571) | < .001 |
| T1 GAD → T2 Inflammation → T3 EF (c path) | −0.180* (0.078) | .021 |
Note.
p < .001
p < .01
p < .05.
Model Fit Indices – χ2(132)=150.890, p=.132, CFI=.997, RMSEA=.013. CFI=confirmatory fit index; β=Regression weight estimate; EF=executive functioning; GAD=generalized anxiety disorder symptom severity; RMSEA=root mean square error of approximation; SE=standard error.
Unit loading identification was used in the confirmatory factor analysis portion of the structural equation model.
Highlights.
Time 1 MDD and GAD severity predicted increased inflammation 9 years later.
T1 PD severity did not forecast future IL-6, CRP, and fibrinogen levels.
Heightened T2 inflammation predicted lower executive function after 9 years.
T1 MDD, GAD, and PD independently related to less EF capacity following 18 years.
Findings remained after adjusting for BMI, sociodemographic, comorbid MDD, GAD, and PD, and lifestyle factors.
Acknowledgements
Each author has (1) made substantial contributions to the conception or design of the work, or to the acquisition, analysis, or interpretation of data for the work; (2) participated in drafting the work or revising it critically for important intellectual content; (3) approved the final version to be published; and (4) agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding Sources
The data used in this publication were made available by the Data Archive on University of Wisconsin - Madison Institute on Aging, 1300 University Avenue, 2245 MSC, Madison, Wisconsin 53706-1532. Since 1995 the Midlife Development in the United States (MIDUS) study has been funded by the following: John D. and Catherine T. MacArthur Foundation Research Network; National Institute on Aging (P01-AG020166); National Institute on Aging (U19-AG051426). The original investigators and funding agency are not responsible for the analyses or interpretations presented here.
Statement of Ethics
This study was conducted in compliance with the American Psychological Association (APA) ethical standards in the treatment of human participants and approved by the institutional review board (IRB). Further, this research was conducted was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Informed consent was obtained from participants as per IRB requirements at Harvard University, Georgetown University, University of California at Los Angeles, and University of Wisconsin. Since this study used a publicly available dataset, it was exempt from IRB approval.
Footnotes
Conflict of Interest Statement
The authors, Nur Hani Zainal and Dr. Michelle G. Newman do not have any conflicts of interest or financial disclosures. (Declaration of Interest: None).
Conflict of Interest Statement
The authors do not have any conflicts of interest or financial disclosures. (Declaration of Interest: None)
Refer to Tables S1 to S3 in the online supplementary materials for more information on all of the unique direct effect models for T1 MDD, GAD, and PD severity predicting for T3 EF.
The pattern of results remained similar even if the levels of CRP, fibrinogen, and IL-6 were summed to create an inflammatory marker composite (vs. using CFA) or if each biomarker was tested in separate models. Moreover, we determined that the pattern of findings held if diagnostic status of MDD, GAD, or PD was entered as a predictor into distinct models. Also, the results remained similar if cognitive functioning-related items within the diagnoses were added into the analyses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nur Hani Zainal, The Pennsylvania State University.
Michelle G. Newman, The Pennsylvania State University
References
- American Psychiatric Association. (1987). Diagnostic and statistical manual of mental disorders (3rd, rev. ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Arnold BF, Hogan DR, Colford JM Jr., & Hubbard AE (2011). Simulation methods to estimate design power: An overview for applied research. BMC Medical Research Methodology, 11, 94. doi: 10.1186/1471-2288-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107, 238–246. doi: 10.1037/0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Dore GA, Canas J-A, Liang H, Beydoun HA, Evans MK, & Zonderman AB (2018). Systemic inflammation is associated with longitudinal changes in cognitive performance among urban adults. Frontiers in Aging Neuroscience, 10, 313. doi: 10.3389/fnagi.2018.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Weiss J, Obhi HK, Beydoun HA, Dore GA, Liang H, … Zonderman AB. (2019). Cytokines are associated with longitudinal changes in cognitive performance among urban adults. Brain, Behavior, and Immunity, 80, 474–487. doi: 10.1016/j.bbi.2019.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan JM, Cundiff JM, Fuller-Rowell TE, & Ryff CD (2020). Childhood socioeconomic status and inflammation: Psychological moderators among Black and White Americans. Health Psychology, 39, 497–508. doi: 10.1037/hea0000866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburn S, Sarginson J, & Murgatroyd CA (2018). Association of peripheral interleukin-6 with global cognitive decline in non-demented adults: A meta-analysis of prospective studies. Frontiers in Aging Neuroscience, 9, 438. doi: 10.3389/fnagi.2017.00438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR, … Shweder RA (2019). Midlife in the United States (MIDUS 1), 1995–1996: Inter-university Consortium for Political and Social Research [distributor]. [Google Scholar]
- Brodaty H, & Connors MH (2020). Pseudodementia, pseudo-pseudodementia, and pseudodepression. Alzheimer’s & Dementia, 12, e12027–e12027. doi: 10.1002/dad2.12027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GW, & Lau RS (2008). Testing mediation and suppression effects of latent variables: Bootstrapping with structural equation models. Organizational Research Methods, 11, 296–325. doi: 10.1177/1094428107300343 [DOI] [Google Scholar]
- Clauss A (1957). Gerinnungsphysiologische schnellmethode zur bestimmung des fibrinogens. Acta Haematologica, 17, 237–246. doi: 10.1159/000205234 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power for the social sciences. Hillsdale, NJ: Laurence Erlbaum and Associates. [Google Scholar]
- Colasanto M, Madigan S, & Korczak DJ (2020). Depression and inflammation among children and adolescents: A meta-analysis. Journal of Affective Disorders, 277, 940–948. doi: 10.1016/j.jad.2020.09.025 [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, & Costello EJ (2012). Generalized anxiety and C-reactive protein levels: A prospective, longitudinal analysis. Psychological Medicine, 42, 2641–2650. doi: 10.1017/S0033291712000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels TE, Olsen EM, & Tyrka AR (2020). Stress and psychiatric disorders: The role of mitochondria. Annual Review of Clinical Psychology, 16, 165–186. doi: 10.1146/annurev-clinpsy-082719-104030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, & Hofman A (2018). Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimer’s & Dementia, 14, 1450–1459. doi: 10.1016/j.jalz.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Deverts DJ, Cohen S, DiLillo VG, Lewis CE, Kiefe C, Whooley M, & Matthews KA (2010). Depressive symptoms, race, and circulating C-reactive protein: The coronary artery risk development in young adults (CARDIA) study. Psychosomatic Medicine, 72, 734–741. doi: 10.1097/PSY.0b013e3181ec4b98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64, 135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, & Burke MJ (1996). Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods, 1, 170–177. doi: 10.1037/1082-989x.1.2.170 [DOI] [Google Scholar]
- Dunst CJ, Hamby DW, & Trivette CM (2004). Guidelines for calculating effect sizes for practice-based research syntheses. Centerscope, 3, 1–10. [Google Scholar]
- Eyre H, & Baune BT (2012). Neuroimmunological effects of physical exercise in depression. Brain, Behavior, and Immunity, 26, 251–266. doi: 10.1016/j.bbi.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, … Murray CJL (2018). Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. The Lancet, 392, 2052–2090. doi: 10.1016/S0140-6736(18)31694-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, & Herd P (2010). Income, education, and inflammation: Differential associations in a national probability sample (The MIDUS Study). Psychosomatic Medicine, 72, 290–300. doi: 10.1097/psy.0b013e3181cfe4c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaus J, von Känel R, Lasserre AM, Strippoli M-PF, Vandeleur CL, Castelao E, … Merikangas KR (2018). The bidirectional relationship between anxiety disorders and circulating levels of inflammatory markers: Results from a large longitudinal population-based study. Depression and Anxiety, 35, 360–371. doi: 10.1002/da.22710 [DOI] [PubMed] [Google Scholar]
- Graham JW (2009). Missing data analysis: Making it work in the real world. Annual Review of Psychology, 60, 549–576. doi: 10.1146/annurev.psych.58.110405.085530 [DOI] [PubMed] [Google Scholar]
- Gustavson DE, Franz CE, Panizzon MS, Reynolds CA, Xian H, Jacobson KC, … Kremen WS (2019). Genetic and environmental associations among executive functions, trait anxiety, and depression symptoms in middle age. Clinical Psychological Science, 7, 127–142. doi: 10.1177/2167702618805075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U-K (2002). Microglia as a source and target of cytokines. Glia, 40, 140–155. doi: 10.1002/glia.10161 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Lachman M, Mroczek D, Seeman T, & Miller G (2015). Additive contributions of childhood adversity and recent stressors to inflammation at midlife: Findings from the MIDUS study. Developmental Psychology, 51 11, 1630–1644. doi: 10.1037/dev0000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, & Trivedi MH (2018). Personalized antidepressant selection and pathway to novel treatments: Clinical utility of targeting inflammation. International Journal of Molecular Sciences, 19, 233. doi: 10.3390/ijms19010233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun B, & Wittchen H-U (1998). The World Health Organization Composite International Diagnostic Interview short-form (CIDI-SF). International Journal of Methods in Psychiatric Research, 7, 171–185. doi: 10.1002/mpr.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Zammit S, Lewis G, & Jones PB (2016). Association between serum C-reactive protein and DSM-IV generalized anxiety disorder in adolescence: Findings from the ALSPAC cohort. Neurobiology of Stress, 4, 55–61. doi: 10.1016/j.ynstr.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, & Fagundes CP (2015). Inflammation: Depression fans the flames and feasts on the heat. American Journal of Psychiatry, 172, 1075–1091. doi: 10.1176/appi.ajp.2015.15020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, & Glaser R (2011). Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain, Behavior, and Immunity, 25, 1725–1734. doi: 10.1016/j.bbi.2011.07.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, & Glaser R (2003). Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences, 100, 9090. doi: 10.1073/pnas.1531903100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kop WJ, Stein PK, Tracy RP, Barzilay JI, Schulz R, & Gottdiener JS (2010). Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosomatic Medicine, 72. doi: 10.1097/PSY.0b013e3181eadd2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Tun PA, & Weaver SL (2014). Monitoring cognitive functioning: Psychometric properties of the Brief Test of Adult Cognition by Telephone (BTACT). Assessment, 21, 404–417. doi: 10.1177/1073191113508807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. doi: 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, & Ryff CD (2010). Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health, 22, 1059–1080. doi: 10.1177/0898264310374355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Mõttus R, Starr JM, McNeill G, Jia X, Craig LCA, & Deary IJ (2012). Depressive symptoms and diet: Their effects on prospective inflammation levels in the elderly. Brain, Behavior, and Immunity, 26, 717–720. doi: 10.1016/j.bbi.2011.10.007 [DOI] [PubMed] [Google Scholar]
- Mac Giollabhui N, Swistun D, Murray S, Moriarity DP, Kautz MM, Ellman LM, … Alloy LB (2020). Executive dysfunction in depression in adolescence: The role of inflammation and higher body mass. Psychological Medicine, 50, 683–691. doi: 10.1017/S0033291719000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majd M, Saunders EFH, & Engeland CG (2020). Inflammation and the dimensions of depression: A review. Frontiers in Neuroendocrinology, 56, 100800. doi: 10.1016/j.yfrne.2019.100800 [DOI] [PubMed] [Google Scholar]
- Matthews KA, Schott LL, Bromberger JT, Cyranowski JM, Everson-Rose SA, & Sowers M (2010). Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain, Behavior, and Immunity, 24, 96–101. doi: 10.1016/j.bbi.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Schott LL, Bromberger J, Cyranowski J, Everson-Rose SA, & Sowers MF (2007). Associations between depressive symptoms and inflammatory/hemostatic markers in women during the menopausal transition. Psychosomatic Medicine, 69, 124–130. doi: 10.1097/01.psy.0000256574.30389.1b [DOI] [PubMed] [Google Scholar]
- Maxwell SE, & Cole DA (2007). Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods, 12, 23–44. doi: 10.1037/1082-989X.12.1.23 [DOI] [PubMed] [Google Scholar]
- McAfoose J, & Baune BT (2009). Evidence for a cytokine model of cognitive function. Neuroscience & Biobehavioral Reviews, 33, 355–366. doi: 10.1016/j.neubiorev.2008.10.005 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress- and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–445. doi: 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, & Jovanovic T (2017). Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology, 42, 254–270. doi: 10.1038/npp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, & Raison CL (2016). The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Reviews Immunology, 16, 22–34. doi: 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions. Current Directions in Psychological Science, 21, 8–14. doi: 10.1177/0963721411429458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, & Ryff CD (2010). Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychology, 29, 626–635. doi: 10.1037/a0021360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson MW (2005). Fibrinogen and fibrin structure and functions. Journal of Thrombosis and Haemostasis, 3, 1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x [DOI] [PubMed] [Google Scholar]
- Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, … Zlokovic BV (2019). Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nature Medicine, 25, 270–276. doi: 10.1038/s41591-018-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddy WH, Allen KL, Trapp GSA, Ambrosini GL, Black LJ, Huang R-C, … Mori TA (2018). Dietary patterns, body mass index and inflammation: Pathways to depression and mental health problems in adolescents. Brain, Behavior, and Immunity, 69, 428–439. doi: 10.1016/j.bbi.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Watson DR, Meeten F, Makovac E, Garfinkel SN, & Critchley HD (2016). Neurobiological substrates of cognitive rigidity and autonomic inflexibility in generalized anxiety disorder. Biological Psychology, 119, 31–41. doi: 10.1016/j.biopsycho.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Otto MW, Eastman A, Lo S, Hearon BA, Bickel WK, Zvolensky M, … Doan SN (2016). Anxiety sensitivity and working memory capacity: Risk factors and targets for health behavior promotion. Clinical Psychology Review, 49, 67–78. doi: 10.1016/j.cpr.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Pozzoli S, De Carlo V, & Madonna D (2019). Depression, dementia, and pseudodementia. In Altamura AC & Brambilla P (Eds.), Clinical Cases in Psychiatry: Integrating Translational Neuroscience Approaches (pp. 171–188). Cham: Springer International Publishing. doi: 10.1007/978-3-319-91557-9_10 [DOI] [Google Scholar]
- Preacher KJ, & Kelley K (2011). Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods, 16, 93–115. doi: 10.1037/a0022658 [DOI] [PubMed] [Google Scholar]
- Renna ME, O’Toole MS, Spaeth PE, Lekander M, & Mennin DS (2018). The association between anxiety, traumatic stress, and obsessive–compulsive disorders and chronic inflammation: A systematic review and meta-analysis. Depression and Anxiety, 35, 1081–1094. doi: 10.1002/da.22790 [DOI] [PubMed] [Google Scholar]
- Rose-John S (2018). Interleukin-6 family cytokines. Cold Spring Harbor Perspectives in Biology, 10, a028415. doi: 10.1101/cshperspect.a028415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y (2012). Lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48, 1–36. doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, & Polk MJ (2012). Depressive symptoms predict longitudinal change in executive control but not memory. International Journal of Geriatric Psychiatry, 27, 89–96. doi: 10.1002/gps.2697 [DOI] [PubMed] [Google Scholar]
- Ryff C, Almeida DM, Ayanian J, Carr DS, Cleary PD, Coe C, … Williams D (2017). Midlife in the United States (MIDUS 2), 2004–2006. ICPSR04652-v7. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2017–11-20. doi: 10.3886/ICPSR04652.v7 [DOI] [Google Scholar]
- Ryff CD, & Lachman ME (2019). Midlife in the United States (MIDUS 3): Cognitive Project, 2013–2017 ki: Inter-university Consortium for Political and Social Research [distributor]. [Google Scholar]
- Ryff CD, & Lachman ME (2018). Midlife in the United States (MIDUS 3): Cognitive project, 2013–2017. doi: 10.3886/ICPSR37095.v1 [DOI] [Google Scholar]
- Ryff CD, Seeman T, & Weinstein M (2019). Midlife in the United States (MIDUS 2): Biomarker Project, 2004–2009: Inter-university Consortium for Political and Social Research [distributor]. [Google Scholar]
- Sanada K, Montero-Marin J, Barceló-Soler A, Ikuse D, Ota M, Hirata A, … Iwanami A (2020). Effects of mindfulness-based interventions on biomarkers and low-grade inflammation in patients with psychiatric disorders: A meta-analytic review. International Journal of Molecular Sciences, 21, 2484. doi: 10.3390/ijms21072484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, & Launer LJ (2002). Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia aging study. Annals of Neurology, 52, 168–174. doi: 10.1002/ana.10265 [DOI] [PubMed] [Google Scholar]
- Schweizer S, Satpute AB, Atzil S, Field AP, Hitchcock C, Black M, … Dalgleish T (2019). The impact of affective information on working memory: A pair of meta-analytic reviews of behavioral and neuroimaging evidence. Psychological Bulletin, 145, 566–609. doi: 10.1037/bul0000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sild M, Ruthazer ES, & Booij L (2017). Major depressive disorder and anxiety disorders from the glial perspective: Etiological mechanisms, intervention and monitoring. Neuroscience & Biobehavioral Reviews, 83, 474–488. doi: 10.1016/j.neubiorev.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Simes RJ (1986). An improved Bonferroni procedure for multiple tests of significance. Biometrika, 73, 751–754. doi: 10.2307/2336545 [DOI] [Google Scholar]
- Smith KJ, Au B, Ollis L, & Schmitz N (2018). The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: A systematic review and meta-analysis. Experimental Gerontology, 102, 109–132. doi: 10.1016/j.exger.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139, 81–132. doi: 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328. doi: 10.3389/fpsyg.2015.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyridaki EC, Avgoustinaki PD, & Margioris AN (2016). Obesity, inflammation and cognition. Current Opinion in Behavioral Sciences, 9, 169–175. doi: 10.1016/j.cobeha.2016.05.004 [DOI] [Google Scholar]
- Steiger JH (1990). Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research, 25, 173–180. doi: 10.1207/s15327906mbr2502_4 [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, & Kamarck TW (2009). A prospective evaluation of the directionality of the depression–inflammation relationship. Brain, Behavior, and Immunity, 23, 936–944. doi: 10.1016/j.bbi.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, … Kubzansky LD (2017). Cross-sectional and longitudinal associations of chronic posttraumatic stress disorder with inflammatory and endothelial function markers in women. Biological Psychiatry, 82, 875–884. doi: 10.1016/j.biopsych.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, & Ruiz-Padial E (2006). Neurovisceral integration, emotions and health: An update. International Congress Series, 1287, 122–127. doi: 10.1016/j.ics.2005.12.018 [DOI] [Google Scholar]
- Tomarken AJ, & Waller NG (2005). Structural equation modeling: Strengths, limitations, and misconceptions. Annual Review of Clinical Psychology, 1, 31–65. doi: 10.1146/annurev.clinpsy.1.102803.144239 [DOI] [PubMed] [Google Scholar]
- Warren KN, Beason-Held LL, Carlson O, Egan JM, An Y, Doshi J, … Resnick SM (2018). Elevated markers of inflammation are associated with longitudinal changes in brain function in older adults. Journals of Gerontology: Medical Sciences, 73, 770–778. doi: 10.1093/gerona/glx199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, & Fan X (2015). Monotonicity of effect sizes: Questioning kappa-squared as mediation effect size measure. Psychological Methods, 20, 193–203. doi: 10.1037/met0000029 [DOI] [PubMed] [Google Scholar]
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, & Ali S (2007). Depression and inflammation in patients with coronary heart disease: findings from the heart and soul study. Biological Psychiatry, 62, 314–320. doi: 10.1016/j.biopsych.2006.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Zhao S, Kessler RC, & Eaton WW (1994). DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Archives of General Psychiatry, 51, 355–364. doi: 10.1001/archpsyc.1994.03950050015002 [DOI] [PubMed] [Google Scholar]
- Wu Y, Potempa LA, El Kebir D, & Filep JG (2015). C-reactive protein and inflammation: conformational changes affect function. Biological Chemistry, 396, 1181. doi: 10.1515/hsz-2015-0149 [DOI] [PubMed] [Google Scholar]
- Yan Z, Hong S, Liu F, & Su Y (2020). A meta-analysis of the relationship between empathy and executive function. PsyCh Journal, 9, 34–43. doi: 10.1002/pchj.311 [DOI] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (2020). Within-person increase in pathological worry predicts future depletion of unique executive functioning domains. Psychological Medicine, 1–11. doi: 10.1017/S0033291720000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (2021a). Mindfulness enhances cognitive functioning: A meta-analysis of 100 randomized controlled trials. doi: 10.31234/osf.io/vzxw7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (2018). Executive function and other cognitive deficits are distal risk factors of generalized anxiety disorder 9 years later. Psychological Medicine, 48, 2045–2053. doi: 10.1017/S0033291717003579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (in press). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychological Medicine, 1–11. doi: 10.1017/s0033291721000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (2021b). Larger increase in trait negative affect is associated with greater future cognitive decline and vice versa across 23 years. Depression and Anxiety, 38, 146–160. doi: 10.1002/da.23093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, & Xie W (2018). High-sensitivity C-reactive protein and cognitive decline: The English longitudinal study of ageing. Psychological Medicine, 48, 1381–1389. doi: 10.1017/S0033291717003130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.