Abstract
Background
COVID-19-associated coagulopathy is incompletely understood.
Objectives
To characterize thrombin generation, Von Willebrand Factor (VWF), neutrophil extracellular traps (NETs), and their role in COVID-19 risk stratification in the emergency department (ED).
Patients/methods
Plasma samples from 67 ED COVID-19 patients were compared to 38 healthy volunteers (HVs). Thrombin generation (calibrated automated thrombogram, CAT) was expressed as lag time (LT, min), peak height (PH, min), and time to peak (ttPeak, min). Citrullinated nucleosomes and histones were quantified with ELISA, VWF antigen and activity (IU/dL) through latex immunoassay, Factor VIII (IU/dL) through one-stage optical clot detection, and VWF multimers with Western blot densitometry. Wilcoxon testing and multivariable logistic regression were performed. Results presented as median [Q1, Q3]; p < 0.05 significant.
Results
COVID-19 patients had longer LT (4.00 [3.26, 4.67]; 2.95 [2.67, 3.10], p < 0.001) and ttPeak (7.33 [6.33, 8.04]; 6.45 [6.00, 7.50], p = 0.004), greater VWF antigen (212 [158, 275]; 110 [91, 128], p < 0.001) and Factor VIII levels (148 [106, 190]; 106 [86, 129], p < 0.001), with decreased high molecular weight multimers (Normalized multimer ratio 0.807 [0.759, 0.869]; 0.891 [0.858, 0.966], p < 0.001), than HVs. COVID-19 patients requiring admission from the ED had longer LT and ttPeak with greater VWF antigen and Factor VIII levels than those not admitted. Two and three variable models of CAT parameters and VWF correlated with COVID-19 and admission status (C-statistics 0.677 to 0.922).
Conclusions
Thrombin generation kinetics and VWF levels, independent of NETs, may have a role in predicting admission need for COVID-19 patients.
Keywords: COVID-19, Extracellular traps, Thrombin, Von Willebrand factor
1. Introduction
Coagulopathy remains a major source of morbidity and mortality in patients infected with Coronavirus 2019 (SARS-CoV-2, COVID-19). Reported venous thromboembolism (VTE) rates in COVID-19 infection range from 2.9% to 31% in patients across the spectrum of severity and remained high even when mortality levels were 50% lower in the fall of 2020 [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. Autopsy studies have identified diffuse macro- and microvascular thrombosis, suggesting dysregulated coagulation in these patients [10,11]. It is hypothesized that SARS-CoV-2 binding to ACE-2 receptors induces endothelial injury that triggers local and systemic inflammatory response with resultant macro- and microvascular thrombosis [12,13]. Cytokine storm, complement activation, and neutrophil extracellular traps (NETs) have also been implicated in COVID-19-associated coagulopathy [[14], [15], [16], [17]]. The complex interactions among inflammation, endothelial injury, and coagulation, termed “thromboinflammation” or “immunothrombosis”, significantly impact clinical outcomes [18,19]; however, the exact clinical role of plasma biomarkers of thromboinflammation in COVID-19 is yet to be determined.
Case series have shown greater levels of von Willebrand Factor (VWF) and Factor VIII in COVID-19 patients and hypothesized their role in COVID-19-associated coagulopathy through endothelial activation and propagation of the intrinsic pathway [[20], [21], [22], [23], [24]]. Endothelial injury mediates development of VTE and stimulates neutrophils to form neutrophil extracellular traps (NETs), which induce a positive feedback cycle by triggering further endothelial damage [25,26]. Cohort studies have demonstrated increased levels of cell-free DNA, citrullinated histone-3 (citH3), and myeloperoxidase-DNA complexes (MPO-DNA) in patients with COVID-19, suggesting increased NETosis [27,28]. Autopsy studies have demonstrated the presence of NETs in the microvasculature of the kidneys, lungs, and liver in deceased patients with COVID-19 [16,29,30]. It is theorized that the interplay between endothelial injury and NETosis contributes to macro- and micro-vessel thrombosis in COVID-19.
Thrombin (Factor IIa) is a key serine protease in the coagulation cascade. Presently, there is limited information on the clinical utility of measuring thrombin generation and markers of thromboinflammation in COVID-19-associated coagulopathy. In this pilot study, we characterized COVID-19-associated coagulopathy in a heterogeneous cohort of patients requiring emergency department evaluation, by quantifying plasma thrombin generation kinetics, NETosis, and VWF activity, as compared to healthy volunteers. We also sought to determine the role of these plasma biomarkers in risk stratifying COVID-19 patients for admission need in the emergency department (ED).
2. Methods
2.1. Study design and database
Patients presenting to the Emergency Department (ED) at Mayo Clinic in Rochester, MN who tested positive for COVID-19 were prospectively enrolled. This study was approved by the Mayo Clinic Institutional Review Board and all subjects provided written informed consent. Exclusion criteria included: Refusal or inability to obtain informed consent from a patient or their Legal Authorized Representative (LAR), therapeutic anticoagulation (e.g., warfarin, dabigatran etexilate, rivaroxaban, apixaban), an inherited or acquired coagulation disorder, active malignancy, renal failure, or high-dose immunosuppression (including active chemotherapy, biologic medications, chronic immune suppression for prior transplant, and high dose steroids). Patients on anti-platelet medications, including aspirin, clopidogrel, ticagrelor, and non-steroidal anti-inflammatory medications were included. Patients who had blood drawn after the receipt of VTE chemoprophylaxis in-hospital were also excluded, to best assess baseline coagulation profiles. We analyzed a total of 67 citrated plasma samples from enrolled COVID-19 patients and 38 citrated plasma samples from healthy volunteers. Volunteer samples utilized in this study were collected under a parent study, as previously described, and samples were randomly selected to include in this cohort, in order to avoid introducing bias through specific patient selection [31]. Volunteers were recruited from the local community, screened for major medical conditions, and written informed consent was obtained prior to sample collection [31]. The electronic medical record was reviewed to obtain information on patient demographics and clinical course.
2.2. Sample collection, processing, and storage
Whole blood was collected by venipuncture or via existing indwelling catheters into 4.5 mL citrated Vacutainer tubes (0.105 M buffered sodium citrate, 3.2% Becton Dickinson, Plymouth, UK) and processed (within 1 h of collection) to platelet-poor plasma by double centrifugation (3000g, 15 min), and stored in multiple aliquots at −80° Celsius until analysis.
2.3. Calibrated automated thrombogram (CAT)
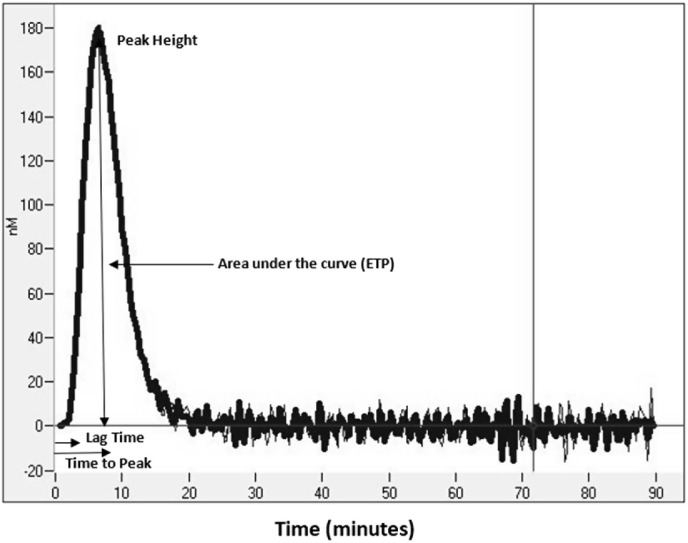
Thrombin generation kinetics were measured with the Calibrated Automated Thrombogram assay (CAT, Thrombinoscope BV, Maastricht, Netherlands), utilizing a Fluoroskan Ascent plate reader (390 nm excitation, 460 nm emission, Thermo Electron Corp, Vantaa, Finland), as previously described [32,33]. Assays were performed in triplicate. Corn trypsin inhibitor (50 μg/mL final concentration) was added to each plasma sample prior to analysis. Thrombin generation was initiated using two different reagents: 20 μL of PPP (5 pM re-lipidated human tissue factor and 4 μM phospholipids, Diagnostica Stago Inc., Parsippany, NJ) reagent. Then, 80 μL of platelet poor plasma was added to each well of U-bottom 96-well microtiter plates (Nunc, Thermo Fischer Scientific, Rochester, NY) using a single channel pipette. After incubation for 10 min at 37 °C, 20 μL of warmed FluCa reagent (FluCa kit, Diagnostica Stago Inc., Parsippany, NJ), which contains the fluorogenic substrate and CaCl2 was added to each well via an automated dispenser. Thrombin generation curves were recorded continuously for 90 min at a rate of three readings per minute. Separate wells containing the thrombin calibrator, which corrects for inner filter effects and quenching variation among individual plasmas were also measured in parallel. A dedicated software program, Thrombinoscope (Thrombinoscope BV, Maastricht, Netherlands) was used to calculate thrombin activity over time. As shown in Fig. 1 , the parameters derived were lag time (LT, minutes), peak height (PH, nM), time to peak (ttPeak, minutes), and endogenous thrombin potential (ETP, nM*minutes).
Fig. 1.
Representative plasma thrombin generation curve as generated by the calibrated automated thrombogram (CAT) assay. Parameters generated include: Lag time (LT – time to initiation of thrombin generation), peak height (PH – maximum thrombin generation at a given point in the assay), time to peak (ttPeak – time to maximum thrombin generation), and endogenous thrombin potential (ETP or area under the curve – total thrombin generated).
2.4. Markers of NETosis (H3NUC and H3Free)
Nucleosome-based ELISA was used to quantify citrullinated histones (H3NUC) using the H3R8Cit ELISA Capture Kit (EpiCypher Catalog No. R&D143001) [34,35]. Plates were coated with monoclonal detection antibody to H3R8Cit (EpiCypher Catalog No. 13-0046) overnight at 4 °C, followed by three washes with 50 mM Tris-HCl pH 7.5, 0.01% (w/v) BSA, 0.01% (v/v) Tween-20, 1 h of blocking (1X PBS, 1% (w/v) BSA), and three additional washes. Calibration standards (1000–15.6 ng/mL and 0 ng/mL) were prepared using serial dilutions of H3R2,8,17Cit recombinant designer nucleosome (dNucs, EpiCypher Catalog No. 16-1362). Plasma samples at 1:4 dilution and standards were added in triplicate at a volume of 100 μL, incubated for 2 h at room temperature, and washed three times. Biotinylated H3R8Cit detection antibody was added for a 1-h incubation. Signal detection was performed using Pierce™ High Sensitivity Streptavidin-HRP (Thermo Fisher Scientific Catalog No. 21130), 1-Step™ Ultra TMB-ELISA Substrate Solution (Thermo Fisher Scientific Catalog No. 34028) and Stop Solution for TMB Substrates (Thermo Fisher Scientific Catalog No. N600). Signal (absorbance at 450 nm) was read using EnVision™ Multilabel Plate Reader (Perkin Elmer). H3R8Cit recoveries were determined by using plate-specific standard curve (sigmoidal 5 PL regression analysis for absorbance vs. log ([H3R2,8,17Cit dNuc], ng/mL) with a lower limit of detection of 16.7 ng/mL. Citrullinated Histone H3 ELISA kit was used (Cayman Chemical, Clone 11D3) to measure free histones (H3Free) following the manufacturer's instructions at 1:4 dilutions.
2.5. VWF antigen, VWF activity, and factor VIII levels
VWF antigen (VWF: Ag) was measured using HemosIL von Willebrand Factor Antigen latex immunoassay kits (Instrumentation Laboratory, Bedford, MA) with two ACL TOP coagulation system analyzers, following the manufacturer's instructions. VWF activity in plasma samples was measured using Hemosil von Willebrand Factor Activity latex immunoassay kits (Instrumentation Laboratory, Bedford, MA) a previously described [36]. Factor VIII levels were quantified using one-stage optical clot detection.
2.6. VWF multimer analysis
Quantitative loss of high molecular weight multimers (HMWM) was determined with in-gel Western blot gel electrophoresis as previously described [37]. Multimer ratios were calculated by dividing the measured density of the sample range of interest (VWF multimers >10) by the remaining bands in the column. The normalized multimer ratio (NMR10) is calculated by dividing the patient multimer ratio for bands >10 by the pooled control multimer ratio for bands >10. A control column utilizing pooled plasma from normal donors (separate from the volunteers used in this study) was used to normalize VWF multimers. In addition, plasma from 27 normal donors was used to derive the normalized multimer ratio band 10 (NMR10) normal range (0.84–1.13). NMR10 for each subject was calculated using the following equation:
The first multimer band is ignored in this formula as it often contains proteins unassociated with VWF, is most likely to be artifact, and has the highest quantitative inter-observer variability of all the bands [38]. An NMR10 below the normal range (i.e., low NMR10 ratio) indicates loss of high molecular weight multimers in subject plasma relative to pooled plasma from normal donors.
2.7. Statistical analysis
Data analysis was performed using SAS Version 9.4 (Cary, NC). The Wilcoxon rank sum test was used to detect differences in values between COVID-19 patients and healthy volunteers, as well as between COVID-19 patients who were admitted to the hospital and those who were not admitted. Spearman coefficients were used to determine correlations between quantitative variables. Results are presented as median and quartiles [Q1, Q3], unless otherwise specified. Two-variable and three-variable logistic regression models were fitted with either COVID-19 (yes/no) or admission (yes/no) as the variables to be predicted. Results are expressed as odds ratio (OR) with 95% confidence interval and concordance statistic (C-stat) to quantify the overall strength of the model. A p-value of <0.05 was considered statistically significant. With 67 enrolled patients and 38 healthy volunteers, our study had 80% power to detect a standardized effect of 0.569.
3. Results
3.1. Baseline characteristics
A total of 67 patients with COVID-19 and 38 healthy volunteers were included in this study. Baseline characteristics are shown in Table 1 . In comparison to healthy volunteers, patients with COVID-19 were older, more commonly on anti-platelet agents, and had greater incidences of hypertension and chronic kidney disease. Of the 67 COVID-19 patients, 66 (98.5%) presented to the ED with symptoms directly related to the virus, with the most common patient reported symptoms being dyspnea, chest pain, cough, and fevers. The one asymptomatic patient had known COVID-19 infection and presented to the emergency room with an unrelated urgent care problem. Patients presented to the ED with a median of 8 [4,10] days of symptoms prior to sample collection (Table 1).
Table 1.
Baseline Characteristics of COVID-19 Patients vs. Healthy Volunteers.
| COVID-19 (n = 67) | Healthy Volunteers (n = 38) | p-valuea | |
|---|---|---|---|
| Age (years) | 58 [33, 71] | 41 [30, 51] | 0.002 |
| Sex (% male) | 30 (44.8%) | 19 (50.0%) | 0.686 |
| Taking anti-platelets at the time of sample collection (%) | 11 (16.4%) | 0 (0%) | 0.007 |
| Days since COVID-19 PCR test | 5 [1,9] | – | – |
| Symptomatic (%) | 66 (98.5%) | – | – |
| Days of symptoms prior to sample collection | 8 [4,10] | – | – |
| Hypertension (%) | 23 (34.3%) | 4 (10.5%) | 0.010 |
| Hyperlipidemia (%) | 15 (22.4%) | 9 (23.7%) | >0.999 |
| Diabetes Mellitus (%) | 11 (16.4%) | 3 (7.9%) | 0.251 |
| Chronic Kidney Disease (%) | 8 (11.9%) | 0 (0.0%) | 0.049 |
| COPD/Asthma (%) | 17 (25.4%) | 5 (13.2%) | 0.212 |
p-value < 0.05 is considered statistically significant, and noted in bold text.
4. Clinical features of patients with COVID-19
Clinical features of the 67 enrolled COVID-19 patients are shown in Table 2 . Nearly half of the admitted COVID-19 patients received corticosteroid therapy, mostly in the form of intravenous dexamethasone during their treatment course. None of the patients were administered convalescent plasma. All but one patient received VTE prophylaxis or therapeutic anticoagulation within 24 h of admission. All samples were collected prior to administration of any chemoprophylaxis, which was verified through review of the medical record. The one patient who did not was discharged after less than 24 h. Among all enrolled COVID-19 patients, D-dimer levels were increased (lab normal reference <220 ng/mL), as described in Table 2. Neutrophil to lymphocyte ratio (NLR) on initial labs was significantly greater in admitted COVID-19 patients than non-admitted patients (5.23 [2.63, 7.18] vs. 2.79 [1.82, 5.22], p = 0.02). Five of the 67 COVID-19 patients developed symptomatic VTE (incidence 7.5%) within 90 days, including 4 with symptomatic PE found on CT angiography, early after admission. One patient developed an isolated DVT in the intensive care unit. Four of the five VTE patients reported >7 days of COVID-19 symptoms prior to sample collection. Median D-dimer for patients who developed VTE was markedly increased at 2380 ng/mL with range 957–9658 ng/mL. All patients with VTE were started on and discharged on therapeutic anticoagulation. There was one mortality in our cohort, and this was an elderly patient, who required ICU care but did not develop VTE.
Table 2.
Clinical features of COVID-19 patients (n = 67).
| Admitted (%) | 34 (53.1%) |
| LOS (days)* | 3 [3, 6] |
| ICU (%)* | 3 (8.8%) |
| Mechanical ventilation (%)* | 1 (2.9%) |
| Inpatient steroids (%)* | 16 (47.1%) |
| Convalescent plasma (%)* | 0 (0%) |
| VTE prophylaxis or anticoagulation | 33 (97.1%) |
| within 24 h of admission* | |
| Supplemental Oxygen Requirement | 21 (61.8%) |
| Laboratory values | |
| Initial D-dimer level (ng/mL) | 536 [291, 857] |
| Initial Platelet count (x 10^9/L) | 215 [173.5, 256.5] |
| Initial WBC (x 10^9/L) | 6.5 [4.7, 7.8] |
| Initial INR | 1.2 [1.1, 1.3] |
| Initial NLR | 3.4 [2.1, 6.0] |
| VTE (%) | 5 (7.5%) |
| DVT (%) | 1 (1.5%) |
| PE (%) | 4 (6.0%) |
| Discharge disposition | |
| Home | 60 (89.6%) |
| SNF or assisted living | 5 (7.5%) |
| Respiratory care unit | 1 (1.5%) |
| Death | 1 (1.5%) |
4.1. Thrombin generation kinetics
COVID-19 patients had longer LT and ttPeak, and greater PH as compared to healthy volunteers (Table 3 ). COVID-19 patients who developed VTE had similar thrombin generation parameters as compared to those without. COVID-19 patients who required hospital admission had longer LT and ttPeak than those who were discharged from the ER, while PH and ETP were not significantly different between admitted patients and those who were not admitted (Table 4 ). There were no significant differences in thrombin generation parameters when COVID-19 patients were examined by duration of symptoms prior to sample collection. Initial D-dimer level correlated to LT (Spearman coefficient 0.336, p = 0.013), but not to any other thrombin generation parameter.
Table 3.
Summary of Plasma Thrombin Generation Kinetics and NETosis in COVID-19 Patients vs. Healthy Volunteers.
| COVID-19* (n = 67) | Healthy Volunteers^ (n = 38) | p-valuea | |
|---|---|---|---|
| LT (min) | 4.00 | 2.95 | < 0.001 |
| [3.26, 4.67] | [2.67, 3.10] | ||
| PH (nM) | 233.9 | 206.2 | < 0.001 |
| [210.2, 288.8] | [168.1, 248.8] | ||
| ttPeak (min) | 7.33 | 6.45 | 0.004 |
| [6.33, 8.04] | [6.00, 7.50] | ||
| ETP (nM*min) | 1475 | 1318 | 0.053 |
| [1221, 1776] | [1173, 1556] | ||
| H3NUC (ng/mL) | 79.5 | 66.8 | 0.050 |
| [66.8, 213.6] | [66.8, 113.8] | ||
| Patients with H3NUC above LLD (%) | 36 (53.7%) | 16 (42.1%) | – |
| H3Free (ng/mL) | 1.086 | 0.784 | 0.093 |
| [0.744, 1.716] | [0.400, 2.204] | ||
| VWF: Ag (IU/dL) | 212 | 110 | < 0.001 |
| [158, 275] | [91, 128] | ||
| VWF Activity (IU/dL) | 165 | 89 | < 0.001 |
| [128, 221] | [73, 107] | ||
| Factor VIII (IU/dL) | 148 | 106 | < 0.001 |
| [106, 190] | [86, 129] | ||
| VWF Activity to Antigen Ratio | 0.803 | 0.813 | 0.287 |
| [0.754, 0.844] | [0.777, 0.849] | ||
| VWF Multimers (NMR10) | 0.807 | 0.891 | < 0.001 |
| [0.759, 0.869] | [0.858, 0.966] | ||
| Patients with low NMR | 50 (74.6%) | 6 (16.2%) | – |
p-value < 0.05 is considered statistically significant, and noted in bold text.
Table 4.
Thrombin generation, NETosis, and VWF by admission status.
| Admitted |
Not admitted |
p-valuea | |
|---|---|---|---|
| (n = 34) | (n = 33) | ||
| LT (min) | 4.33 | 3.33 | < 0.001 |
| [3.67, 5.67] | [3.00, 4.00] | ||
| PH (nM) | 233.3 | 243.2 | 0.940 |
| [215.3, 276.2] | [209.8, 302.2] | ||
| ttPeak (min) | 7.73 | 7.15 | 0.012 |
| [6.89, 8.33] | [6.10, 7.67] | ||
| ETP (nM*min)* | 1476 | 1472 | 0.622 |
| [1241, 1650] | [1222, 1848] | ||
| H3NUC (ng/mL)* | 74.1 [66.8, 213.6] | 84.7 | 0.770 |
| [66.8, 200.9] | |||
| H3Free (ng/mL) | 1.314 | 0.934 [0.586, 1.663] | 0.092 |
| [0.836, 1.982] | |||
| VWF: Ag (IU/dL) | 247 | 168 | < 0.001 |
| [210, 295] | [104, 212] | ||
| VWF Activity (IU/dL) | 196 | 130 | < 0.001 |
| [165, 238] | [95, 155] | ||
| Factor VIII (IU/dL) | 159 | 119 | 0.023 |
| [130, 193] | [90, 168] | ||
| VWF Activity to Antigen Ratio | 0.808 | 0.793 | 0.684 |
| [0.761, 0.844] | [0.743, 0.835] | ||
| VWF Multimers (NMR10) | 0.780 | 0.831 | 0.002 |
| [0.734, 0.817] | [0.793, 0.899] |
p-value < 0.05 is considered statistically significant, and noted in bold text.
4.2. Neutrophil extracellular traps
H3NUC trended toward greater levels in COVID-19 patients as compared to healthy volunteers, but this did not reach statistical significance. There was no difference in H3Free levels between COVID-19 patients and healthy volunteers (Table 3). Both COVID-19 patients with and without VTEs had a wide distribution of H3NUC levels, including one non-VTE patient with H3NUC levels >5000 ng/mL, in comparison to a relatively narrow distribution in healthy volunteers. Of the 34 COVID-19 patients who were admitted, H3Free levels were greater as compared to healthy volunteers (1.314 ng/mL [0.834, 2.021]; 0.784 ng/mL [0.400, 2.232], p = 0.048). There were no significant differences in H3NUC or H3Free levels between COVID-19 patients who required hospital admission and those who did not (Table 4).
4.3. Von Willebrand Factor and factor VIII
Patients with COVID-19 had significantly greater VWF antigen levels, VWF activity levels and Factor VIII levels, as compared to healthy volunteers (Table 3). There was no significant difference in VWF Activity to Antigen ratio between COVID-19 patients and volunteers (Table 3). Consistent with high VWF levels, Factor VIII levels were also greater in COVID-19 patients than in volunteer subjects (Table 3). VWF multimers, expressed as NMR10, were quantitatively lower in COVID-19 patients as compared to healthy volunteers (Table 3). NMR was low in 6 (16.2%) volunteers compared to 48 (73.8%) COVID-19 patients. Admitted patients were noted to have greater VWF antigen, VWF activity, Factor VIII levels, and decreased loss of VWF multimers as compared to those who were not admitted (Table 4). VWF antigen and activity levels showed a strong correlation to Factor VIII levels in the whole cohort (n = 105) including COVID-19 patients and volunteers (Spearman coefficients 0.765 (p < 0.001) and 0.775 (p < 0.001) respectively). Additionally, VWF Antigen, Activity and Factor VIII were correlated to D-dimers in patients with COVID-19 (Spearman coefficients 0.553 (p < 0.001), 0.573 (p < 0.001), and 0.517 (p < 0.001), respectively).
4.4. Two and three variable logistic regression of thrombin generation kinetics and VWF antigen fitted to COVID-19 infection
The univariate results (longer LT, ttPeak, and greater PH in COVID-19 patients) display both hypo- and hypercoagulable thrombin profiles, suggesting a combination of variables could be even more powerful in discriminating COVID-19 patients. Two variable models combining CAT parameters or CAT parameters with VWF antigen fitted to COVID-19 infection status as the dependent variable showed high concordance statistics, which ranged from 0.805 to 0.901 (Table 5a ). With a three-variable logistic regression model using LT, PH, and VWF antigen, the c-statistic of 0.922 was achieved (Table 5 b).
Table 5a.
Two variable logistic regression models correlating thrombin generation kinetics parameters and von Willebrand factor antigen to COVID-19 status.
| Parameters | Odds Ratio (95% CI) | p-valuea | C-statistic |
|---|---|---|---|
| Lag time (LT) | 9.52 (3.58, 28.33) | <0.001 | 0.879 |
| Peak Height (PH) | 3.07 (1.54, 6.13) | 0.002 | |
| Time to Peak (ttPeak) | 3.15 (1.72, 5.82) | < 0.001 | 0.805 |
| Peak Height (PH) | 3.72 (1.92, 7.20) | < 0.001 | |
| Lag time (LT) | 5.55 (2.03, 15.12) | < 0.001 | 0.901 |
| VWF Antigen (VWF: Ag) | 4.34 (2.01, 9.35) | < 0.001 | |
| Time to Peak (ttPeak) | 1.68 (0.92, 3.08) | 0.094 | 0.872 |
| VWF Antigen (VWF: Ag) | 5.85 (2.83, 12.06) | < 0.001 | |
| Peak Height (PH) | 1.96 (1.12, 3.46) | 0.020 | 0.871 |
| VWF Antigen (VWF: Ag) | 6.10 (2.84, 13.10) | < 0.001 |
p-value < 0.05 is considered statistically significant, and noted in bold text.
Table 5b.
Three variable logistic regression model correlating thrombin generation kinetics parameters and von Willebrand factor antigen to COVID-19 status.
| Parameters | Odds Ratio (95% CI) | p-valuea | C-statistic |
|---|---|---|---|
| Lag time (LT) | 8.64 (2.57, 28.98) | <0.001 | 0.922 |
| Peak Height (PH) | 2.65 (1.29, 5.46) | 0.008 | |
| VWF Antigen (VWF: Ag) | 4.19 (1.74, 10.05) | 0.001 |
p-value < 0.05 is considered statistically significant, and noted in bold text.
4.5. Two and three variable logistic regression for thrombin generation kinetics and VWF antigen fitted to admission status for COVID-19 patients
Logistic regression models using two variables (LT and PH, ttPeak and PH, LT and VWF antigen, ttPeak and VWF antigen, PH and VWF antigen, ttPeak and D-dimer) used to predict admission status of COVID-19 patients also had strong concordance, with c-statistics ranging from 0.677 to 0.846 (Table 6 a). A three variable logistic regression model showed no change in concordance to admission status when PH was added to variables, LT and VWF antigen (Table 6 b).
Table 6a.
Two variable logistic regression models correlating thrombin generation kinetics parameters and von Willebrand factor antigen to admission status for COVID-19 patients.
| Parameters | Odds Ratio (95% CI) | p-valuea | C-statistic |
|---|---|---|---|
| Lag time (LT) | 3.70 (1.79, 7.66) 0.97 (0.56, 1.68) |
< 0.001 | 0.792 |
| Peak Height (PH) | 0.91 | ||
| Time to Peak (ttPeak) | 2.23 (1.23, 4.04) | 0.008 | 0.677 |
| Peak Height (PH) | 1.13 (0.66, 1.94) | 0.66 | |
| Lag time (LT) | 2.57 (1.25, 5.29) | 0.010 | 0.846 |
| VWF Antigen (VWF: Ag) | 2.95 (1.25, 6.95) | 0.013 | |
| Time to Peak (ttPeak) | 1.86 (1.02, 3.42) | 0.045 | 0.804 |
| VWF Antigen (VWF: Ag) | 3.78 (1.65, 8.62) | 0.002 | |
| Peak Height (PH) | 0.91 (0.51, 1.64) | 0.76 | 0.774 |
| VWF Antigen (VWF: Ag) | 4.09 (1.83, 9.14) | < 0.001 | |
| Time to Peak (ttPeak) | 2.72 (1.30, 5.68) | 0.008 | 0.794 |
| D-dimer | 2.13 (1.01, 4.52) | 0.048 |
p-value < 0.05 is considered statistically significant, and noted in bold text.
Table 6b.
Three variable logistic regression model correlating thrombin generation kinetics parameters and von Willebrand factor antigen to admission status for COVID-19 patients.
| Parameters | Odds Ratio (95% CI) | p-valuea | C-statistic |
|---|---|---|---|
| Lag time (LT) | 2.56 (1.25, 5.27) | 0.011 | 0.846 |
| Peak Height (PH) | 0.95 (0.52, 1.73) | 0.86 | |
| VWF Antigen (VWF: Ag) | 2.98 (1.25, 7.10) | 0.014 |
p-value < 0.05 is considered statistically significant, and noted in bold text.
5. Discussion
Studies on the clinical utility of thrombin generation kinetics in COVID-19-associated coagulopathy are limited [[39], [40], [41]]. In this pilot study, we found that in vitro thrombin generation profiles of COVID-19 patients have features of both hypocoagulability (longer LT and ttPeak) and hypercoagulability (increased PH). Our data are similar to those of Chistolini et al. who showed increased LT and ttPeak along with greater ETP and PH in mechanically ventilated COVID-19 patients [39]. Prolonged LT and ttPeak for admitted COVID-19 patients in comparison to healthy volunteers was shown in another small study [42]. Morena-Barrio et al. demonstrated hypocoagulable thrombin generation parameters in hospitalized COVID-19 patients, as compared to patients hospitalized with non-SARS-CoV-2 pneumonia [41]. They found greater PH in patients with COVID-19 infection as compared to 12 healthy volunteers and pooled plasma [41]. Our pilot study adds a larger, heterogenous cohort of ED patients, that is likely reflective the population presenting for urgent care at many centers, with varying degrees of severity and symptom duration. Thrombin generation profiles in these patients differ from those seen in healthy volunteers. We hypothesize that COVID-19 infection results in an early cascade of accelerated coagulation and consumptive coagulopathy in vivo leading to the prolonged initiation of thrombin generation seen with greater symptom duration. The ongoing cycle of endothelial activation and dysregulated coagulation, both evidenced by our findings of increased VWF, may predispose these patients to thrombotic complications, including a high rate of VTE. We additionally identify a number of predictive models for admission status using thrombin generation and other plasma biomarkers, particularly VWF, at initial patient evaluation. A recently published study has shown that increased thrombin peak, in association with clinical features, was associated with clinical worsening (escalation of care or death) in a multivariable logistic regression model [43]. Ultimately, ours and similar models may also play a role in determining level of care, as studies have shown longer LT and ttPeak in critically ill patients with COVID-19 as compared to non-critically ill patients [40].
Our demonstration of a wide range of greater H3NUC levels in patients with COVID-19 infection, as compared to a relatively narrow range in healthy volunteers, supports the role of NETosis in COVID-19 related thromboinflammation. While H3NUC levels trended toward greater levels in COVID-19 patients, this did not reach significance, and we did not observe a difference in H3Free levels. This contrasts with the findings of Ng et al. who demonstrated significantly greater H3NUC levels in COVID-19 patients as compared to healthy volunteers, but this difference was only true of patients requiring high levels of respiratory support (>5 L oxygen by nasal cannula) [43]. Similarly, Zuo et al. demonstrated increased H3Free levels in hospitalized COVID-19 patients, nearly one-third of whom required mechanical ventilation [27]. Unlike these studies, only 31% of our patients required any respiratory support. It is likely that greater differences would be seen in a more severely ill patient cohort, as indicated by the observation that patients in our cohort that required admission did have greater H3Free levels than healthy volunteers. It is important to note that we assessed only two independent markers of NETosis, specifically citrullinated histones (H3NUC) and H3Free. There are multiple additional methods of quantifying NETosis, including MPO-DNA complexes and indirect measurement through cell-free DNA levels, which have been previously shown to be increased in COVID-19 infection [27,28]. While circulating cell-free DNA levels can be non-specific and from cells other than neutrophils, the measurement of MPO-DNA complexes may not reliably reflect in vivo NETosis [44]. Our ELSA-based assay for measuring H3NUC has been recently shown to have high antibody specificity, superior nucleosome-based calibration standards, negligible intra- and inter-lot variability, and robust recovery for measuring circulating NETs in human plasma [34]. We have also recently demonstrated that the commercially available assay for H3Free positively correlates to this newly validated assay for H3NUC [34,45].
Our quantitative analysis of VWF multimers revealed a decrease in high molecular weight (HMW) VWF multimers in COVID-19 patients, suggesting a possible consumptive process. This is similar to the findings of other recently published cohort studies that have shown a decrease in HMW VWF multimers and reduced ADAMTS-13 activity in COVID-19 infection [46,47]. It has been proposed that the relative decrease in VWF multimers in COVID-19 patients may be due to an early increase in VWF proteolysis, due to in vivo consumption of HMW multimers in VWF-platelet aggregates, or due to altered regulation of VWF multimers by ADAMTS-13 [46,47]. In our study, COVID-19 patients who required hospital admission had greater levels of VWF antigen, activity, and Factor VIII, as well as lower relative levels of HMW VWF multimers than those who did not. This suggests that VWF levels and multimer size may be markers of disease severity, particularly in combination with thrombin generation profiles. VWF antigen levels, along with thrombin generation kinetics, have potential clinical utility in the ED for predicting need for admission with symptomatic COVID-19 infection.
This study has several limitations. First, this is a pilot study with a small number of patients, limiting our ability to link plasma biomarkers with clinical outcomes, such as VTE. Additionally, because we are using samples from a single time point, at patient presentation in ED, we have captured a heterogeneous population of COVID-19 patients with varying disease severity and symptom duration. It is likely that symptom duration and disease severity influence inflammation, endothelial activation, and coagulation. We also had relatively few critically ill patients in this cohort, so our results may be under-representing the coagulopathy seen in that patient population. Immunothrombosis is modulated by a complex interaction of additional pathways, including complement activation, which we did not evaluate in this pilot study. Complement mediated microangiopathies (TMA) and a robust complement driven immune response to SARS-CoV-2 infection may be responsible for much of the end-organ damage observed in severe COVID-19 infection, including renal and pulmonary dysfunction [48,49]. We were unable to assess the contribution of complement activation and resultant cytokine release in COVID-associated coagulopathy in our cohort. COVID-19-associated coagulopathy remains an urgent clinical challenge, and ongoing prospective studies and clinical trials are needed to gain mechanistic insight, design algorithms of individualized risk stratification, and identify potential novel therapeutic targets.
6. Conclusions
Symptomatic COVID-19 patients presenting to the ED have prolonged initiation of thrombin generation, especially those who required hospital admission. Greater VWF, Factor VIII and a trend toward increased NETosis in these patients suggest thromboinflammation as a driving force in COVID-19-associated coagulopathy. Plasma biomarkers, particularly the combination of thrombin generation parameters and VWF, may have utility in risk stratification during urgent evaluation of COVID-19 patients in the ED.
Addendum (Author contributions)
J. Goswami, T. MacArthur, M. Sridharan, and M.S. Park contributed substantially to concept and design, analysis and interpretation of data, critical writing, and final approval of this manuscript. A. Kirmse, T.Y. Chon, R.H. Hurt, B. B. Salonen, and R. Ganesh assisted with study concept and design. D. Chen and J. Tange were involved in data analysis and interpretation. K. Lundell aided with study concept and design as well as analysis and interpretation of data. M. Auton, Y.M. Erben, C.P. Marquez, J.F. Dong, R.A. Kozar, S. Heller, and E.A. Loomis participated in analysis and interpretation of data, critical revising of intellectual content, and final approval.
Funding
This project was supported by T32 AG049672 from the National Institute of Aging (NIA) and Robert and Arlene Kogod Center for Aging, Mayo Clinic (JG), R38HL150086 Stimulating Access to Research in Residency (TAM) from the National Heart, Lung, and Blood Institute (NHLBI), HL146508 from the NHLBI (MA), UM1 HL120877-06 (MSP) by the Trans-Agency Consortium for Trauma-Induced Coagulopathy (TACTIC), EpiCypher (ALJ) is supported by the NIH under award numbers R43AI134162 from the National Institute of Allergy and Infectious Diseases (NIAID), R43GM131560 from the National Institute of General Medical Sciences (NIGMS), and R44AI134162 (NIAID). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: EpiCypher is a commercial developer and supplier of platforms like those used in this study: recombinant semi-synthetic nucleosomes (dNucs), antibody validation platforms, and nucleosome based H3Cit assays (e.g. EpiCypher Catalog No. R&D143001). ALJ is an inventor on patents covering use of recombinant nucleosomes carrying histone or DNA modifications for antibody validation and assay quantification. All other authors declare no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the Clinical Research Unit (CRU) of the Center for Translational Science Activities (CTSA) at Mayo Clinic for their 24-h support in sample collection. We thank our research coordinators Michael J. Ferrara, Joseph M. Immermann, and Joel Anderson for their hard work. We are grateful for the technical assistance received from Tammy L. Price-Troska and Timothy M. Halling for sample analysis.
Footnotes
Co-First Authors: Dr. Julie Goswami and Dr. Taleen A. MacArthur.
References
- 1.Roberts L.N., Whyte M.B., Georgiou L., Giron G., Czuprynska J., Rea C., Vadher B., Patel R.K., Gee E., Arya R. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136:1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hippensteel J.A., Burnham E.L., Jolley S.E. Prevalence of venous thromboembolism in critically ill patients with COVID-19. Br. J. Haematol. 2020;190:e134–e137. doi: 10.1111/bjh.16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi G., Lee J.J., Jamil A., Gunnam V., Najafi H., Memar Montazerin S., Shojaei F., Marszalek J. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. J. Clin. Med. 2020;9 doi: 10.3390/jcm9082489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demelo-Rodriguez P., Cervilla-Munoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macias M., Toledo-Samaniego N., Garcia-Garcia A., Garcia-Fernandez-Bravo I., Ji Z., de-Miguel-Diez J., Alvarez-Sala-Walther L.A., Del-Toro-Cervera J., Galeano-Valle F. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb. Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mestre-Gomez B., Lorente-Ramos R.M., Rogado J., Franco-Moreno A., Obispo B., Salazar-Chiriboga D., Saez-Vaquero T., Torres-Macho J., Abad-Motos A., Cortina-Camarero C., Such-Diaz A., Ruiz-Velasco E., Churruca-Sarasqueta J., Munoz-Rivas N., Infanta Leonor Thrombosis Research G. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J. Thromb. Thrombolysis. 2021;51:40–46. doi: 10.1007/s11239-020-02190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhary R., Padrnos L., Wysokinska E., Pruthi R., Misra S., Sridharan M., Wysokinski W., McBane R.D., 2nd, Houghton D.E. Macrovascular thrombotic events in a Mayo clinic enterprise-wide sample of hospitalized COVID-19-positive compared with COVID-19-negative patients. Mayo Clin. Proc. 2021;96:1718–1726. doi: 10.1016/j.mayocp.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutch C., Thrombosis C., Kaptein F.H.J., Stals M.A.M., Grootenboers M., Braken S.J.E., Burggraaf J.L.I., van Bussel B.C.T., Cannegieter S.C., Ten Cate H., Endeman H., Gommers D., van Guldener C., de Jonge E., Juffermans N.P., Kant K.M., Kevenaar M.E., Koster S., Kroft L.J.M., Kruip M., et al. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID-19 in the second and first wave. Thromb. Res. 2021;199:143–148. doi: 10.1016/j.thromres.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edler C., Schroder A.S., Aepfelbacher M., Fitzek A., Heinemann A., Heinrich F., Klein A., Langenwalder F., Lutgehetmann M., Meissner K., Puschel K., Schadler J., Steurer S., Mushumba H., Sperhake J.P. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int. J. Leg. Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapkiewicz A.V., Mai X., Carsons S.E., Pittaluga S., Kleiner D.E., Berger J.S., Thomas S., Adler N.M., Charytan D.M., Gasmi B., Hochman J.S., Reynolds H.R. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joly B.S., Siguret V., Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1603–1606. doi: 10.1007/s00134-020-06088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goswami J., MacArthur T.A., Sridharan M., Pruthi R.K., McBane R.D., 2nd, Witzig T.E., Park M.S. A review of pathophysiology, clinical features, and management options of COVID-19 associated coagulopathy. Shock. 2021;55:700–716. doi: 10.1097/SHK.0000000000001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trimaille A., Curtiaud A., Marchandot B., Matsushita K., Sato C., Leonard-Lorant I., Sattler L., Grunebaum L., Ohana M., Von Hunolstein J.J., Andres E., Goichot B., Danion F., Kaeuffer C., Poindron V., Ohlmann P., Jesel L., Morel O. Venous thromboembolism in non-critically ill patients with COVID-19 infection. Thromb. Res. 2020;193:166–169. doi: 10.1016/j.thromres.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuels J.M., Coleman J.R., Moore E.E., Bartley M., Vigneshwar N., Cohen M., Silliman C.C., Sauaia A., Banerjee A. Alternative complement pathway activation provokes a hypercoagulable state with diminished fibrinolysis. Shock. 2020;53:560–565. doi: 10.1097/SHK.0000000000001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P., Bacon C.L., Gaule R., Gillett A., Byrne M., Ryan K., O'Connell N., O'Sullivan J.M., Conlon N., O'Donnell J.S. More on COVID-19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020;189:1060–1061. doi: 10.1111/bjh.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikdeli B., Madhavan M.V., Gupta A., Jimenez D., Burton J.R., Der Nigoghossian C., Chuich T., Nouri S.N., Dreyfus I., Driggin E., Sethi S., Sehgal K., Chatterjee S., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Bertoletti L., Giri J., et al. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb. Haemostasis. 2020;120:1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonaventura A., Vecchie A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., Dentali F., Montecucco F., Massberg S., Levi M., Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., Dela Cruz C.S., Dumont A., Halene S., Hwa J., Koff J., Menninger H., Neparidze N., Price C., Siner J.M., Tormey C., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escher R., Breakey N., Lammle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escher R., Breakey N., Lammle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb. Res. 2020;192:174–175. doi: 10.1016/j.thromres.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemostasis. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzeffi M.A., Chow J.H., Tanaka K. COVID-19 associated hypercoagulability: manifestations, mechanisms, and management. Shock. 2021;55:465–471. doi: 10.1097/SHK.0000000000001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi H., Yang S., Zhang L. Neutrophil extracellular traps and endothelial dysfunction in atherosclerosis and thrombosis. Front. Immunol. 2017;8:928. doi: 10.3389/fimmu.2017.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta A.K., Joshi M.B., Philippova M., Erne P., Hasler P., Hahn S., Resink T.J. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo Y., Zuo M., Yalavarthi S., Gockman K., Madison J.A., Shi H., Woodard W., Lezak S.P., Lugogo N.L., Knight J.S., Kanthi Y. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis. 2021;51:446–453. doi: 10.1007/s11239-020-02324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., Herrmann I., Sturzl M., Staats L., Mahajan A., Schauer C., Kremer A.N., Volkl S., Amann K., Evert K., Falkeis C., Wehrfritz A., Rieker R.J., Hartmann A., Kremer A.E., Neurath M.F., et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., Muenchhoff M., Hellmuth J.C., Ledderose S., Schulz H., Scherer C., Rudelius M., Zoller M., Hochter D., Keppler O., Teupser D., Zwissler B., von Bergwelt-Baildon M., Kaab S., Massberg S., et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park M.S., Spears G.M., Bailey K.R., Xue A., Ferrara M.J., Headlee A., Dhillon S.K., Jenkins D.H., Zietlow S.P., Harmsen W.S., Ashrani A.A., Heit J.A. Thrombin generation profiles as predictors of symptomatic venous thromboembolism after trauma: a prospective cohort study. J. Trauma Acute Care Surg. 2017;83:381–387. doi: 10.1097/TA.0000000000001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemker H.C. Recollections on thrombin generation. J. Thromb. Haemostasis. 2008;6:219–226. doi: 10.1111/j.1538-7836.2008.02864.x. [DOI] [PubMed] [Google Scholar]
- 33.Hemker H.C., Al Dieri R., De Smedt E., Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb. Haemostasis. 2006;96:553–561. [PubMed] [Google Scholar]
- 34.Thalin C., Aguilera K., Hall N.W., Marunde M.R., Burg J.M., Rosell A., Daleskog M., Mansson M., Hisada Y., Meiners M.J., Sun Z.W., Whelihan M.F., Cheek M.A., Howard S.A., Saxena-Beem S., Noubouossie D.F., Key N.S., Sheikh S.Z., Keogh M.C., Cowles M.W., et al. Quantification of citrullinated histones: development of an improved assay to reliably quantify nucleosomal H3Cit in human plasma. J. Thromb. Haemostasis. 2020;18:2732–2743. doi: 10.1111/jth.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goswami J., MacArthur T., Bailey K., Spears G., Kozar R.A., Auton M., Dong J.F., Key N.S., Heller S., Loomis E., Hall N.W., Johnstone A.L., Park M.S. Neutrophil extracellular trap formation and syndecan-1 shedding are increased after trauma. Shock. 2021;56:433–439. doi: 10.1097/SHK.0000000000001741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen D., Tange J.I., Meyers B.J., Pruthi R.K., Nichols W.L., Heit J.A. Validation of an automated latex particle-enhanced immunoturbidimetric von Willebrand factor activity assay. J. Thromb. Haemostasis. 2011;9:1993–2002. doi: 10.1111/j.1538-7836.2011.04460.x. [DOI] [PubMed] [Google Scholar]
- 37.Austin C.O., Chen D., Thomas C.S., Safford R.E., Shapiro B.P., Bryan J.A., Ray J.C., Blackshear J.L. Von Willebrand factor multimer quantitation for assessment of cardiac lesion severity and bleeding risk. Res. Pract. Thromb Haemost. 2018;2:155–161. doi: 10.1002/rth2.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackshear J.L., Wysokinska E.M., Safford R.E., Thomas C.S., Stark M.E., Shapiro B.P., Ung S., Johns G.S., Chen D. Indexes of von Willebrand factor as biomarkers of aortic stenosis severity (from the Biomarkers of Aortic Stenosis Severity [BASS] study) Am. J. Cardiol. 2013;111:374–381. doi: 10.1016/j.amjcard.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chistolini A., Ruberto F., Alessandri F., Santoro C., Barone F., Cristina Puzzolo M., Ceccarelli G., De Luca M.L., Mancone M., Alvaro D., Pulcinelli F.M., Martelli M., Foa R., Pugliese F., Policlinico Umberto I.C.-G. Effect of low or high doses of low-molecular-weight heparin on thrombin generation and other haemostasis parameters in critically ill patients with COVID-19. Br. J. Haematol. 2020;190:e214–e218. doi: 10.1111/bjh.17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White D., MacDonald S., Edwards T., Bridgeman C., Hayman M., Sharp M., Cox-Morton S., Duff E., Mahajan S., Moore C., Kirk M., Williams R., Besser M., Thomas W. Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int. J. Lab. Hematol. 2021;43:123–130. doi: 10.1111/ijlh.13329. [DOI] [PubMed] [Google Scholar]
- 41.de la Morena-Barrio M.E., Bravo-Perez C., Minano A., de la Morena-Barrio B., Fernandez-Perez M.P., Bernal E., Gomez-Verdu J.M., Herranz M.T., Vicente V., Corral J., Lozano M.L. Prognostic value of thrombin generation parameters in hospitalized COVID-19 patients. Sci. Rep. 2021;11:7792. doi: 10.1038/s41598-021-85906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benati M., Salvagno G.L., Nitto S., Gelati M., Lavorgna B., Fava C., Minuz P., Lippi G. Thrombin generation in patients with Coronavirus disease 2019. Semin. Thromb. Hemost. 2021;47:447–450. doi: 10.1055/s-0041-1722844. [DOI] [PubMed] [Google Scholar]
- 43.Ng H., Havervall S., Rosell A., Aguilera K., Parv K., von Meijenfeldt F.A., Lisman T., Mackman N., Thalin C., Phillipson M. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler. Thromb. Vasc. Biol. 2021;41:988–994. doi: 10.1161/ATVBAHA.120.315267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayden H., Ibrahim N., Klopf J., Zagrapan B., Mauracher L.M., Hell L., Hofbauer T.M., Ondracek A.S., Schoergenhofer C., Jilma B., Lang I.M., Pabinger I., Eilenberg W., Neumayer C., Brostjan C. ELISA detection of MPO-DNA complexes in human plasma is error-prone and yields limited information on neutrophil extracellular traps formed in vivo. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goswami J., MacArthur T., Bailey K., Spears G., Kozar R.A., Auton M., Dong J.F., Key N.S., Heller S., Loomis E., Hall N.W., Johnstone A.L., Park M.S. Neutrophil extracellular trap formation and syndecan-1 shedding are increased after trauma. Shock. 2021 doi: 10.1097/SHK.0000000000001741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mancini I., Baronciani L., Artoni A., Colpani P., Biganzoli M., Cozzi G., Novembrino C., Boscolo Anzoletti M., De Zan V., Pagliari M.T., Gualtierotti R., Aliberti S., Panigada M., Grasselli G., Blasi F., Peyvandi F. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J. Thromb. Haemostasis. 2021;19:513–521. doi: 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward S.E., Fogarty H., Karampini E., Lavin M., Schneppenheim S., Dittmer R., Morrin H., Glavey S., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Mallon P.W., Curley G.F., Baker R.I., Budde U., O'Sullivan J.M., JS OD, Irish C-VSi. ADAMTS13 regulation of VWF multimer distribution in severe COVID-19. J. Thromb. Haemostasis. 2021 doi: 10.1111/jth.15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gavriilaki E., Brodsky R.A. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br. J. Haematol. 2020;189:e227–e230. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]