Barrett’s esophagus (BE) is a precancerous condition in which normal squamous esophageal mucosa is replaced by specialized intestinal metaplasia.1 It is the main risk factor for esophageal adenocarcinoma. Patients who are high risk for BE and who meet screening criteria are eligible for endoscopy.2 If dysplastic BE is found, then patients undergo ablation therapy, which can decrease the progression to advanced neoplasia.3 , 4
The coronavirus disease 2019 (COVID-19) has affected health care throughout the world. With healthcare efforts geared toward curtailing the virus, cancer screening program resources have decreased,5 especially those involving elective procedures in the United States.6 , 7 It is unclear how the pandemic has affected the diagnosis and therapy of BE and esophageal cancer in the United States.
We conducted a retrospective study using the large claims database, Premier Healthcare Database (Charlotte, NC). Details regarding the Premier Healthcare Database can be found in Supplementary Methods and Appendix 1. Forty months (January 2018 to April 2021) of Premier data were used. This study included 4 distinct cohorts: patients with newly diagnosed BE, patients with BE with endoscopic ablation, patients with newly diagnosed esophageal cancer, and patients with esophagectomy for esophageal and gastric cardia cancer. Cohort details and full definitions can be found in Supplementary Methods.
The primary outcome was the change in the monthly number of newly diagnosed BE cases, number of endoscopic ablation cases performed, number of new diagnosed esophageal cancer cases, and number of esophagectomies performed during the pandemic compared with baseline. Average and absolute numbers of cases per month were reported.
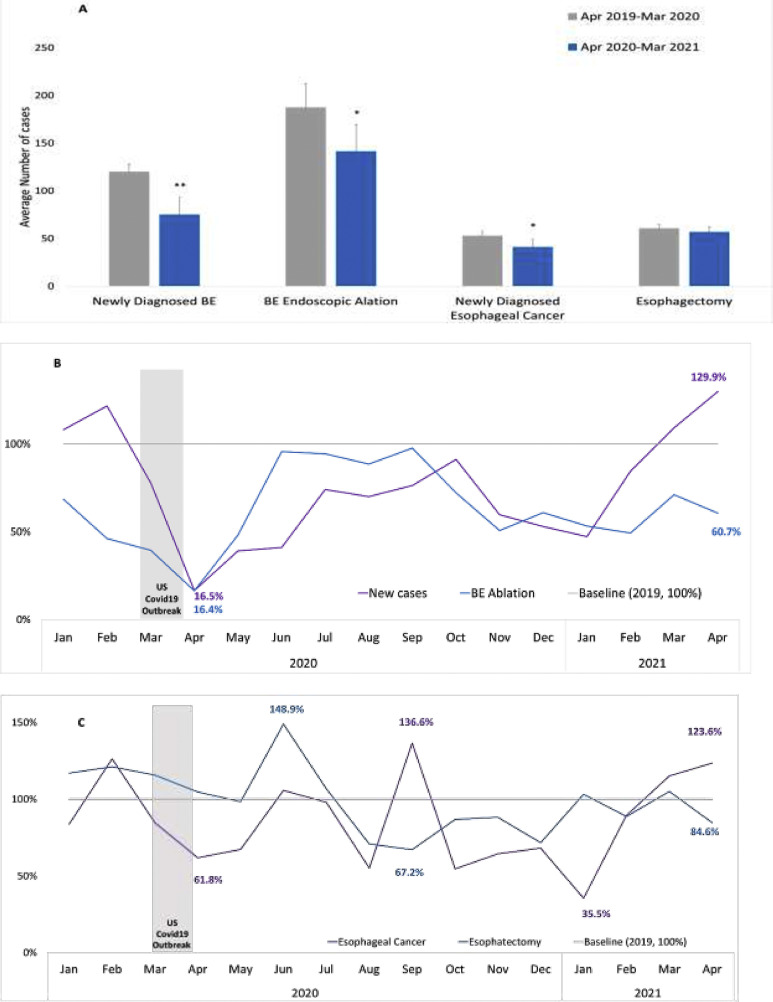
Figure 1A compares the average number of monthly cases before (April 2019 to March 2020) and after (April 2020 to March 2021) the COVID-19 pandemic for newly diagnosed BE, BE endoscopic ablation, newly diagnosed esophageal cancer, and patients with esophagectomy. Compared with data before the pandemic, except esophagectomy (P = .2373), patient monthly volume in all cohorts decreased significantly since the pandemic (P < .0001, P = .0135, and P = .0145 for newly diagnosed BE, BE endoscopic ablation, and newly diagnosed esophageal cancer, respectively).
Figure 1.
(A) Average monthly cases before and during COVID-19 and monthly temporal trends. (B) Newly diagnosed BE cases and BE ablation cases. (C) Newly diagnosed esophageal cancer and esophagectomy cases. ∗∗P < .0001, ∗P < .05.
Figure 1B shows the temporal trends in the monthly newly diagnosed BE patient volume and BE endoscopic ablation between January 2019 and April 2021. The monthly number of newly diagnosed BE cases or BE endoscopic ablation cases in 2019 was used as a baseline (100% line). The number of newly diagnosed BE cases were 16.5% and 129.9% of the baseline in April 2020 and April 2021, respectively. The number of BE endoscopic ablation cases was 16.4% and 60.7% of the baseline in April 2020 and April 2021, respectively.
Figure 1C shows the temporal trends for newly diagnosed esophageal cancer and patients with esophagectomy between January 2019 and April 2021. The monthly number of newly diagnosed esophageal cancer cases or esophagectomy cases in 2019 was used as a baseline (100% line). The number of newly diagnosed esophageal cancer patients was 61.8% and 123.6% of the baseline in April 2020 and April 2021, respectively. The number of esophagectomy cases was 148.9% and 84.6% of the baseline in June 2020 and April 2021, respectively.
Supplementary Table 1A shows the average number of monthly cases yearly and quarterly, and Supplementary Table 1B shows the average number monthly. Compared with the corresponding months in 2019, between April 2020 and June 2020 (second quarter) the average number of monthly cases for newly diagnosed BE and BE endoscopic ablation deceased by 68.3% (P = .0009) and 51.8% (P = .0685), respectively. Compared with the corresponding months in 2020, between January and March 2021 (first quarter) the average number of monthly cases for all cohorts had no significant difference (P > .05).
The results indicate that early esophageal cancer likely was undiagnosed or untreated during COVID-19. Meanwhile, those undergoing esophagectomy were more likely to be advanced cancers that are symptomatic. One would expect a rising trend for the diagnosis of BE and esophageal cancer for the first quarter of 2021 to compensate for the decrease. This is seen in our data; however, this trend is not statistically significant compared with 2020 data. In addition, the decrease in ablation of BE is concerning because dysplastic lesions are high risk for progression to cancer. The expected consequence of our findings would be a sharp rise of diagnosis of esophageal cancer, which is likely too early to see in our study period.
To date 1 Irish study examined the impact of COVID-19 on the diagnosis of esophageal cancer and BE.8 A 26% decline in esophagogastric cancer and a 59% decrease in BE diagnosis was observed. This study only examined the pandemic period from March to September 2020 and did not examine ablation and esophagectomy trends.
Our study has several strengths. It uses a large database of BE patients that capture unique patients who can be tracked between visits. In addition, we only included patients in which the hospitals reported data for the entire study period. This allows for a more accurate estimation of the impact of COVID-19 on the endoscopic or surgical therapy utilization for diagnosis and therapy of BE and esophageal cancer.
Our study does have limitations. The billing codes cannot distinguish between esophageal adenocarcinoma and squamous cell cancer or the stage of cancer. However, our aim was to examine all esophageal cancer in the United States. In addition, this accuracy of this dataset depends on the accuracy of billing codes, as is the case for all large insurance claims databases.
In conclusion, we show from a large database that the new diagnosis of BE and esophageal cancer decreased during COVID-19 along with endoscopic ablation therapy; however, the number of esophagectomies was not significantly impacted. In addition, when comparing the first quarter of 2021 with 2020, there was no significant difference in the average number of cases across all cohorts noted that would compensate for the decreased number of cases.
Acknowledgments
CRediT Authorship Contributions
Conception and design (AJT, JZ). Analysis and interpretation of the data (AJT, JZ). Drafting of the article (AJT, JZ). Critical revision of the article for important intellectual content (AJT, JZ, JH, CL, PI). Final approval of the article (AJT, JZ, JH, CL, PI).
Footnotes
Conflicts of interest These authors disclose the following: Arvind J. Trindade is a consultant for Pentax Medical. Jianying Zhang and John Hauschild are employees of Medtronic. Prasad G. Iyer receives research funding from Exact Sciences and Pentax Medical and is a consulting for Medtronic. The remaining author discloses no conflicts.
Funding None.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org and at https://doi.org/10.1053/j.gastro.2021.11.020.
Supplementary Methods
Premier Healthcare Database Information
The Premier Healthcare Database is a large, US hospital–based, service-level, all-payer database. Inpatient admission data include over 127 million visits with more than 11 million per year since 2012, representing approximately 25% of annual US inpatient admissions. Outpatient encounters include over 947 million outpatient visits with more than 102 million visits per year since 2012. Outpatient visits to emergency departments, ambulatory surgery centers, and alternate sites of care are included. The Premier Healthcare Database contains data from over 244 million unique patients. Patients can be tracked in the same hospital across the inpatient and hospital-based outpatient settings. More than 700 hospitals provide yearly data since 2012.1 In terms of data quality, for most data elements, less than 1% of patient records have missing information, and for key elements, such as demographics and diagnostic information, less than 0.01% have missing data.2 This database was chosen becasue it includes both inpatients and outpatients, which allows the estimation of the number of cases of newly diagnosed BE, endoscopic ablation procedures, which are usually outpatient procedures, and newly diagnosed esophageal cancer; and surgical esophagectomy, which requires an inpatient recovery.
The Premier Healthcare Database is considered exempt from Institutional Review Board oversight as dictated by Title 45 Code of Federal Regulations, Part 46 of the United States, specifically 45 CFR 46.101(b)(4). In accordance with the HIPAA Privacy Rule, disclosed data from Premier are considered deidentified per 45 CFR 164.506(d)(2)(ii)(B) through the “Expert Determination” method.
Patients Cohorts
For patients with newly diagnosed BE, we included all adult patients who were newly diagnosed with BE in each month during the study period. BE was identified by using International Classification of Diseases, 10th revision (ICD-10-CM) diagnosis codes of BE (Appendix 1). “Newly diagnosed BE” was defined as no BE diagnosis was found within 12 months before the new BE diagnosis or when an esophagogastroduodenoscopy (EGD) was performed within 7 days to 3 months before new BE diagnosis.
For BE patients with endoscopic ablation, we included all adult patients who were diagnosed with BE and had an endoscopic ablation procedure during our study period. Exclusion criteria were patients who had a diagnosis of esophageal or stomach cancer 6 months before the index diagnosis, who had an esophageal ablation procedure within 6 months before the index diagnosis, and who had an endoscopic resection on the same day of endoscopic esophagus ablation. Endoscopic esophagus ablation and endoscopic resection were identified by using the Current Procedural Terminology (CPT) codes (Appendix 1). Billing codes for radiofrequency ablation and cryotherapy are the same and thus cannot be differentiated within a dataset.
For patients with esophagectomy, we included all adult patients who had an esophagectomy procedure for esophageal cancer (including gastric cardia cancer). Esophagectomy was identified by using ICD-10-PCS surgical codes. Esophageal cancer was identified by using ICD-10-CM diagnosis codes of esophageal cancer (Appendix 1).
For patients with newly diagnosed esophageal cancer, we included all adult patients who were newly diagnosed with esophageal cancer each month during the study period. The newly diagnosed esophageal cancer diagnosis was defined as 12 months before the new diagnosis, no esophageal or stomach cancer found, and EGD performed within 7 days to 3 months before the new diagnosis. Esophageal cancer was identified by using ICD-10-CM diagnosis codes of esophageal cancer (Appendix 1).
Data from January 2019 to April 2021 was used to identify the cases for each cohort. We used calendar year 2018 data to identify preceding diagnosis or procedures to meet our selection criteria for new diagnosed cases. Claims with service date within 12 months before new diagnosed cases were examined for previous BE or cancer diagnosis.
On March 18, 2020, the Centers for Medicare & Medicaid Services issued guidance that all nonurgent surgeries and medical procedures should be delayed. On April 19, 2020, the Centers for Medicare & Medicaid Services issued the first in a series of recommendations on how states and regions with stabilized COVID-19 outbreaks that meet certain criteria can begin reinstituting elective surgeries and medical procedures.
Statistics
To examine the impact of COVID-19 on patient volume in selected cohorts, first we compared the average monthly patient volume before (April 2019 to March 2020) and during (April 2020 to March 2021) the pandemic. Second, we broke down each year into 4 quarters and compared the average monthly patient volume within each quarter with the corresponding quarter of the prior year (such as 2019 quarter 1 vs 2020 quarter 2). Finally, we reported the absolute number of cases in each month during our study period; we also used 2019 volumes as baseline (treated number of cases in 2019 as 1 [100%]) and calculated the proportion of 2020 and 2021 volumes to the baseline (number of cases in 2020–2021 divided by number of cases in 2019 in each corresponding month) to illustrate the change in patient volumes. Only hospitals with continuous data input for the entire study period (defined as at least 1 claim in 2019, 1 claim in 2020, and 1 claim in January to April 2021) were included.
Bivariate analysis was used to compare the difference in the number of cases between different time periods. A 2-tailed t test was used for testing the statistical significance and was set at P < .05. Data preparation and analyses were performed using SAS software (version 9.4; SAS Institute, Inc, Cary NC).
Appendix 1.
Related Codes for Identifying Disease and Procedures
| ICD-10 diagnosis codes | CPT codes | |
|---|---|---|
| BE | K22.70 Barrett's esophagus without dysplasia K22.710 Barrett's esophagus with low-grade dysplasia K22.711 Barrett's esophagus with high-grade dysplasia K22.719 Barrett's esophagus with dysplasia, unspecified D00.1 carcinoma in situ of esophagus |
|
| Esophageal cancer | C15.3 Malignant neoplasm of upper third of esophagus C15.4 Malignant neoplasm of middle third of esophagus C15.5 Malignant neoplasm of lower third of esophagus C15.8 Malignant neoplasm of overlapping sites of esophagus C15.9 Malignant neoplasm of esophagus, unspecified |
|
| Stomach cancer | C16.0 Malignant neoplasm of cardia C16.1 Malignant neoplasm of fundus of stomach C16.2 Malignant neoplasm of body of stomach C16.3 Malignant neoplasm of pyloric antrum C16.4 Malignant neoplasm of pylorus C16.5 Malignant neoplasm of lesser curvature of stomach, unspecified C16.6 Malignant neoplasm of greater curvature of stomach, unspecified C16.8 Malignant neoplasm of overlapping sites of stomach C16.9 Malignant neoplasm of stomach, unspecified |
|
| ICD-10 surgical procedure codes | ||
| Esophagectomy | 0DT10ZZ Resection of upper esophagus, open approach 0DT14ZZ Resection of upper esophagus, percutaneous endoscopic approach 0DT17ZZ Resection of upper esophagus, via natural or artificial opening; resection of upper esophagus, via natural or artificial opening endoscopic; 0DT20ZZ Resection of middle esophagus, open approach 0DT24ZZ Resection of middle esophagus, percutaneous endoscopic approach 0DT27ZZ Resection of middle esophagus, via natural or artificial opening 0DT28ZZ Resection of middle esophagus, via natural or artificial opening endoscopic 0DT30ZZ Resection of lower esophagus, open approach 0DT34ZZ Resection of lower esophagus, percutaneous endoscopic approach 0DT37ZZ Resection of lower esophagus, via natural or artificial opening 0DT38ZZ Resection of lower esophagus, via natural or artificial opening endoscopic 0DT50ZZ Resection of esophagus, open approach 0DT54ZZ Resection of esophagus, percutaneous endoscopic approach 0DT57ZZ Resection of esophagus, via natural or artificial opening 0DT58ZZ Resection of esophagus, via natural or artificial opening endoscopic |
|
| ICD-10 surgical procedure codes | CPT codes | |
|---|---|---|
| Esophageal endoscopic ablation | 43228 Esophagoscopy, with ablation of tumor(s), polyp(s), or other lesion(s), not amenable to removal by hot biopsy forceps, bipolar cautery or snare technique 43229 Esophagoscopy, flexible, transoral; with ablation of tumor(s), polyp(s), or other lesion(s) (includes pre- and post-dilation and guide wire passage, when performed) 43258 Esophagoscopy, rigid or flexible; diagnostic with ablation of tumor(s), polyp(s), or other lesion(s) not amenable to removal by hot biopsy forceps, bipolar cautery or snare technique 43270 Esophagogastroduodenoscopy, flexible, transoral; with ablation of tumor(s), polyp(s), or other lesion(s) (includes pre- and post-dilation and guide wire passage, when performed) |
|
| Earlier endoscopic resection of nodular BE | 43211 Esophagoscopy, flexible, transoral; with endoscopic mucosal resection 43216 Esophagoscopy, flexible, transoral; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps 43217 Esophagoscopy, flexible, transoral; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique 43250 Esophagogastroduodenoscopy, flexible, transoral; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps 43251 Esophagogastroduodenoscopy, flexible, transoral; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique 43254 Esophagogastroduodenoscopy, flexible, transoral; with EMR (endoscopic mucosal resection) |
|
| EGD | 43239 Esophagogastroduodenoscopy, flexible, transoral; with biopsy, single or multiple 43202 Esophagoscopy, flexible, transoral; with biopsy, single or multiple 43251 EGD with snare polypectomy (remove nodular Barrett’s) 43254 EGD with EMR (remove nodular or flat Barrett’s) 43211 Esophagoscopy, flexible, transoral; with endoscopic mucosal resection 43217 Esophagoscopy, flexible, transoral; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique. 43193 Esophagoscopy, rigid, transoral; with biopsy, single or multiple 43198 Esophagoscopy, flexible, transnasal; with biopsy, single or multiple |
EMR, endoscopic mucosal resection.
Supplementary Table 1.
Average Number of Monthly Cases During Year 2019 to April 2021
| A (Yearly and Quarterly) | |||||
|---|---|---|---|---|---|
| N (95% CI) |
N (95% CI) |
%change∗ | P value | ||
| Apr 2019-Mar 2020 | Apr 2020-Mar 2021 | ||||
| Newly diagnosed BE | 119.8 (111.7-128.0) | 75.0 (56.5-93.5) | 37.4 | <0.0001 | |
| BE endoscopic ablation | 187.3 (162.4-212.1) | 141.4 (113.3-169.6) | 24.5 | 0.0135 | |
| Newly diagnosed esophageal cancer | 52.7 (47.6-57.7) | 40.9 (32.6-49.3) | 22.3 | 0.0145 | |
| Esophagectomy | 60.5 (56.4-64.6) | 56.6 (50.8-62.3) | 6.5 | 0.2373 | |
| CY2019∗∗ | CY2020 | ||||
| Jan-Mar | Newly diagnosed BE | 105.0 (87.611-122.4) | 107.0 (57.5-156.5) | -1.9 | 0.8777 |
| BE endoscopic ablation | 310.7 (249.1-372.2) | 160.7 (30.7-290.6) | 48.3 | 0.0109 | |
| Newly diagnosed esophageal cancer | 53.0 (20.5-85.5) | 50.0 (45.0-55.0) | 5.7 | 0.7145 | |
| Esophagectomy | 56.7 (49.1-64.3) | 65.3 (59.6-71.1) | -15.3 | 0.0173 | |
| Apr-Jun | Newly diagnosed BE | 119.7 (103.7-135.6) | 38.0 (1.2-74.8) | 68.3 | 0.0009 |
| BE endoscopic ablation | 223.3 (107.9-338.7) | 107.7 (-56.9-272.2) | 51.8 | 0.0685 | |
| Newly diagnosed esophageal cancer | 52.0 (44.5-59.5) | 40.7 (9.8-71.5) | 21.8 | 0.1994 | |
| Esophagectomy | 62.0 (37.5-86.5) | 69.7 (52.2-78.1) | -12.4 | 0.334 | |
| Jul-Sept | Newly diagnosed BE | 123.0 (105.6-140.4) | 90.3 (80.3-100.4) | 26.6 | 0.0022 |
| BE endoscopic ablation | 174.3 (159.4-189.3) | 163.0 (144.2-181.8) | 6.5 | 0.112 | |
| Newly diagnosed esophageal cancer | 52.3 (19.3-85.4) | 47.0 (23.3-70.7) | 10.2 | 0.6029 | |
| Esophagectomy | 68.0 (50.6-85.4) | 58.0 (31.7-84.3) | 14.7 | 0.244 | |
| Oct-Dec | Newly diagnosed BE | 129.7 (119.6-139.7) | 87.7 (28.4-146.9) | 32.4 | 0.0397 |
| BE endoscopic ablation | 190.7 (154.5-226.9) | 117.0 (-56.3-177.7) | 38.6 | 0.0109 | |
| Newly diagnosed esophageal cancer | 56.3 (28.4-84.3) | 34.7 (24.3-45.0) | 38.5 | 0.0352 | |
| Esophagectomy | 59.0 (43.9-74.1) | 50.7 (32.7-68.6) | 14.1 | 0.2014 | |
| CY2020 | CY2021 | ||||
| Jan-Mar | Newly diagnosed BE | 107.0 (57.5-156.5) | 84.0 (1.9-166.1) | 21.5 | 0.3602 |
| BE endoscopic ablation | 160.7 (30.7-290.6) | 178/0 (126.1-229.9) | -10.8 | 0.6223 | |
| Newly diagnosed esophageal cancer | 50.0 (45.0-55.0) | 41.3 (-17.9-100.6) | 17.3 | 0.5647 | |
| Esophagectomy | 65.3 (59.6-71.1) | 64.0 (56.5-71.5) | 2.0 | 0.5748 | |
| B (Monthly) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Newly diagnosed BE |
BE endoscopic ablation |
Newly diagnosed esophageal cancer |
Esophagectomy |
||||||||
| 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | |
| Month | ||||||||||||
| Jan | 110 | 119 | 52 | 315 | 216 | 168 | 62 | 52 | 22 | 53 | 62 | 64 |
| Feb | 97 | 118 | 82 | 333 | 154 | 164 | 38 | 48 | 34 | 52 | 63 | 56 |
| Mar | 108 | 84 | 118 | 284 | 112 | 202 | 59 | 50 | 68 | 51 | 59 | 62 |
| Apr | 127 | 21 | 165 | 275 | 45 | 167 | 55 | 34 | 68 | 62 | 65 | 55 |
| May | 115 | 45 | 210 | 101 | 49 | 33 | 63 | 62 | ||||
| Jun | 117 | 48 | 185 | 177 | 52 | 55 | 47 | 70 | ||||
| Jul | 116 | 86 | 180 | 170 | 49 | 48 | 59 | 63 | ||||
| Aug | 130 | 91 | 175 | 155 | 67 | 37 | 72 | 51 | ||||
| Sep | 123 | 94 | 168 | 164 | 41 | 56 | 67 | 45 | ||||
| Oct | 126 | 115 | 201 | 145 | 66 | 36 | 61 | 53 | ||||
| Nov | 129 | 77 | 197 | 100 | 59 | 38 | 51 | 45 | ||||
| Dec | 134 | 71 | 174 | 106 | 44 | 30 | 60 | 43 | ||||
Percentage change from later period to the corresponding prior period. Negative (-) indicates the volume increase.
CY, calendar year. Bolded number indicates P value < 0.05.
References
- 1.Shaheen N.J., et al. Am J Gastroenterol. 2016;111:30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qumseya B., et al. Gastrointest Endosc. 2019;90:335–359. doi: 10.1016/j.gie.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen N.J., et al. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 4.Phoa K.N., et al. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 5.Alkatout I., et al. Front Oncol. 2021;11:675038. doi: 10.3389/fonc.2021.675038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro Filho E.C., et al. Gastrointest Endosc. 2020;92:440–445. doi: 10.1016/j.gie.2020.03.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu P.W.Y., et al. Gut. 2020;69:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turkington R.C., et al. Gastroenterology. 2021;160:2169–2171. doi: 10.1053/j.gastro.2021.01.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary References
- 1.Premier Incorporated. Prem Appl Sci. 2020;2:1–15. [Google Scholar]
- 2.Fisher B.T., et al. In: Pharmacoepidemiology. 2012:244–258. https://onlinelibrary.wiley.com/doi/10.1002/9781119959946.ch16. [Google Scholar]