Abstract
Colorectal cancer (CRC) represents one of the most frequent malignancies in terms of incidence and mortality, thus representing the third leading cause of cancer death worldwide. In the last decade, few drugs have enriched the treatment landscape of metastatic CRC and have significantly affected prognosis. Unlike other neoplasms, metastatic CRC patients who have exhausted treatment options often still maintain a good performance status. There are many challenges to increasing potential treatment options, notably a better understanding of disease biology and the mechanisms of resistance underlying cancer treatment failure. The development of new drugs for metastatic CRC certainly represents one of the most important challenges in medical oncology. This article discusses the main limitations in the development of new drugs and potential future scenarios. In particular, we addressed three questions: (1) The main limitations of targeted therapy in the treatment of metastatic CRC (mCRC); (2) New target armamentarium that could escape primary and secondary resistance and lead to more personalized mCRC therapy; and (3) Future directions.
Keywords: Colon cancer, Colon rectal cancer, New drugs, Drug resistance, Metastatic colorectal cancer
Core Tip: Although metastatic colorectal cancer (CRC) is a relevant oncological issue, few drugs have changed clinical practice in the last decade. In fact, there are many difficulties in the development of new drugs closely related to the biology of CRC; however, improved knowledge of the molecular biology of this cancer has led to a few steps forward and the hope for more targeted cancer treatments for metastatic CRC patients in the near future.
INTRODUCTION
Targeted therapy has drastically changed the oncological landscape by modifying the natural history of numerous oncological pathologies. Colorectal cancer (CRC) patients were among the first to benefit from the introduction of targeted therapy a decade ago, following a better understanding of the molecular biology of metastatic colorectal cancer (mCRC) and the advent of anti-vascular endothelial growth factor (VEGF) and anti-epidermal growth factor receptor (EGFR) drugs, such as bevacizumab, cetuximab and panitumumab. This was followed by the introduction of a multikinase molecule, regorafenib. Despite these advances, CRC is still one of the leading causes of cancer-related deaths, being the world's fourth most deadly cancer, with almost 900000 deaths annually[1]. Furthermore, the 5-year survival for metastatic colon cancer patients to date remains below 15%[2]. For this reason, it is of fundamental importance to enrich the therapeutic scenario of mCRC, and drugs that can impact not only mCRC overall survival but also quality of life are desperately needed.
MAIN LIMITATIONS OF TARGETED THERAPY IN THE TREATMENT OF MCRC
The use of anti-EGFR target drugs, such as cetuximab and panitumumab, and anti-angiogenesis drugs, such as bevacizumab and aflibercept, are consolidated in the clinical practice of metastatic CRC as first- and second-line treatments. Regorafenib is a multikinase drug approved for third-line treatment. In recent years, numerous new agents have emerged that block various critical pathways; however, many studies involving drugs that have led to excellent results in other tumours have not yielded the expected results in the treatment of mCRC. The main causes of treatment failure in mCRC are complex downstream signalling, difficulties in completely inhibiting specific biological interactions for the compensatory activation of other signalling pathways, and innate or acquired resistance to treatment (Figure 1).
Figure 1.
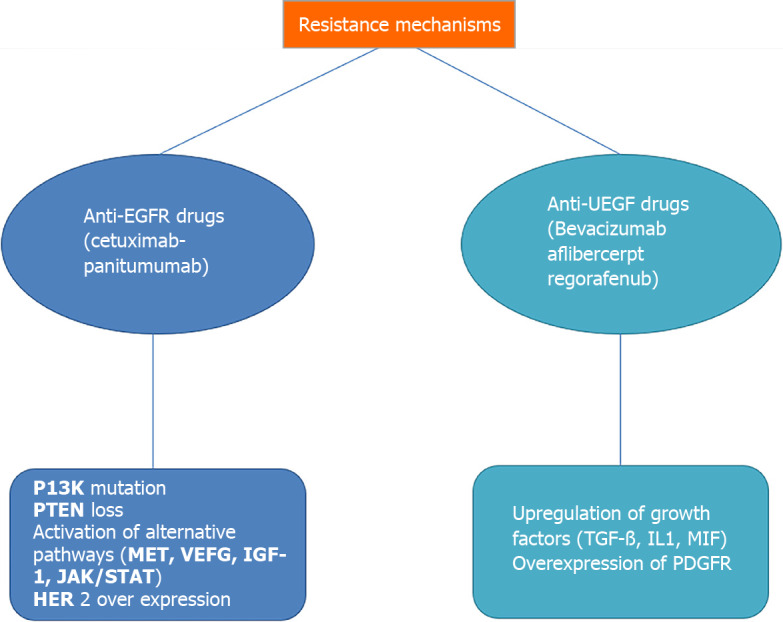
Main resistance mechanisms in targeted treatment for metastatic colorectal cancer. EGFR: Epidermal growth factor receptor; VEGF: Vascular endothelial growth factor; TGF-β: Transforming growth factor-β; IL1: Interleukin 1.
An emblematic example of the compensatory activation of other signalling pathways is explained by the history of anti-BRAF drugs in BRAF V600E-mutated mCRC compared to melanoma. Mutations in the BRAF isoform, especially V600E, of the RAF protein are present in approximately 5% to 10% of CRCs[3]. The results of BRAF inhibitors in melanoma have led to an enthusiastic development of anti-BRAF/MEK drugs in CRC; however, blockade of BRAF or BRAF/MEK did not lead to a gain in PFS (progression-free survival) or OS (overall survival) of metastatic CRC patients, although it did result in inhibition of downstream MAPK activity[4]. A possible explanation is that blocking BRAF/MEK could trigger EGFR feedback reactivation, which would bypass MAPK activation via RAS[5]. Based on this evidence, subsequent studies have focused on the combined use of BRAF inhibitors and EGFR inhibitors[6], ultimately leading to FDA and EMA approval of the combination encorafenib, binimetinib and cetuximab in second- or third-line mCRC. This indication is the result of the BEACON study, which demonstrated a benefit in terms of overall survival (OS: 9 vs 5.4 mo, HR = 0.52, P < 0.001) and response rate (RR: 26% vs 2%, P < 0.01) with a good safety profile[7].
Innate and acquired resistance mechanisms are very complex and affect both anti-EGFR and anti-VEGF drugs.
Concerning anti-EGFR drugs, the main known mechanisms of resistance are RAS mutations, PI3K mutations, PTEN loss, HER2 overexpression, and compensative activation of alternative pathways.
HER2 is a protein member of the Erb family (erythroblastosis oncogene B Erb)/human epidermal growth factor receptor HER). In patients with CRC, the HER2 overexpression rate is 2%–3% and it is independent of the RAS or RAF mutation. HER2 acts similarly to EGFR (HER1), sharing many downstream pathways, such as RAS/RAF/MEK and PI3K/AKT. For this reason, HER overexpression provides a logical explanation for anti-EGFR resistance[8]. Preclinical and clinical studies have shown that combined targeting of HER2 and EGFR can lead to a better result than those gained with the use of a single agent alone[9]. For Her2-overexpressing disease, unlike in breast cancer, the single inhibition of her2 does not seem to be effective in mCRC, which is likely linked to compensatory mechanisms and the activation of other pathways[10]. Further research is needed to better understand the clinical significance of HER2 gene amplification in mCRC. An illustrative example has been reported in MyPathway, a clinical trial investigating the activity of pertuzumab + trastuzumab in patients with HER2-amplified mCRC, in which eight (8/57) patients had no response[11].
Compensatory activation of alternative pathways, such as c-MET, VEGF, insulin-like growth factor receptor 1 (IGF-1R), and JAK/STAT, could be linked to acquired resistance to anti-EGFR drugs[12].
Regarding antiangiogenic drugs, there are currently three approved drugs for mCRC: bevacizumab, a humanized monoclonal antibody that binds to vascular endothelial growth factor (VEGF-A) administered as a first- and second-line treatment; aflibercept, a recombinant fusion protein composed of fragments of VEGF receptors fused with the Fc portion of human IgG1 approved for second-line treatment; and regorafenib, an oral multikinase inhibitor approved for third-line treatment. There are several intrinsic and secondary known resistance mechanisms for antiangiogenic drugs.
In particular, these resistance mechanisms underlie the difficulty in translating preclinical successes into actual clinical advantage. For example, unlike preclinical studies, bevacizumab improves clinical outcomes only when combined with chemotherapy, with a short disease response to the withdrawal of antiangiogenic drugs, as tumour vessels rapidly grow back after stopping treatment. Compensatory mechanisms in antiangiogenic drug-resistant disease could be the upregulation of growth factors such as TGF-β, IL-1,231 MIF (macrophage migration inhibitory factor) and the overexpression of other growth factor receptors such as PDGFR[13].
Ultimately, the complexity of intrinsic and secondary resistance mechanisms in the targeted treatment of patients with metastatic CRC makes this pathology a challenging oncological dilemma.
NEW TARGET ARMAMENTARIUM THAT COULD ESCAPE PRIMARY AND SECONDARY RESISTANCE AND LEAD TO MORE PERSONALIZED MCRC THERAPY
Primary and secondary drug resistance represents the main limitation of CRC care, especially concerning targeted therapies; however, new promising drugs and drug combinations are expected to modify this complex scenario.
As mentioned before, patients with the BRAF V600E mutation have a worse prognosis, and the median overall survival (OS) is less than 1 year vs 2 years for patients without the non-BRAF V600E mutation[3]. In the phase III study BEACON trial, the small molecule BRAF V600E inhibitor encorafenib was combined with binimetinib, a MEK1/2 inhibitor, and cetuximab. The trial showed improved overall survival in both the triplet arm (cetuximab, binimetinib and encorafenib) and doublet arm (cetuximab and encorafenib). The median OS was 9.0 and 8.4 mo, respectively; however, the PFS was approximately 4 mo in both arms[7]. A possible explanation for this short PFS could be the reactivation of MEK and ERK signalling. An ERK 1/2 inhibitor, ulixertinib, is under investigation in a phase I trial, although data from a CRC cohort have not been reported[14].
The EGFR family also includes the HER2 receptor. Activating alterations of this receptor have been detected in approximately 2%–3% of RAS and RAF wild-type colon cancer cases[15]. Many phase II trials have explored the potential use of HER2 inhibitors in mCRC, including a combination of drugs such as trastuzumab and lapatinib in the HERACLES trial[16], trastuzumab and tucatinib, an orally administered HER2–3 inhibitor, in the MOUNTANEER trial[17], and pertuzumab and trastuzumab-emtansine (TDM1) in the HERACLES-B trial[18]. All these trials showed potential activity in terms of ORR and PFS in pretreated metastatic CRC HER2-amplificated patients with the combination of Her2 inhibitor blockade. To date, no drugs have been approved for Her2-amplified CRC.
New promising molecules are also being explored in the VEGF inhibitor setting. In particular, a multicentre phase III study, FRESCO-2, comparing placebo vs fruquintinib (NCT04322539), is ongoing. Fruquintinib is a highly selective small molecule inhibitor of VEGFR 1, 2, 3[19]. In the first FRESCO trial, the fruquintinib group showed a median OS of 9.3 mo vs 6.6 in the placebo group (P < 0.001) and a PFS of 3.7 mo vs 1.8 mo. Due to the encouraging results obtained with this trial in China, it has been extended and is now recruiting in Europe and the United States[20].
Current studies are also investigating the potential role of the combination of VEGF with conventional chemotherapy. Trifluridine/tipiracil (TAS-102) was associated with bevacizumab in a phase II study of 93 patients. The association improved both PFS and OS compared with TAS-102 alone[21]. Trifluridina/tipiracil is under investigation with many other drugs, probably due to its low toxicity profile and the absence of cross-resistance with 5-fluorouracil in pretreated patients.
Another crucial new perspective for patients with mCRC is immunotherapy. Currently, immunotherapy has been approved in the United States and will be approved in Europe for patients with microsatellite-deficient mismatch repair/microsatellite instability-high (dMMR/MSI-high), which affects approximately 15% of all patients with mCRC[22]. The clinical trial KEYNOTE 177 comparing pembrolizumab, a PD-1 inhibitor, with standard chemotherapy showed a substantial improvement in PFS with pembrolizumab as the first line in dMMR/MSI-high mCRC[23]. This trial thus represents a practice-changing approach in the first-line therapy of patients with dMMR/MSI metastatic CRC. In the same way, the CheckMate 142 phase II trial investigated the association of nivolumab and ipilimumab in pretreated dMMR/MSI metastatic CRC. The combined treatment, such as for melanoma cancer, showed high response rates and favourable progression-free survival and OS at 12 mo with a low toxicity profile[24].
Unfortunately, immunotherapy is a missed opportunity for patients with proficient mismatch repair and microsatellite stable (pMMR/MSS) mCRC. Many ongoing studies are exploring the possibility of combining immune checkpoint inhibitors with VEGF inhibitors to enhance lymphocyte activation. In preclinical models, VEGF inhibitors showed synergistic action with immune checkpoint inhibition[25]. Based on this evidence, regorafenib was combined with nivolumab in a phase Ib trial (REGONIVO) in patients with refractory metastatic gastric and CRC, obtaining a median PFS of 5.6 and 7.9 mo, respectively[26]. Similarly, in the REGOMUNE phase II trial, the combination of regorafenib and avelumab showed a median progression-free survival of 3.6 mo and overall survival of 10.8 mo[27]. Bevacizumab has been combined with atezolizumab and the triplet chemotherapy regimen FOLFOXIRI (oxaliplatin, irinotecan and 5-fluorouracil) in the AtezoTRIBE trial for patients with unresectable or metastatic CRC. The results are not yet available[28].
Recent data showed a promising combination of avelumab and cetuximab in a rechallenge strategy for RAS and RAF wild-type mCRC patients. In a preliminary analysis, the CAVE study showed a median OS of 13.1 mo and a median PFS of 3.6 mo[29]).
The complexity of the resistance mechanisms, multiple escape pathways and disease biology make metastatic CRC a challenging disease in terms of therapeutic strategies. Fortunately, patients with mCRC maintain a good performance status even during disease progression. A very large number of new drugs or combinations are under investigation to reach an even more personalized cancer cure.
FUTURE DIRECTIONS
Currently, negative predictive markers for the response to EGFR-targeted therapies (KRAS, NRAS mutations), anti-BRAF targeted therapies (BRAF mutation) and positive predictive markers for immune checkpoint inhibitors (microsatellite instability) are standard of care in the treatment of mCRC. In all the main arms of medical oncology, the future seems to lead towards a great and ambitious goal represented by personalized medicine. A large area of research is concentrated on this trend, focusing on next-generation sequencing (NGS)[30] (Table 1).
Table 1.
Main ongoing studies (clinicaltrial.gov) for metastatic colorectal cancer
|
Study
|
Treatment
|
Phase of study
|
Primary objectives
|
| COLOMATE trial | |||
| NCT03765736 | Specific targeted treatment arms based on the molecular profiles | Phase II prospective trial | (1) To perform blood-based genomic profiling on patients with treatment refractory metastatic colorectal cancer (CRC) to facilitate accrual to molecularly assigned therapies; and (2) To facilitate clinically annotated genomic analyses |
| CALGB (Alliance)/SWOG 80405 | |||
| NCT00265850 | Bevacizumab or cetuximab combined with the same chemotherapy | Phase III, randomized, open-label, multicentre study | To determine if the addition of cetuximab to FOLFIRI or FOLFOX chemotherapy prolongs survival compared to FOLFIRI or FOLFOX with bevacizumab in patients with untreated, advanced or metastatic colorectal cancer who have K-ras wild type tumours |
| KRYSTAL 10 | |||
| NCT04793958 | MRTX849 (inhibitor of KRAS G12C) in Combination with Cetuximab vs Chemotherapy | Phase III, open-label, randomized | Comparing the efficacy of MRTX849 administered in combination with cetuximab vs chemotherapy in the second-line treatment setting in patients with CRC with KRAS G12C mutation |
| C-PRECISE-01 | |||
| NCT04495621 | MEN1611 + Cetuximab | Phase Ib/II, open-label, multicentre study | MEN1611, a PI3K Inhibitor, and Cetuximab in Patients With PIK3CA Mutated Metastatic Colorectal Cancer Failing Irinotecan, Oxaliplatin, 5-FU and Anti-EGFR Containing Regimens |
| NCT04096417 | Pemigatinib | phase II, multicentre, single-Arm study | To assess overall response rate (ORR) of pemigatinib in patients with metastatic or unresectable CRC harbouring activating FGFR alterations |
| MOUNTAINEER | |||
| NCT03043313 | Trastuzumab+tucatinib | Phase II open label study | Tucatinib combined with trastuzumab in patients with HER2+ metastatic colorectal cancer |
| NAVIGATE | |||
| NCT02576431 | Larotrectinib | Phase II open label study | Investigate the efficacy of larotrectinib for the treatment of advanced solid tumours harbouring a fusion of neurotrophic tyrosine receptor kinase (NTRK) of types 1–3 in children and adults |
| NCT03829410 | Onvansertib (PCM-075) | Phase Ib/II open label study | Determine the safety and efficacy of Onvansertib in combination with FOLFIRI + Avastin, as second-line treatment in adult patients who have metastatic colorectal cancer with a Kras mutation |
| STARTRK-2 | |||
| NCT02568267 | Entrectinib (RXDX-101) | Phase 2 basket study | Treatment of patients with Locally Advanced or metastatic solid tumours that harbour NTRK1/2/3, ROS1, or ALK gene rearrangements |
| NCT03724851 | Vactosertib (TGF-β receptor I kinase inhibitor) + pembrolizumab | Phase 2, open label study | Safety, tolerability, pharmacokinetics and antitumour activity of vactosertib in combination with pembrolizumab in patients with mCRC including CMS4 or diffuse GC/GEJC |
EGFR: Epidermal growth factor receptor; TGF-β: Transforming growth factor-β.
According to the European Society for Medical Oncology (ESMO) guidelines, in colon cancers, NGS could be an alternative to PCR[31]. This method has led to the identification of mutations that could explain greater resistance to standard treatments[32,33] as well as new targets whose therapeutic effects are being studied.
Concerning the use of NGS to identify patients who are likely to respond to standard treatments, few interesting studies have been conducted. One example is the study conducted by Innocenti and colleagues that analysed the response to standard treatments with cetuximab or bevacizumab-based regimens and the results that emerged from NGS. Mutated genes that conferred worse overall survival (OS) than wild-type (WT) tumours and mutations that conferred better survival were highlighted. For example, FANCI-mutated tumours (4%) conferred worse OS than WT tumours [HR 2.0 (1.2–3.3), P = 0.005; OR 5.0 (1.9–14.8), P = 0.002][34]. These findings are very interesting, as they could provide new genes that could become predictors of response to chemotherapy regimens with cetuximab and bevacizumab combinations. If validated in other phase III trials, these mutated genes could be used to guide treatment decisions in mCRC patients.
Another interesting ongoing trial is the COLOMATE umbrella trial, which uses the genomic profiling Guardant360 NGS assay, a plasma-based assay of more than 70 genes, to detect colorectal tumour cfDNA to assign patients with advanced CRC to specific targeted treatment arms based on the molecular profiles of their tumours (NCT03765736). It is fascinating to think that this approach could become our clinical practice in the very near future.
Listed below are some new targets we believe could represent a potential innovation in the near future: KRAS, PI3K, NTRK fusions, ALK, ROS1, RET, and FGFR.
KRAS and NRAS mutations occur in a consistent percentage of mCRC cases (approximately 50% of cases) and identify tumours with a poor prognosis. Mutated KRAS tumours are also inherently resistant to anti-EGFR drugs. KRAS is considered a challenging therapeutic target (Cox AD). Nevertheless, among the different RAS mutations, the KRAS pG12C mutation, which represents approximately 1%–4% of RAS mutations in CRC[35], has been considered potentially druggable. In particular, KRAS-dependent signalling is inhibited by binding to a pocket near the nucleotide binding site and locking it in an inactive guanosine diphosphate (GDP)-bound state[36]. Two drugs are currently under investigation in colon cancer, sotorasib (AMG510) and adagrasib (MRTX849). The first phase I study, CodeBreack100, investigated the activity of sotorasib in 129 pretreated patients with the KRAS G12C mutation, including 42 patients with mCRC[37]. In the colorectal cohort, the overall response rate (ORR) was 7.1% and the disease control rate was 73.8% (DCR). The median duration of stable disease was 4 mo. Overall, these results were considered disappointing in terms of quality, duration, and adaptive signalling response to drug treatment. The authors postulated that KRAS G12C-mutant cancer cells may still become activated upstream by EGFR[38]. For this reason, ongoing studies combining KRAS G12C inhibitors and EGFR inhibitors, such as the randomized phase 3 clinical trial comparing MRTX849 in combination with cetuximab vs chemotherapy in patients with advanced CRC, KRYSTAL 10 (NCT04793958), are ongoing.
Another fundamental oncological driver in CRCs is the PTEN/PI3K/mTOR pathway (20% of cases). The presence of the PI3K mutation confers resistance to anti-EGFR treatments. For this reason, combinations of PI3K oral inhibitors with cetuximab are being studied. This approach, which has been successful in patients with a BRAF mutation[7], another driver that confers resistance to treatments with anti EGFR, could be applicable in patients with a PI3K mutation[39].
Neurotrophic receptor tyrosine kinase (NTRK) fusions are chromosomal abnormalities that result in uncontrolled TRK signalling that can lead to cancer. NTRK fusions can be identified with NGS, immunohistochemistry (IHC), polymerase chain reaction (PCR), and fluorescent in situ hybridization (FISH) techniques. NTRK fusion-positive mCRC is rare (0.9%)[40]. For this reason, a clinical indication to seek the presence of a NTRK fusion is lacking; however, with increasingly accessible NGS, this target will be necessary to evaluate. In mCRC, NTRK fusions are more frequent in elderly patients, in females, and in right-sided tumours. From the point of view of molecular biology, they are often associated with MSI-H, RAS and BRAF wild-type, as well as poor prognosis with a median overall survival (OS) of approximately 15 mo[41]. Regarding the efficacy of treatments with the oral TRK-selective inhibitors larotrectinib and entrectinib, registration studies of these two molecules, including a very low number of patients with mCRC (4 patients for larotrectinib and only 1 patient for entrectinib), are scarce given the rarity of NTRK fusion in colon cancer; however, the data are encouraging. For example, in the 4 patients included in the single-arm study that evaluated the efficacy of larotrectinib, a partial response and disease control rate were achieved in 2 and 4 cases, respectively[42]. The data are too scarce to draw any conclusions but favourable given the poor prognosis of this category of patients.
Other very rare mutations in mCRC are rearrangements of anaplastic lymphoma kinase (ALK) and v-ros avian UR2 sarcoma virus oncogene homologue (ROS1)[43]. The future use of oral tyrosine kinase inhibitors, such as alectinib and crizotinib, would be possible in patients with mCRC who present such rearrangements. RET (rearranged during transfection) fusions are even rarer in mCRC (2% of cases)[44], although the message is the same: could a mutation, albeit rare, be successfully treated with a specific drug already used in clinical practice for other solid tumours? These are the considerations we will need to be increasingly familiar with in the near future. Unfortunately, we have no data from clinical trials on the use of selective RET inhibitor drugs in mCRC. A case report of a patient with mCRC harbouring a RET fusion treated with a selective RET inhibitor achieved a complete response to the selective RET inhibitor drug RXDX-105 and a significant PFS of 19 mo[45].
Aberrant activation of fibroblast growth factor (FGFR) signalling has been implicated in the development of various cancers, including colon cancer. Several studies have been conducted to validate the efficacy of FGFR inhibitors in mCRC and other solid tumours (NCT04096417, NCT01976741, NCT03410693, NCT03473756).
Given all these data, the use of NGS will be essential in clinical practice for the treatment of mCRC.
CONCLUSION
Limitations in the development of novel CRC drugs are due to the mechanisms of resistance to target treatments, namely, EGFR antibodies and antiangiogenic treatments, which currently represent therapeutic options for mCRC. A better understanding of mCRC molecular biology has elucidated the resistance mechanisms and consequently enabled the development of combined treatments geared towards precision medicine. The advent of new effective therapies has been very slow in CRCs; in fact, the complexity of the mechanisms involved in the carcinogenesis of CRC makes it difficult to use single biological targets for the development of new drugs. Currently, many signs give hope for new potential possibilities in the treatment of this challenging cancer.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review started: April 2, 2021
First decision: June 23, 2021
Article in press: September 8, 2021
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mirallas O, Sano W S-Editor: Ma YJ L-Editor: A P-Editor: Li X
Contributor Information
Sara Cherri, Department of Oncology, Fondazione Poliambulanza, Brescia 25124, Italy. sara.cherri@poliambulanza.it.
Michela Libertini, Department of Oncology, Fondazione Poliambulanza, Brescia 25124, Italy.
Alberto Zaniboni, Department of Oncology, Fondazione Poliambulanza, Brescia 25124, Italy.
References
- 1.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med. 2020;9:361–373. doi: 10.1002/cam4.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tie J, Gibbs P, Lipton L, Christie M, Jorissen RN, Burgess AW, Croxford M, Jones I, Langland R, Kosmider S, McKay D, Bollag G, Nolop K, Sieber OM, Desai J. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer. 2011;128:2075–2084. doi: 10.1002/ijc.25555. [DOI] [PubMed] [Google Scholar]
- 4.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, Brown RD, Della Pelle P, Dias-Santagata D, Hung KE, Flaherty KT, Piris A, Wargo JA, Settleman J, Mino-Kenudson M, Engelman JA. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 6.Corcoran RB, André T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, Hollebecque A, McRee AJ, Siena S, Middleton G, Muro K, Gordon MS, Tabernero J, Yaeger R, O'Dwyer PJ, Humblet Y, De Vos F, Jung AS, Brase JC, Jaeger S, Bettinger S, Mookerjee B, Rangwala F, Van Cutsem E. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Cancer. Cancer Discov. 2018;8:428–443. doi: 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Pfeiffer P, Orlov S, Lonardi S, Elez E, Kim TW, Schellens JHM, Guo C, Krishnan A, Dekervel J, Morris V, Calvo Ferrandiz A, Tarpgaard LS, Braun M, Gollerkeri A, Keir C, Maharry K, Pickard M, Christy-Bittel J, Anderson L, Sandor V, Tabernero J. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 8.Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M, Fujisaka Y, Philips J, Shimizu T, Maenishi O, Cho Y, Sun J, Destro A, Taira K, Takeda K, Okabe T, Swanson J, Itoh H, Takada M, Lifshits E, Okuno K, Engelman JA, Shivdasani RA, Nishio K, Fukuoka M, Varella-Garcia M, Nakagawa K, Jänne PA. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luca T, Barresi V, Privitera G, Musso N, Caruso M, Condorelli DF, Castorina S. In vitro combined treatment with cetuximab and trastuzumab inhibits growth of colon cancer cells. Cell Prolif. 2014;47:435–447. doi: 10.1111/cpr.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramanathan RK, Hwang JJ, Zamboni WC, Sinicrope FA, Safran H, Wong MK, Earle M, Brufsky A, Evans T, Troetschel M, Walko C, Day R, Chen HX, Finkelstein S. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest. 2004;22:858–865. doi: 10.1081/cnv-200039645. [DOI] [PubMed] [Google Scholar]
- 11.Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R, Burris H, Sweeney C, Bose R, Spigel DR, Beattie MS, Blotner S, Stone A, Schulze K, Cuchelkar V, Hainsworth J. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–530. doi: 10.1016/S1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gacche RN, Assaraf YG. Redundant angiogenic signaling and tumor drug resistance. Drug Resist Updat. 2018;36:47–76. doi: 10.1016/j.drup.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan RJ, Infante JR, Janku F, Wong DJL, Sosman JA, Keedy V, Patel MR, Shapiro GI, Mier JW, Tolcher AW, Wang-Gillam A, Sznol M, Flaherty K, Buchbinder E, Carvajal RD, Varghese AM, Lacouture ME, Ribas A, Patel SP, DeCrescenzo GA, Emery CM, Groover AL, Saha S, Varterasian M, Welsch DJ, Hyman DM, Li BT. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study. Cancer Discov . 2018;8:184–195. doi: 10.1158/2159-8290.CD-17-1119. [DOI] [PubMed] [Google Scholar]
- 15.Nowak JA. HER2 in Colorectal Carcinoma: Are We There yet? Surg Pathol Clin . 2020;13:485–502. doi: 10.1016/j.path.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738–746. doi: 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 17.Strickler JH, Zemla T, Ou FS, Cercek A, Wu C, Sanchez FA, Hubbard J, Jaszewski B, Bandel L, Schweitzer B, Niedzwiecki D, Kemeny N, Boland PM, Ng K, Bekaii-Saab T. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): Initial results from the MOUNTAINEER trial. Ann Oncol. 2019;30:V200. [Google Scholar]
- 18.Sartore-Bianchi A, Lonardi S, Martino C, Fenocchio E, Tosi F, Ghezzi S, Leone F, Bergamo F, Zagonel V, Ciardiello F, Ardizzoni A, Amatu A, Bencardino K, Valtorta E, Grassi E, Torri V, Bonoldi E, Sapino A, Vanzulli A, Regge D, Cappello G, Bardelli A, Trusolino L, Marsoni S, Siena S. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open. 2020;5:e000911. doi: 10.1136/esmoopen-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Q, Zhou J, Zhang Z, Guo M, Liang J, Zhou F, Long J, Zhang W, Yin F, Cai H, Yang H, Gu Y, Ni L, Sai Y, Cui Y, Zhang M, Hong M, Sun J, Yang Z, Qing W, Su W, Ren Y. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther. 2014;15:1635–1645. doi: 10.4161/15384047.2014.964087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486–2496. doi: 10.1001/jama.2018.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeiffer P, Yilmaz M, Möller S, Zitnjak D, Krogh M, Petersen LN, Poulsen LØ, Winther SB, Thomsen KG, Qvortrup C. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:412–420. doi: 10.1016/S1470-2045(19)30827-7. [DOI] [PubMed] [Google Scholar]
- 22.Arnold CN, Goel A, Compton C, Marcus V, Niedzwiecki D, Dowell JM, Wasserman L, Inoue T, Mayer RJ, Bertagnolli MM, Boland CR. Evaluation of microsatellite instability, hMLH1 expression and hMLH1 promoter hypermethylation in defining the MSI phenotype of colorectal cancer. Cancer Biol Ther. 2004;3:73–78. doi: 10.4161/cbt.3.1.590. [DOI] [PubMed] [Google Scholar]
- 23.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 24.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 25.Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60. doi: 10.1186/s12943-019-0974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603) J Clin Oncol. 2020;38:2053–2061. doi: 10.1200/JCO.19.03296. [DOI] [PubMed] [Google Scholar]
- 27.Cousin S, Cantarel C, Guegan JP, Gomez-Roca C, Metges JP, Adenis A, Pernot S, Bellera C, Kind M, Auzanneau C, Le Loarer F, Soubeyran I, Bessede A, Italiano A. Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE): A Single-arm, Open-label, Phase II Trial. Clin Cancer Res. 2021;27:2139–2147. doi: 10.1158/1078-0432.CCR-20-3416. [DOI] [PubMed] [Google Scholar]
- 28.Antoniotti C, Borelli B, Rossini D, Pietrantonio F, Morano F, Salvatore L, Lonardi S, Marmorino F, Tamberi S, Corallo S, Tortora G, Bergamo F, Brunella DS, Boccaccino A, Grassi E, Racca P, Tamburini E, Aprile G, Moretto R, Boni L, Falcone A, Cremolini C. AtezoTRIBE: a randomised phase II study of FOLFOXIRI plus bevacizumab alone or in combination with atezolizumab as initial therapy for patients with unresectable metastatic colorectal cancer. BMC Cancer. 2020;20:683. doi: 10.1186/s12885-020-07169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinelli E, Martini G, Troiani T, Pietrantonio F, Avallone A, Normanno N, Nappi A, Maiello E, Falcone A, Santabarbara G, Pinto C, Santini D, Ciardiello D, Terminiello M, Borrelli C, Napolitano S, Renato D, Famiglietti V, Esposito L, Ciardiello F. Avelumab plus cetuximab in pre-treated RAS wild type metastatic colorectal cancer patients as a rechallenge strategy: The phase II CAVE (cetuximab-avelumab) mCRC study. Ann Oncol. 2020;31:(suppl_4). [Google Scholar]
- 30.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, Schnall-Levin M, White J, Sanford EM, An P, Sun J, Juhn F, Brennan K, Iwanik K, Maillet A, Buell J, White E, Zhao M, Balasubramanian S, Terzic S, Richards T, Banning V, Garcia L, Mahoney K, Zwirko Z, Donahue A, Beltran H, Mosquera JM, Rubin MA, Dogan S, Hedvat CV, Berger MF, Pusztai L, Lechner M, Boshoff C, Jarosz M, Vietz C, Parker A, Miller VA, Ross JS, Curran J, Cronin MT, Stephens PJ, Lipson D, Yelensky R. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, Normanno N, Scarpa A, Robson M, Meric-Bernstam F, Wagle N, Stenzinger A, Bonastre J, Bayle A, Michiels S, Bièche I, Rouleau E, Jezdic S, Douillard JY, Reis-Filho JS, Dienstmann R, André F. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Ciardiello F, Normanno N, Maiello E, Martinelli E, Troiani T, Pisconti S, Giuliani F, Barone C, Cartenì G, Rachiglio AM, Montesarchio V, Tonini G, Rizzi D, Cinieri S, Bordonaro R, Febbraro A, De Vita F, Orditura M, Fenizia F, Lambiase M, Rinaldi A, Tatangelo F, Botti G, Colucci G. Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next-generation sequencing: findings from the CAPRI-GOIM trial. Ann Oncol. 2014;25:1756–1761. doi: 10.1093/annonc/mdu230. [DOI] [PubMed] [Google Scholar]
- 33.Del Vecchio F, Mastroiaco V, Di Marco A, Compagnoni C, Capece D, Zazzeroni F, Capalbo C, Alesse E, Tessitore A. Next-generation sequencing: recent applications to the analysis of colorectal cancer. J Transl Med. 2017;15:246. doi: 10.1186/s12967-017-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Innocenti N, Rashid M, Wancen F, Ou X, Qu S, Denning M, Bertagnolli CD, Blanke A, Venook O, Kabbarah HJ. 4878 - Next-generation sequencing (NGS) in metastatic colorectal cancer (mCRC): novel mutated genes and their effect on response to therapy (Alliance) Ann Oncol. 2019;30 (suppl_5):v198–v252. [Google Scholar]
- 35.Neumann J, Zeindl-Eberhart E, Kirchner T, Jung A. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract. 2009;205:858–862. doi: 10.1016/j.prp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Nagasaka M, Li Y, Sukari A, Ou SI, Al-Hallak MN, Azmi AS. KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat Rev. 2020;84:101974. doi: 10.1016/j.ctrv.2020.101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, Bang YJ, Dy GK, Krauss JC, Kuboki Y, Kuo JC, Coveler AL, Park K, Kim TW, Barlesi F, Munster PN, Ramalingam SS, Burns TF, Meric-Bernstam F, Henary H, Ngang J, Ngarmchamnanrith G, Kim J, Houk BE, Canon J, Lipford JR, Friberg G, Lito P, Govindan R, Li BT. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amodio V, Yaeger R, Arcella P, Cancelliere C, Lamba S, Lorenzato A, Arena S, Montone M, Mussolin B, Bian Y, Whaley A, Pinnelli M, Murciano-Goroff YR, Vakiani E, Valeri N, Liao WL, Bhalkikar A, Thyparambil S, Zhao HY, de Stanchina E, Marsoni S, Siena S, Bertotti A, Trusolino L, Li BT, Rosen N, Di Nicolantonio F, Bardelli A, Misale S. EGFR Blockade Reverts Resistance to KRASG12C Inhibition in Colorectal Cancer. Cancer Discov. 2020;10:1129–1139. doi: 10.1158/2159-8290.CD-20-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong S, Kim S, Kim HY, Kang M, Jang HH, Lee WS. Targeting the PI3K signaling pathway in KRAS mutant colon cancer. Cancer Med. 2016;5:248–255. doi: 10.1002/cam4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamura R, Boichard A, Kato S, Sicklick JK, Bazhenova L, Kurzrock R. Analysis of NTRK Alterations in Pan-Cancer Adult and Pediatric Malignancies: Implications for NTRK-Targeted Therapeutics. JCO Precis Oncol. 2018;2018 doi: 10.1200/PO.18.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietrantonio F, Di Nicolantonio F, Schrock AB, Lee J, Tejpar S, Sartore-Bianchi A, Hechtman JF, Christiansen J, Novara L, Tebbutt N, Fucà G, Antoniotti C, Kim ST, Murphy D, Berenato R, Morano F, Sun J, Min B, Stephens PJ, Chen M, Lazzari L, Miller VA, Shoemaker R, Amatu A, Milione M, Ross JS, Siena S, Bardelli A, Ali SM, Falcone A, de Braud F, Cremolini C. ALK, ROS1, and NTRK Rearrangements in Metastatic Colorectal Cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx089. [DOI] [PubMed] [Google Scholar]
- 42.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houang M, Toon CW, Clarkson A, Sioson L, de Silva K, Watson N, Singh NR, Chou A, Gill AJ. ALK and ROS1 overexpression is very rare in colorectal adenocarcinoma. Appl Immunohistochem Mol Morphol. 2015;23:134–138. doi: 10.1097/PAI.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 44.Le Rolle AF, Klempner SJ, Garrett CR, Seery T, Sanford EM, Balasubramanian S, Ross JS, Stephens PJ, Miller VA, Ali SM, Chiu VK. Identification and characterization of RET fusions in advanced colorectal cancer. Oncotarget. 2015;6:28929–28937. doi: 10.18632/oncotarget.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietrantonio F, Di Nicolantonio F, Schrock AB, Lee J, Morano F, Fucà G, Nikolinakos P, Drilon A, Hechtman JF, Christiansen J, Gowen K, Frampton GM, Gasparini P, Rossini D, Gigliotti C, Kim ST, Prisciandaro M, Hodgson J, Zaniboni A, Chiu VK, Milione M, Patel R, Miller V, Bardelli A, Novara L, Wang L, Pupa SM, Sozzi G, Ross J, Di Bartolomeo M, Bertotti A, Ali S, Trusolino L, Falcone A, de Braud F, Cremolini C. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann Oncol. 2018;29:1394–1401. doi: 10.1093/annonc/mdy090. [DOI] [PubMed] [Google Scholar]


