Abstract
Introduction:
Treatments for brain cancer have radically evolved in the past decade due to a better understanding of the interplay between the immune system and tumors of the central nervous system (CNS). However, glioblastoma multiforme (GBM) remains the most common and lethal CNS malignancy affecting adults.
Areas covered:
The authors review the literature on glioblastoma pharmacologic therapies with a focus on trials of combination chemo-/immunotherapies and drug delivery platforms from 2015 to 2021.
Expert opinion:
Few therapeutic advances in GBM treatment have been made since the Food and Drug Administration (FDA) approval of the BCNU-eluting wafer, Gliadel, in 1996 and oral temozolomide (TMZ) in 2005. Recent advances in our understanding of GBM have promoted a wide assortment of new therapeutic approaches including combination therapy, immunotherapy, vaccines, and Car T-cell therapy along with developments in drug delivery. Given promising preclinical data, these novel pharmacotherapies for the treatment of GBM are currently being evaluated in various stages of clinical trials.
Keywords: Chimeric antigen receptor (CAR) t-cell therapy, chemotherapy, drug delivery, glioblastoma, immunotherapy, liposomes, polymersomes, vaccine
1. Introduction
Glioblastoma multiforme (GBM) is the most common type Q4 of adult primary brain tumor [1,2]. Its aggressive and heterogeneous nature presents a tremendous challenge to the management of patient care [1,2]. Despite decades of pre-clinical research and innovations in surgical, radiation and chemical therapeutics, the prognosis of GBM remains poor with median survival just under two years [1,2]. Several tumor characteristics specific to GBM render most standard chemotherapeutics ineffective, including its high level of invasiveness, elevated proliferative index, immunological escape capabilities, genetic heterogeneity, and genetic instability [3]. Furthermore, many novel treatment options face steep challenges in blood–brain barrier (BBB) permeability [4] and also targeting glioma stem-like cells (GSC) [5], a common mechanism of drug resistance. There is a desperate need for novel therapeutics and strategies that directly address the unique challenges of GBM treatment. Recent studies have focused on optimizing chemo and immunotherapeutic interventions, engineering more effective drug delivery systems, and implementing personalized care based on the genomic profile of the tumor. In this review, we focus on these innovations to discuss how optimizing pharmacological strategies can be translated into better patient care.
2. Optimization of current treatment strategies
Current strategies for GBM combine surgical resection and radiotherapy with concurrent and neo/adjuvant temozolomide and/or bevacizumab 6. Recent innovations in isocitrate dehydrogenase (IDH) inhibitors, nivolumab, and ipilimumab serve as interesting prospects in optimizing chemo- and immunotherapeutics. Here, we review the literature supporting first-line therapies and repurposed treatments, and explore their combination with up-and-coming therapeutics.
2.1. Temozolomide
Temozolomide (TMZ) is one of the most common therapeutics offered to patients with glioblastoma [7]. As a small lipophilic molecule, it has the ability to cross the blood–brain barrier with high fidelity, making it a useful drug for the treatment of brain tumors [8]. TMZ can damage DNA and trigger death in rapidly proliferating cells. After being rapidly converted to 3-methyl-(triazen-1-yl) imidazole-4-carboxamide (MTIC) by non-enzymatic chemical conversion, MTIC works by alkylating DNA and forcing repair mechanisms to take place in order to avert cell death. Temozolomide is responsible for increasing the median survival curve for patients with GBM to 14.6 months and has been shown to increase two year survival from 10.4% to 26.5%[8]. Despite its high fidelity in crossing the blood–brain barrier, TMZ’s short half-life warrants high dosages contributing to significant hematologic and hepatotoxicity [9]. Modern therapies aim to improve the efficacy of TMZ by exploring the efficiency of its delivery through liposomes and nanoparticles [10,11] as well as local delivery via BCNU wafers through in vitro and in vivo models [9]. MTIC methylates, among other sites, the O6-position of guanine, yielding the minor DNA adduct O6-methylguanine, which is a cytotoxic lesion. This therapy is effective for 45% of patients who down regulate expression of O6-methylguanine-DNA methyltransferase (MGMT), a gene which confers drug resistance by reversing the 06-methylation of guanine [6]. Fifty-five percent of patients, however, express O-methylguanine-DNA methyltransferase (MGMT), a gene which promotes drug resistance in these patients [6]. Several strategies are being studied to increase efficiency of TMZ via MGMT inhibition in order to decrease chemoresistance and improve survival [12].
2.2. Bevacizumab
Bevacizumab (BVZ) is a monoclonal antibody that targets vascular endothelial growth factor (VEGF) in order to inhibit tumor angiogenesis. The success of BVZ in slowing GBM tumor progression and increasing progression-free survival has already elevated it to a frontline therapy, though phase III clinical trials have yet to be completed [6]. Bevacizumab showed significant potential in preclinical work. Studies utilizing intravital multiphoton microscopy in mouse models showed that higher doses of BVZ reduced tumor growth and tumor cell viability [13] (Table 2). Furthermore, work done in nude mice demonstrated that BVZ checked growth in implanted gliomas [14,15] (Table 2) and decreased cerebellar edema [16] (Table 2). Taken together, these preclinical results and phase II trials indicate the potential use of BVZ in the management of GBM. Assessing treatment response to BVZ, however, presents significant imaging challenges due to the fact that pseudo response and non-enhancing tumor progression are common in T2-Flair [17] (Table 2). Additionally, negligible changes in overall patient survival limit the use of BVZ as a standalone therapy. More work needs to be done to explore its combined success with other chemotherapeutics, radiation, and surgery, and to quantify its ability to increase progression free survival and overall survival for patients with GBM.
Table 2.
Summary of Preclinical Studies on Glioblastoma Treatment.
| Title | Model | Treatment | Results |
|---|---|---|---|
|
| |||
| Bevacizumab Has Differential and Dose-Dependent Effects on Glioma Blood Vessels and Tumor Cells[11] | Orthotopic glioma nude mouse model | Bevacizumab 1) Subclinical dose 2) Medium clinical dose 3) High clinical dose |
Low (subclinical) doses of bevacizumab - reduced total vascular volume, no effect on tumor cell viability or overall tumor growth rates Medium and high doses - vascular regression, decreased tumor growth and prolonged survival |
| Anticancer Therapies Combining Antiangiogenic and Tumor Cell Cytotoxic Effects Reduce the Tumor Stem-Like Cell Fraction in Glioma Xenograft Tumors[14] | Xenograft C6 rat glioma cells in athymic nude mouse model | 1) Targeted antiangiogenic agent 2) Combination therapies of antiangiogenic agents and chemotherapy 3) Cytotoxic schedule of maximum tolerated dose chemotherapy using cyclophosphamide |
Targeted antiangiogenic therapy or cytotoxic chemotherapy: - no reduction in fraction of tumor sphere-forming units (SFU) tumors All combination antiangiogenic and cytotoxic drug treatments: - significant reduction in SFU |
| Edema Control by Cediranib, a Vascular Endothelial Growth Factor Receptor–Targeted Kinase Inhibitor, Prolongs Survival Despite Persistent Brain Tumor Growth in Mice[15] | 1) Orthotopic mouse model utilizing human glioblastoma (GBM) cell line U87 2) Orthotopic mouse model utilizing human GBM cell line U118 3) Orthotopic mouse model utilizing rat GBM cell line CNS-1 |
1) Cediranib 2) Dexamethasone |
Cediranib: - significantly decreased tumor vessel permeability and diameter - induced normalization of perivascular cell coverage and thinning of the basement membrane with increase in plasma collagen IV - edema alleviation - no effect on tumor growth - significantly increased survival of mice despite persistent tumor growth. |
| Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo[16] | Xenograft GBM, leiomyosarcoma, and rhabdomyosarcoma nude mouse models | Anti-VEGF monoclonal antibody | Anti-VEGF monoclonal antibody: - inhibited growth of tumors, but no effect on growth rate of tumor cells in vitro - decreased density of vessels |
| Biodegradable wafers releasing Temozolomide and Carmustine for the treatment of brain cancer[2] | Orthotopic gliosarcoma rat model | 1) Wafers loaded with 50% Temozolomide (TMZ) in poly(lactic acid-glycolic acid) (PLGA) 2) Wafers with a co-loading of TMZ and BCNU 3) Systemic TMZ 4) BCNU wafer alone 5) TMZ wafer alone |
Untreated/Treated with blank wafer: died within 11 days Systemic TMZ: median survival of 18 days. BCNU wafer alone: median survival of 15 days TMZ wafer alone: median survival of 19 days BCNU-TMZ wafer: median survival of 28 days with 25% of animals living long term |
| Temozolomide combined with PD-1 Antibody therapy for mouse orthotopic glioma model[20] | Syngeneic, orthotopic glioma mouse model | 1) TMZ 2) anti-PD-1 antibody 3) TMZ combined with anti-PD-1 antibody |
TMZ + anti-PD-1 antibody: - increased median survival of mice from 25 days to 42 days - tumor volume was significantly decreased in comparison to other groups - number of CD4 and CD8 T cells infiltrating the brain tumor was increased TMZ and anti-PD-1 antibody alone: - marginal increase in median survival time |
| Impairing temozolomide resistance driven by glioma stem-like cells with adjuvant immunotherapy targeting O-acetyl GD2 ganglioside[21] | Patient derived xenograft (PDX) model in Nonobese diabetic/severe combined immunedeficient gamma (NSG) mice | O-acetyl GD2 ganglioside inhibitor (8B6) + TMZ | 8B6 + TMZ: - 8B6 worked synergistically with TMZ to impair glioma stem-like cell self-renewal - significant decrease in cell proliferation in vitro and flank-tumor size in vivo when compared to either therapy alone |
| Proteomic and immunologic analyses of brain tumor exosomes[48] | - Syngeneic murine (VM/Dk) anaplastic astrocytoma model - Orthotopic mouse model using SMA560VIII cells |
SMA560vIII exosomes | SMA560vIII exosomesr - prophylactically protected mice against subcutaneous tumor challenge - failed to prolong survival in an orthotopic, preestablished tumor setting |
| Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubicin liposomes[61] | Orthotopic, syngeneic GBM rat model | 1) Doxorubicin liposomes 2) Doxorubicin liposomes modified with folate (F-dox liposome) 3) Doxorubicin liposomes modified with transferrin 4) Doxorubicin liposomes modified with both folate and transferrin (Tf(F)-Dox-liposome) |
Tf(F) Dox liposome: - increased survival time, decreased tumor volume - less toxic than the Doxorubicin solution, showing a dual-targeting effect |
| Brain Targeted Gold Liposomes Improve RNAi Delivery for Glioblastoma. International Journal of Nanomedicine[62] | Orthotopic, syngeneic GBM mouse model | 1) Spherical nucleic acids (SNAs) [gold nanoparticles with oligonucleotide miRNA inhibitors] encapsulated into ApoE conjugated liposomes 2) SNAs encapsulated into RVGconjugated liposomes |
Conjugated SNA-Liposomes with ApoE or RVG peptides: - increased systemic delivery of liposomes to brain tumors of mice SNA-Liposome-ApoE: - accumulated at higher extension in brain tumor tissues when compared with non-treated controls, SNALiposomes, and SNA-Liposome-RVG |
| Combating Glioblastoma by Codelivering the Small-Molecule Inhibitor of STAT3 and STAT3siRNA with α5β1 Integrin Receptor-Selective Liposomes[63] | Orthotopic mouse GBM model | 1) RGDK-liposomes containing only WP1066 2) RGDK-liposomes containing only STAT3siRNA 3) RGDK-liposomes containing both WP1066 and STAT3siRNA |
RGDK-lipopeptide co-solubilized with WP1066 and STAT3siRNA: - significant increase in survivability of mice leads - significant inhibition (>350% compared to the untreated mice group) mouse glioblastoma |
| Brain delivery of Plk1 inhibitor via chimeric polypeptide polymersomes for safe and superb treatment of orthotopic glioblastoma[65] | Orthotopic mouse GBM model | 1) Angiopep-2-docked chimeric polypeptide polymersome (ANGCPP) loaded with Volasertib 2) Free Volasertib |
ANG-CPP loaded with Volasertib: - suppressed growth of GBM and significantly increased survival rates in mice - reduced toxicity over free volasertib |
| Oncoprotein Inhibitor Rigosertib Loaded in ApoE-Targeted Smart Polymersomes Reveals High Safety and Potency against Human Glioblastoma in Mice. Molecular Pharmaceutics[66] | Orthotopic mouse GBM model | 1) Rigosertib (RGS) loaded in apolipoprotein E derived peptide (ApoE)-targeted chimeric polymersomes (ApoE-CP) 2) RGS loaded in chimeric polymersomes 3) Free RGS |
ApoE-CP loaded with RGS: - GBM inhibition - greatly prolonged survival time - depleted adverse effects |
| Biodegraded Magnetosomes with Reduced Size and Heating Power Maintain a Persistent Activity against Intracranial U87-Luc Mouse GBM Tumors[69] | Orthotopic mouse model with tumors composed of Luciferase-tagged U87 human glioma cells | 1) Magnetosomes injected + exposure to 15 magnetic sessions (MS) 2) Magnetosomes injected, no exposure to 15 MS |
Magnetosomes + 15 MS: - full tumor disappearance in 50% of treated mice (measured by decrease in bioluminescence intensity emitted by the U87-Luc tumor) |
| Development of Non-Pyrogenic Magnetosome Minerals Coated with Poly-l-Lysine Leading to Full Disappearance of Intracranial U87-Luc Glioblastoma in 100% of Treated Mice Using Magnetic Hyperthermia[70] | Orthotopic mouse model with tumors composed of Luciferase-tagged U87 human glioma cells | 1) Magnetosomes coated with polyl-lysine (M-PLL) + 27 magnetic sessions (MS) 2) M-PLL + 23 MS |
M-PLL + 27 MS: - complete reduction in bioluminescence emitted by mouse tumor GBM cells in 68 days in 100% of treated mice - all mice were still alive at day 350 M-PLL + 23 MS: - full tumor bioluminescence disappearance in 20% of treated mice |
| Metformin and temozolomide act synergistically to inhibit growth of glioma cells and glioma stem cells (GSCs) in vitro and in vivo[33] | Xenograft U87 human glioma stem cells in nude mouse model | 1) TMZ 2) Metformin 3) TMZ + Metformin |
TMZ + Metformin: - significantly reduced tumor growth rates and prolonged median survival of tumor-bearing mice - inhibited GSCs self-renewal capability and partly eliminates GSCs in vitro and in vivo |
| Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma therapeutic[37] | 1) Orthotopic nude mouse model utilizing GBM stem-like cells 2) Orthotopic, syngeneic rat model |
1) Ribavirin 2) Ribavirin + TMZ 3) Ribavirin+ radiation 4)Ribavirin + TMZ + radiation 5)TMZ + radiation |
Ribavirin only: - significantly increased survival compared with vehicle-treated animals Ribavirin + TMZ + radiation: - ribavirin potentiated the effect of TMZ and radiation, extending the median survival from 25 days (TMZ/Radiation) to 29 days (TMZ/Radiation/Ribavirin) |
2.3. Nivolumab and Ipilimumab
Nivolumab and ipilimumab are two immune checkpoint inhibitors that have had recent success treating other difficult, solid tumors including non-small-cell lung cancer (NSCLC). Nivolumab is a monoclonal antibody that targets programmed death-1 (PD-1), a receptor that serves as an inhibitory costimulator in T-helper cell activation. By increasing T cell activation, there was an interest in increasing the level of T cell-mediated cytotoxicity against GBM tumors. Recent results from the CheckMate 143 phase III clinical trials, however, have shown that nivolumab is no better than BVZ at treating GBM [18] (Table 1). A recent review article [3] posited whether resistance mechanisms to common therapies can be targeted through checkpoint inhibitors. In fact, current clinical trials are now focusing on combining nivolumab with TMZ to target tumors that are O-6-methylguanine-DNA methyltransferase methylated (NCT02617589) or unmethylated (NCT02667587) (Table 1). Because MGMT confers resistance to TMZ, the outcomes of these trials may offer insight into tumor response and resistance mechanisms. Ipilimumab is a monoclonal antibody that targets CTLA-4 with a similar intent to activate T cell mediated responses to tumors. Ipilimumab has had similar success against aggressive cancers and is being considered for concomitant therapy with nivolumab for NSCLC [19]. Current clinical trials for ipilimumab in GBM remain in phase I. Many current trials are focused on comparing or combining nivolumab and ipilimumab therapy for GBM in combination with TMZ (NCT02311920) or with radiotherapy (NCT04396860), with high mutational burden (NCT04145115), recurrent GBM (NCT03430791), MGMT unmethylated tumors (NCT03367715), or compared to BVZ (NCT02017717) (Table 1). These studies focus on defining treatment response and effectiveness in progression-free survival and overall survival.
Table 1.
Summary of Current Clinical Trials in Glioblastoma.
| Study | Therapy | Study Type | n | Condition | Primary Outcome | Status/ Initial Results |
|---|---|---|---|---|---|---|
|
| ||||||
| NCT02617589 | Nivolumab + Radiotherapy vs. TMZ + Radiotherapy | Multicenter Randomized Phase III Control T rial | 560 | Newly diagnosed MGMT unmethylated type GBM | OS (assessed up to 36 months) | Active, not recruiting No added benefit |
| NCT02667587 | Nivolumab + TMZ + Radiotherapy vs. TMZ + Radiotherapy | Multicenter Randomized Phase III Single Blind Study | 693 | Newly diagnosed MGMT methylated or indeterminate tumor subtype of GBM | OS and PFS | Active, not recruiting No results |
| NCT02311920 | Nivolumab + TMZ vs. Ipilimumab + TMZ vs. Nivolumab + Ipilimumab + TMZ | Multicenter Randomized Phase I Study | 32 | Newly diagnosed GBM or gliosarcoma | Immune-relateddose-limitingtoxicities | Active, not recruiting No results |
| NCT04145115 | Ipilimumab + Nivolumab | Multicenter Open-Label Phase II Study | 37 | Hypermutated recurrent GBM | Overall response rate (assessed by RANO) | Recruiting No results |
| NCT03430791 | Nivolumab + Ipilimumab vs. Nivolumab | Single Center Open-Label Phase I/II study | 60 | Recurrent GBM | Objective response Active, not rate (assessed by recruiting No modified RANO resultscriteria) | Active, not recruiting No results |
| NCT03367715 | Nivolumab + Ipilimumab + Radiotherapy | Single Center Single Arm Open-Label Phase II trial | 24 | Newly diagnosed, MGMT unmethylated GBM | OS at 1 year | Recruiting No results |
| NCT02017717 | Nivolumab vs. BVZ Nivolumab + Ipilimumab vs. Nivolumab | Multicenter Randomized Phase III Open-Label study (Nivolumab + BVZ) and Multiple Phase 1 studies (Nivolumab vs. Nivolumab + Ipilimumab) | Recurrent GBM (Phase III trial) Newly diagnosed GBM, newly diagnosed unmentylated MGMT GBM (Phase 1 trial) | Drug-Related Adverse Events Adverse Events Serious Adverse Events Liver lab abnormalities T hyroid Lab Abnormalities OS | Active Notrecruiting Recruiting | |
| NCT04396860 | Nivolumab + Ipilimumab + Radiotherapy vs. TMZ + Radiotherapy | Multicenter Randomized Phase II/III Open- Label Study | 485 | Newly Diagnosed MGMT unmethylated type GBM | PFS (Phase II) OS (Phase III) | No results |
| NCT00045968 | Dendritic Cell Immunotherapy (DCVaxL) + TMZ vs. TMZ + placebo | Multicenter Randomized Phase III Control T rial | 348 | Newly diagnosed, unilateral GBM (Grade IV) | PFS | Active, not recruiting Feasible and safe May extend survival |
| NCT02455557 | SVN53–67/M57-keyhole limpet hemocyanin (KLH) peptide vaccine (SurVaxM) +TMZ | Multicenter Phase II Single-Arm Design Study | 66 | Newly diagnosed GBM with survivin positive tumor status | PFS6 | Active, not recruiting 95% PFS6 |
| NCT01814813 | Heat shock protein peptide complex 96 (HSPPC-96) vaccine + BVZ vs. BVZ | Multicenter Phase II Randomized Trial | 90 | Surgically resectable, recurrent GBM | OS | Active, not recruiting No significantdifference |
| NCT02465268 | pp65 Dendritic Cell vaccine vs. placebo | Multicenter Phase II Randomized Control Trial | 120 | Newly diagnosed GBM | Median OS Change | Recruiting No results |
| NCT03395587 | Autologous, tumor lysateloaded, mature dendritic cells vs. Surgical resection, radiotherapy, and TMZ | Multicenter Phase II Randomized Control Trial | 136 | Newly diagnosed GBM | OS (assessed up to 34 months) | Recruiting No results |
Abbreviations: GBM – Glioblastoma, TMZ – Tmozolomide, BVZ – Bevacizumab, OS – Overall Survival, PFS – Progression Free Survival, PFS6 – 6-month Progression Free Survival, RANO – Response Assessment in Neuro-Oncology Criteria.
2.4. Combination studies
Recent research studies have focused on the success of coupled chemo- and immunotherapies. This emerging treatment regimen focuses on adjuvant and neoadjuvant immunotherapy after resection or radiation in order to boost anti-tumor immune response and cytotoxicity. As previously mentioned, many pre-clinical studies and clinical trials with novel therapeutics are similarly focusing on concomitant, neoadjuvant, or adjuvant immuno/chemotherapy, especially in situations where there is conferred resistance to front-line therapies [3]. Here, we highlight interesting combination studies that could potentially shape future management of patient care.
Recent work done in rat GBM models explored TMZ and carmustine BCNU wafers for local delivery of chemotherapeutics. Their results indicated that combination therapy increased the median survival rate of rats by 25% compared to either therapy alone [2,10] (Table 2). A single institutional trial of carmustine wafers and BVZ showed that in humans, combination therapy increased survival by 8 months when compared to TMZ alone [20]. More clinical and multi-institutional research studies are needed to explore these effects in a larger patient cohort. Another preclinical study explored the combination of monoclonal antibodies against PD-1 in combination with TMZ in mouse orthotopic glioma models. Results showed that the combination therapy increased median survival of the mice from 25 days to 42 days, while TMZ or anti-PD-1 antibody alone only marginally increased median survival time. These results demonstrate that prescribing nivolumab, a PD-1 monoclonal antibody, along with TMZ may enhance survival outcomes [21] (Table 2). Additional targets of combination therapy are glioma stem-like cells (GSCs), which comprise a subpopulation of cells responsible for tumor chemoresistance, and ultimately, relapse. A recent study explored targeting O-acetyl GD2 ganglioside, a protein overexpressed in GSCs, as a target to modulate chemoresistance. An O-acetyl GD2 ganglioside inhibitor (8B6) and TMZ were used in combination to impair and target GSCs in GBM. Results from this study indicated that 8B6 worked synergistically with TMZ to impair GSC self-renewal. Furthermore, in vitro and in vivo mouse models were able to significantly decrease cell proliferation and flank-tumor size when compared to either therapy alone [22] (Table 2). More clinical work needs to be done to explore the viability and fidelity of this combination therapy in mouse intracranial tumor models and in patients.
2.5. Repurposing drugs
Another promising chemotherapeutic strategy for tackling glioma stem cell-like (GSC) resistance is drug repurposing, which is the action of using previously approved drugs for novel therapeutic benefits [23–25]. This approach has gained considerable traction in the last 5 years as an alternative to conventional de novo drug identification for use in glioma treatment [26–28]. Repurposed drugs have key advantages that make them viable alternatives to their de novo counterparts, particularly well characterized safety profiles that lead to lower probability of drug failure during clinical trials [23,24,27]. Repurposed drugs can be analyzed through novel-omic-based computational methods leading to feasible, cost-effective identification of leading strategies [29–31]. Another strategy of identifying repurposed drugs is activity-based screening, which looks at the proteomic profile of glioblastoma and identifies treatments based on the mechanism of action of the drug [26]. The goals, therefore, of repurposed drugs for GBM are not only identifying high cytotoxic effect, but also their ability to provide synergistic activation of the immune system, target GSC and resistance mechanisms, and personalize care for the genetic profile of tumors at much lower cost [25–27]. Examples of drugs currently being examined for repurpose in GBM are anti diabetes medication, antihypertensive drugs, NSAIDS, antipsychotic drugs, antimicrobial/antiviral drugs, and even antidepressants.
Drugs that are of increased clinical interest in the future are metformin, celecoxib, and ribavirin [27]. Under clinical evaluation now (Table 1), metformin has shown tremendous possibility in preclinical studies. In vitro, it has been shown to decrease cellular viability and in higher concentrations cause cell death [32,33]. On a molecular level metformin inhibits AKT phosphorylation and mTOR signaling in glioma tumor cells, which inhibits their progression and leads to reduced proliferation and migration in vitro and in vivo [32–34] (Table 2). Metformin was also shown to have synergistic effect with TMZ in reducing cell viability [34]. Celecoxib has been shown to increase overall survival of patients and progression-free survival in patients with Grade III astrocytoma. Furthermore, when celecoxib was applied as an adjutant to TMZ it was shown to have good tolerability [35]. However, without control groups in these studies, more needs to be done to evaluate whether celecoxib is clinically efficacious [35,36]. Ribavirin has emerged as a potential candidate for glioblastoma after its success in ongoing clinical trials in acute myeloid leukemia, oropharyngeal squamous cell carcinoma, and metastatic breast cancer [37]. Particularly, ribavirin has antagonism against eukaryotic translation initiation factor 4E (eIF4E), which decreases cell migration and viability and increases cell arrest [38]. Furthermore, this study showed decreased cell viability of GSC and in vivo inhibition of tumor growth [38] (Table 2).
3. New advances
3.1. Isocitrate dehydrogenase inhibitors
Isocitrate dehydrogenase (IDH) is a crucial enzyme in the tricarboxylic acid (TCA) cycle, which is necessary for cellular respiration. Mutated IDH1 and IDH2 exhibit gain-of-function activity blocking normal cellular differentiation and contributing to tumorigenesis. Mutations in IDH1 and IDH2 have been found in up to 7% and 4–8% of GBMs, respectively [39,40]. There are currently two mutant IDH inhibitors, ivosidenib (AG-120) and enasidenib (AG-221) FDA-approved for refractory or IDH-mutant relapsed acute myeloid leukemia based on phase 1 data [41]. These drugs are being studied in advanced solid tumors and enhancing glioma trials and provide a new opportunity for advancement in GBM treatment.
3.2. Personalized proteomic immunotherapy
One of the biggest challenges researchers face in curbing the aggressive nature of GBM is overcoming its ability to escape immune surveillance. Recent innovations in immunotherapy have focused on three particular interventions (1) passive, (2) active, and (3) adaptive immunity. These three forms of therapy focus on boosting the patient’s immune system to identify tumor-associated antigens (TAA) and tumor-specific antigens (TSA) [3,42,43]. After identification, therapy will either block antigen function (passive), elicit a systemic, innate immune response in vivo (active), or develop an anti-tumor immune response from ex-vivo (adaptive) [3].
The increasing accessibility of proteomic subtyping of GBM provides an interesting avenue of overcoming immune escape and tumor heterogeneity by enhancing the personalization of immunotherapy to fit the unique protein expression profile of a particular tumor [42]. Here we look at novel proteins, transporters, and antigens expressed by GBM that can be utilized to target holistic, individualized treatment.
3.3. Tumor-Specific antigens and tumor-associated antigens
Identifying receptors that are essential to the function of GBM is a primary target for passive immunotherapy. A recent study utilized proteomics to identify a receptor tyrosine kinase (RTK) mutation as a TSA that is expressed on the surface of GBM cells. Immunotherapies targeting RTK could impair GBM cell function and limit proliferation [43]. Future work is needed to elucidate whether this mutant RTK can be selectively targeted and inhibited to curb tumor progression.
Proteomic analysis led to the development of BVZ and cetuximab as monoclonal antibodies that are similarly used to target VEGF and EGFR receptors that are essential for GBM survival [13]. These treatments initially succeed but ultimately succumb to recurrent and resistant GBM. Targeting resistance is a novel therapeutic strategy in GBM research. Some of the most common subtypes of GBM tumors are IDH-mutant or IDH-wildtype, the former being inherently resistant to BVZ treatment. Proteomics can be used to address the heterogeneity and resistance in GBM tumor subtypes. By identifying TAA/TSA present in IDH-mutant GBM cells we can inform more effective and efficient target therapeutics. An analysis of IDH-mutant GBM cells recently identified five new TAA: CRKII, CFL1, CNTN1, NMEZ and TK [43]. Further, the study explored the immunogenicity of these TAA and quantified that, in vitro, they all triggered an immune response marked by a release of interferon-gamma and other inflammatory cytokines [43]. Further in vitro and in vivo studies will elucidate the importance of these TAA for GBM therapeutics. In IDH-mutant primary GBM a recent study utilized proteomic associations in IDH mutant/wild type cells to advise multi-level treatment [44]. It was found that expression of PD-L1 was significantly associated with IDH-wild type GBM. Results suggested that PD-L1 antibodies, when coupled with BVZ, yielded additive and synergistic cytotoxicity in this subtype of GBM tumors [44] (Table 2). Further preclinical and clinical work is needed to explore the proteome-guided combination of these synergistic therapies to determine their efficacy.
3.4. Human Leukocyte Antigen (HLA) peptidome
Another antigen of interest is the tumor-specific human leukocyte antigen (HLA) peptidome. Proteome analysis for the HLA peptidome specific to GBM revealed 52 allotypes. The HLA peptidome was able to identify SOX11 as an antigen with two peptide sequences, AHSASEQQL and NFSDLVFTY, that were observed uniquely in the plasma (sHLA) and tumor membrane (mHLA) of the GBM when compared to healthy controls [45]. Developing active or adaptive immunotherapy against these specific HLA peptides could serve as a synergistic active and adaptive immunotherapeutic target. These allotypes are specific to each patient, however, and therefore unique antibodies would need to be developed for each tumor allotype-a process which has proven to be very expensive [45,46]. More research needs to be done in diverse cohorts to identify common HLA allotypes that could be developed into a more ubiquitous treatment for patients with GBM.
3.5. Responsive immunotherapy
Proteomics can also be uniquely helpful in identifying antigens produced by GBM cells in response to chemotherapy. One analysis examined the HLA proteome in GBM patients treated with decitabine [46]. Results indicated that HLA expression changed in response to decitabine and many new tumor HLA were produced. More studies identified proteome changes in GBM in response to the Signal transducer and activator of transcription-1 (STAT1) inhibitor S31201 [47]. The induction of these antigens in patients treated with decitabine offers a novel therapeutic target that can be utilized for synergistic immunotherapy. The possibility of inducing antigens that can subsequently be targeted by immunotherapy serves as a novel therapeutic strategy for GBM that needs to be further explored in pre-clinical research studies.
3.6. Glioma stem cell targeting
Another explanation for GBM recurrence and resistance are glioma stem-cells (GSC), GBM cells that are pushed into a pluripotent state and have robust adaptive immunity to chemotherapeutics. A recent study was done to assess the proteomic profile of GSCs to identify immunotherapeutic targets against them. The study identifies three proteins, PPIA, ANXA1 and CSTA that are specific and robustly expressed in GBM GSC [48]. These novel T cell target antigens could serve as targets for immunotherapy in passive, adaptive, and active immunotherapy. More research exploring and targeting these antigens is underway and could elucidate therapeutics that lengthen relapse time and decrease the viability of GSC cells. Another interesting approach has used proteomics to develop adoptive immunotherapeutics. In these studies, proteomic analysis identified antigens that were presented to dendritic cells ex vivo. Using murine in vivo models, this therapy was shown to increase progression free survival and survival overall [49,50] (Table 2). These strategies are further explored and delineated below.
3.7. CAR T-Cell therapy
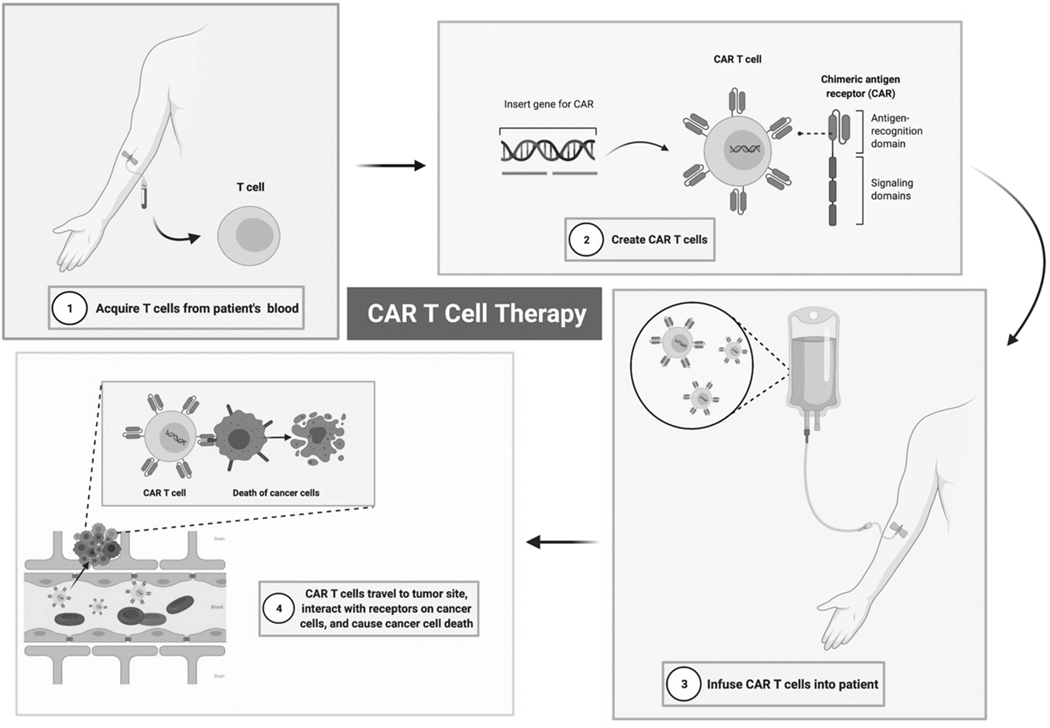
Chimeric antigen receptor (CAR) T-cells are created by engineering donor cells to express surface receptor proteins that recognize TAAs with high specificity [51] (Figure 1). Given recent successes following the use of CAR T-cells engineered to target CD19 in chemotherapy-resistant B-cell malignancies, similar strategies have been applied as immunotherapies against solid tumors [52]. Successful treatment of CNS tumors with CAR T-cell therapy will require overcoming several CNS-specific hurdles, including monitoring for T-cell toxicity in eloquent tissue and ensuring target tissue infiltration past the blood–brain barrier (BBB) [53].
Figure 1. CAR T-Cell Therapy for Glioblastoma.
This therapy is focused on infusion of Chimeric Antigen Receptor (CAR) T-cells into patients. Donor cells are engineered to express surface receptor proteins that recognize tumor-associated antigens (TAAs). Once infused back into the patient, these cells then travel to the site of the tumor, infiltrate the tumor microenvironment, and act on the cancer cell receptors to cause cancer cell death.
In contrast to the challenges chemotherapeutic agents face in reaching tumors of the brain parenchyma, cell-based immunotherapies that can traverse the BBB, such as those using activated T-cells, may allow for increased migration to the tumor location [52]. GBM, for instance, is a highly vascularized tumor which has been shown to permit high levels of immune cell infiltration into the tumor core [52]. Noninvasive, in vivo cell monitoring via 7 T MRI has been used to track nanoparticle-tagged CAR T-cells in mouse GBM models [54]. This type of approach may allow for modification of CAR design to enhance tumor infiltration and persistence within the tumor [54]. Further, the use of chemokines to traffic T-cells to effector locations, an alternative to regional delivery, is currently under investigation [53].
The issue of tumor heterogeneity must be circumvented by identifying tumor-specific, tumor-associated antigens for use in the design of CAR T-cells, and by implementing strategies to counter the exhaustion of transferred CAR T-cells and to overcome antigen loss [52]. CAR T-cells against the following TAAs are currently being tested in glioblastoma clinical trials: IL13Ra2, EGFRvIII, HER2, EphA2, GD2, B7-H3 and Chlorotoxin [51]. Large-scale trials have not demonstrated clinical efficacy to date, primarily due to limited T-cell persistence within the tumor and antigen-negative relapses [51]. These pitfalls may indicate the need for combination therapies and/or genetic modifications of CAR T-cell targets [51]. CRISPR screening of CAR T-cells and patient-derived GSC has revealed genes necessary for tumor susceptibility to CAR-mediated killing, as well as those necessary for CAR T-cell effector function, which may be taken into account when designing future CAR T therapeutics [55]. Further correlation of glioblastoma organoid (GBO) mutation profiles with responses to CAR T may allow for rapid demonstration of the effectiveness of personalized treatments, particularly given the rapidity with which GBOs can be generated [56].
Though these efforts have had varying success and largely remain in the preclinical stage of development, recent innovations in the process of designing, testing, and delivering CAR T therapy may allow for future clinical application in high-grade, chemoradiotherapy-resistant gliomas [52].
3.8. Vaccines
Multiple systematic reviews and meta-analysis studies suggested that the incorporation of dendritic cell vaccines (DCVax-L) into standard approved therapeutic regimens improved median overall survival and 2- and 3-year survival rates in patients with new or recurrent high-grade gliomas [57] (Figure 2). In 2018, Liau et al. published the interim results of a large randomized (2:1) phase III trial of patients newly diagnosed with glioblastoma receiving DCVax-L in addition to temozolomide [58] (Table 1). Two hundred and thirty-two patients received the vaccine intradermally in combination with standard therapy, while 99 received placebo in place of the vaccine. After recurrence, all patients were allowed the DCVax-L. Due to this and high cross-over rates, about 90% of the patient population received the vaccine. At the time of the report, 223 patients were >/ = 30 months past their date of surgery, and 30% of these patients had a Kaplan–Meier (KM)-derived median overall survival (mOS) of 46.5 months. Further, at the time of the report, 182 patients were >/ = 36 months past surgery, and 24.2% of them had a mOS of 40.5 months. Within patients with methylated MGMT, mOS was 37.4 months from surgery, with a three-year survival rate of 46.4%[58]. Taken together, these results are highly promising. Upon publication of the interim report, there was criticism of the trial design and highly selective patient population [59].
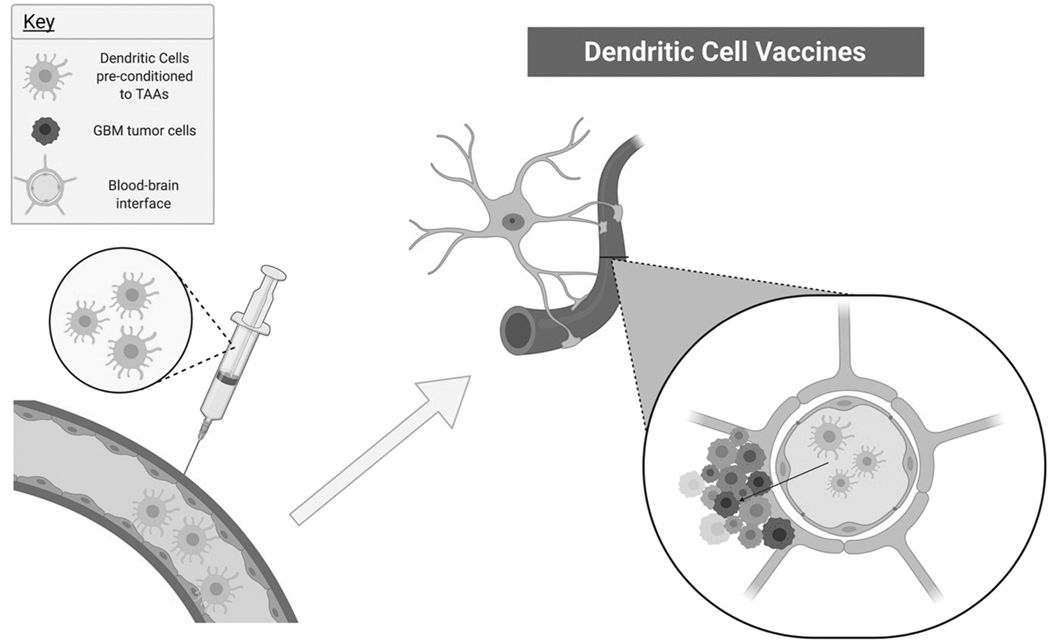
Figure 2. Dendritic Cell Vaccines as Glioblastoma Therapy.
Patient-specific dendritic cells that have been exposed to tumor-associated antigens (TAAs) ex vivo are then injected back into the patient (image on the left), travel to the brain, and cross the blood-brain barrier to enter the tumor microenvironment (image on right).
Recently completed trials include a phase II trial studying the usage of a peptide mimic immunotherapeutic vaccine (SurVaxM) in addition to temozolomide in patients newly diagnosed with GBM (NCT02455557) (Table 1). Vaccine therapy containing heat shock protein-peptide complexes from a patient’s own tumor has also been tested with or without BVZ in patients with recurrent surgical GBM in a phase II trial (NCT01814813) (Table 1). These trials are ongoing, and no conclusions have been determined. Currently, there are a number of other phase II/III vaccine trials to treat GBM, such as an ongoing vaccine trial that includes the pp65 (an antigen marker in GBM) DC vaccine. This is hypothesized to activate the immune system in order to attack GBM tumor cells (NCT02465268), and the lysate-loaded mature DC vaccine in addition to standard therapy for patients with near-complete resection of GBM (NCT03395587) (Table 1). If successful, these therapeutics have the potential to change the landscape of GBM treatment.
3.9. Drug delivery
Effective therapy for GBM requires a BBB penetrable carrier to effectively deliver the drug to site. The FDA-approved polyanhydride: sebacic acid polymeric wafer, Gliadel®, that locally delivers carmustine at the tumor resection site has shown a significant increase in median survival alone and when delivered with oral temozolomide and radiation therapy [60]. This local intracranial delivery platform has led to various drug carriers being investigated as a way to bypass the BBB and to safely and more effectively deliver therapeutic concentrations at the tumor site. Drug carriers currently being studied include liposomes, polymersomes, and iron oxide nanoparticles (Figure 3).
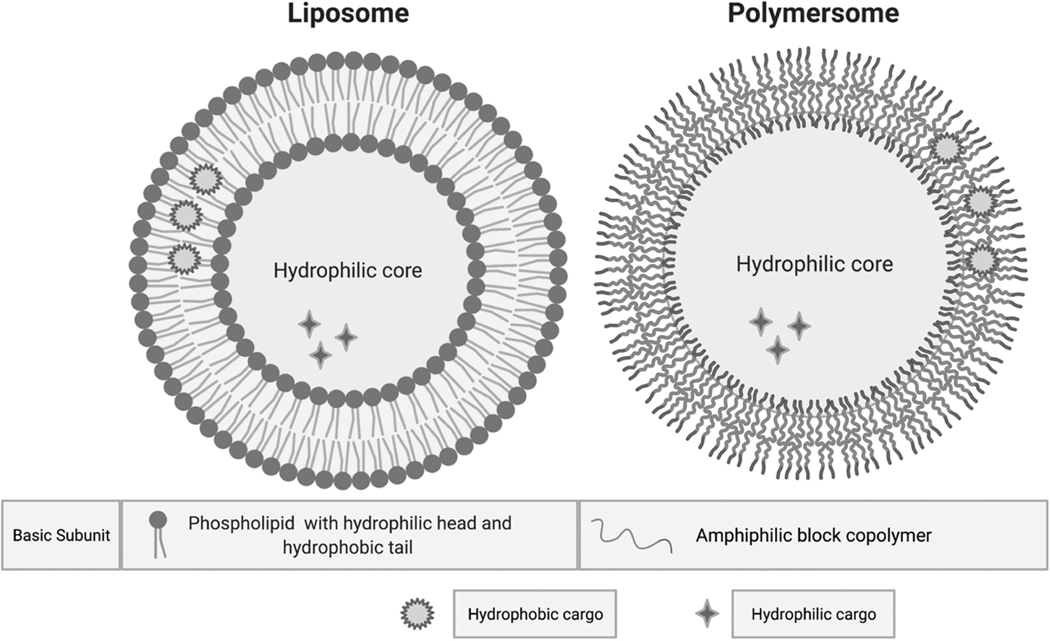
Figure 3. Liposome vs. Polymersome for Drug Delivery.
The basic structure of the liposome is a phospholipid bilayer encompassing a hydrophilic core while the polymersome is composed of a bilayer of amphiphilic block copolymers encompassing a hydrophilic core. Both hydrophobic and hydrophilic cargo can be carried by the liposome or polymersome.
Liposomes are artificial phospholipid bilayer vessels with easily modifiable surfaces to increase their half-life in circulation and enhance their passage across the BBB to deliver their therapeutic payload. They have been widely studied for several decades now. Compared to polymeric nanoparticles, liposomes are easier to manipulate in terms of size and can demonstrate a larger burst release in the first 48 hours [61]. Most likely effective therapy for GBM mandates a dual-function carrier that can penetrate the BBB and target glioma cells. Dual-targeting doxorubicin (Dox) liposomes produced by conjugating liposomes with transferrin and folate have been shown to be effective in targeting tumors and penetrating the BBB in a rodent model of GBM [62] (Table 2). This indicates that liposomes can be used as drug-carriers for GBM chemotherapy.
A recent study by Ruiz et al., demonstrated the usage of gold-liposome nanoparticles conjugated with oligonucleotide miRNA inhibitors (OMIs) and encapsulated into apolipoprotein E (ApoE) or rabies virus glycoprotein (RVG). The liposomes were then administered intravenously in mice implanted with orthotopic GBM. Liposomes, approximately 30–50 nm in size, inhibited the expression of miRNA-92b (an abnormally overexpressed miRNA in GBM). Further, conjugation with ApoE or RVG increased systemic delivery of liposomes to GBM syngeneic mice [63] (Table 2). Another recent study by Vangala et al. depicted that intravenous injection of alpha5beta1 integrin receptor-selective liposomes of RGDK-lipopeptide co-solubilized with WP1066 (a JAK/STAT pathway inhibitor) and STAT3siRNA lead to a significant increase in the survivability of orthotopically established glioblastoma mice [64] (Table 2).
Polymersomes are self-assembled from synthetic amphiphilic block copolymers that have the ability to encapsulate hydrophobic and hydrophilic drugs. They are considered superior to liposomes due to better mechanical and colloidal stability, high drug loading capacity, longer half-life, and less drug leakage [65]. Their surface, like liposomes, is modifiable to increase BBB penetration. A recent study by Fan et al. demonstrated that Plk1 inhibitor volasertib delivery to the brain via angiopep-2-docked chimeric polypeptide polymersome suppressed the growth of orthotopic GBM and significantly increased survival rates in mice [66] (Table 2). Another study utilized ApoE derived peptide targeted chimeric polymersomes to deliver rigosertib to the brain leading to GBM inhibition and increased survival time in an orthotopic U-87 MG GBM model [67] (Table 2).
Iron oxide nanoparticles (IONP) are characterized by a core of crystallized nanoparticulate iron, whose most stable form is maghemite, and are surrounded by an organic stabilizing layer such as lipids, proteins, and lipopolysaccharides. This outer layer determines the nanoparticle’s surface charge and they range in size from 5 to 380 nm [68,69]. Their details have largely been studied in the context of IONPs synthesized by magnetosomes and magnetotactic bacteria[69]. A recent study demonstrated the promising application of IONP magnetosomes in U87-Luc murine GBM tumors. Intratumoral injections of IONP preceding 15 magnetic sessions, each one consisting of a 30 minute application of an alternating magnetic field (AMF) of 27 mT and 198 kHZ resulted in full tumor disappearance in 50% of the treated mice, as measured by the decrease in bioluminescence intensity emitted by the U87-Luc tumor [70] (Table 2). Poly-L-lysine coated magnetosomes injected into U87-Luc tumors and exposed to 27 magnetic sessions, each one consisting of a 30 minute application of an AMF of 27 mT and 202 kHZ, similarly demonstrated a complete reduction in bioluminescence emitted by living GBM cells in 68 days in 100% of the treated mice [71] (Table 2). Another study demonstrated the application of IONP for full disappearance of GL-261 murine GBM tumors with no observable adverse effects by intratumoral injections and subsequent exposure to a series of AMF of 34–47 mT and 198 kHz which caused hyperthermic temperatures in the range of 43–46 C [72]. Taken together, new delivery vehicles and nanoparticles such as liposomes, polymersomes, and magnetosome IONP are paving the way for novel, specific, and nontoxic GBM treatments.
4. Conclusion
The inherent characteristics of GBM, including invasiveness, high proliferative index, immunological escape capabilities, and genetic heterogeneity have led to unique challenges in developing successful therapeutic options. Immunotherapies that have shown success in other cancers are being tested clinically in combination with chemotherapy to increase both the anti-tumor immune response and cytotoxicity. Personalized approaches to GBM therapy allow for the genomic profiling of the tumor to be taken advantage of with regards to treatment choice. CAR T-cell therapy seems to be a promising avenue clinically and is currently undergoing optimization. In addition, dendritic cell vaccines have been shown to improve overall survival in patients with GBM. Combination therapy, attacking the tumor through multiple mechanisms of action, seems to be necessary to have significant results on recurrence. Following in the footsteps of Gliadel, locally delivering carmustine at the site of tumor resection, various delivery systems have been investigated to enhance diffusion and distribution of these chemotherapeutic agents throughout the tumor bed. These delivery platforms include liposomes and polymersomes and have shown promise in preclinical models. Notably, GBMs are particularly challenging to treat due to their intrinsic heterogeneity, evasiveness to treatment, and BBB that often hinders drug delivery. Nevertheless, research is focused on overcoming these barriers, and technical approaches are diverse, allowing multiple therapeutic avenues to be explored.
5. Expert opinion
While the triple combination therapy of Gliadel implanted locally at the time of resection, oral temozolomide, and radiation therapy after maximal tumor resection has significantly increased the median survival for patients with GBM, challenges remain to further this effect for all patients diagnosed with GBM. There is a desperate need for novel therapeutics and strategies which will accurately translate from preclinical testing to clinical utility. There are several hurdles that need to be overcome for this translational goal to be met. Determining optimal drugs of choice, determining the most appropriate animal model, and overcoming the BBB through drug delivery platforms, viral constructs, or through BBB permeabilization are among these obstacles.
The unique selection of drug candidates that are cytotoxic to tumor cells yet spare healthy cells is a necessary qualification for treating tumors in eloquent areas of the brain. High throughput drug screening has led to the identification of several small molecule inhibitors thought to play key roles in tumor growth and invasion. These include PI3K inhibitors, mTOR inhibitors, AKT inhibitors, HIF inhibitors, and Bcl-2 inhibitors, however, several subsequent clinical trials have shown that a multiple pathway attack might be more beneficial. This also leads to the question of determining the best in vitro and in vivo preclinical models to better gauge clinical outcomes. In vitro models and established human and murine models may not sufficiently address the diverse characteristics of in vivo human GBM. While patient-derived xenografts can represent the vast array of common mutations in GBM, they are costly to develop and maintain. Similarly, in vivo models, including genetically modified mouse models and humanized mouse models necessary for studying immunotherapeutic investigations, are often cost prohibitive and may not recapitulate the full human GBM environment.
For some therapeutic interventions, local drug delivery through use of nanoparticles, liposomes, and polymersomes might be the way to achieve therapeutic drug concentrations at the site of residual tumor without additional toxicity. Safety, biodistribution and efficacy are unique considerations for each drug candidate and carrier combination. In addition, devising adequate animal models to test each unique drug delivery method poses a further challenge. One possible technological solution to enhance drug delivery to the brain is high intensity focused ultrasound (HIFU). HIFU, a noninvasive method of focusing ultrasound with transducers to highly targeted areas and used currently for thermal ablation, can also temporarily permeabilize the BBB allowing larger drug molecules and carriers to be delivered in higher concentrations and increase effect locally. HIFU can be utilized as a tool that aids in multiple ways in the preclinical and clinical settings.
While the challenges seem daunting, the current research utilizing inventive drug delivery options, vaccines, and viral and non-viral gene therapy for the treatment of GBM has shown remarkable preclinical results and needs to be tested clinically. Obtaining significant and consistent increases in median and overall survival will require continued thinking outside the box using novel approaches to arrest the invasive growth pattern that is the hallmark of this particular tumor.
Acknowledgments
All figures were created with BioRender.com.
Funding
EE Wicks is a research fellow supported by the Sarnoff Cardiovascular Research Foundation.
Declaration of interest
B Tyler has received research funding from the National Institutes of Health and is co-owner for Accelerating Combination Therapies (including equity and options). B Tyler also declares that Ashvattha Therapeutics Inc. has also licensed one of her patents. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Omuro A. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Weller M, Belanger K, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. In: The New England journal of medicine. 2005. p. 10. [DOI] [PubMed] [Google Scholar]
- 3.Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20 (9):1100–1109. [DOI] [PubMed] [Google Scholar]
- 4.Larsen JM, Martin DR, Byrne ME. Recent advances in delivery through the blood-brain barrier. Curr Top Med Chem. 2014;14 (9):1148–1160. [DOI] [PubMed] [Google Scholar]
- 5.Bou-Gharios J, Assi S, Bahmad HF, et al. The potential use of tideglusib as an adjuvant radio-therapeutic treatment for glioblastoma multiforme cancer stem-like cells. Pharmacol Rep. 2021;73 (1):227–239. [DOI] [PubMed] [Google Scholar]
- 6.Thomas AA, Brennan CW, DeAngelis LM, et al. Emerging Therapies for Glioblastoma. JAMA Neurol. 2014;71(11):1437. [DOI] [PubMed] [Google Scholar]
- 7.Karachi A, Dastmalchi F, Mitchell DA, et al. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol. 2018;20(12):1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas A, Tanaka M, Trepel J, et al. Temozolomide in the era of precision medicine. Cancer Res. 2017;77(4):823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapira-Furman T, Serra R, Gorelick N, et al. Biodegradable wafers releasing temozolomide and carmustine for the treatment of brain cancer. J Control Release. 2019;295:93–101. [DOI] [PubMed] [Google Scholar]
- 10.Lin C-Y, Li R-J, Huang C-Y, et al. Controlled release of liposome-encapsulated temozolomide for brain tumour treatment by convection-enhanced delivery. J Drug Target. 2018;26 (4):325–332. [DOI] [PubMed] [Google Scholar]
- 11.Lee CY. Strategies of temozolomide in future glioblastoma treatment. Onco Targets Ther. 2017;10:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan C-H, Liu W-L, Cao H, et al. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 2013;4(10):e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Baumgarten L, Brucker D, Tirniceru A, et al. Bevacizumab has differential and dose-dependent effects on glioma blood vessels and tumor cells. Clin Cancer Res. 2011;17(19):6192–6205. [DOI] [PubMed] [Google Scholar]
- 14.Im S-A, Gomez-Manzano C, Fueyo J, et al. Antiangiogenesis Treatment for Gliomas: Transfer of Antisense-Vascular Endothelial Growth Factor Inhibits Tumor Growth in Vivo. 7. n.d. [PubMed] [Google Scholar]
- 15.Folkins C, Man S, Xu P, et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67(8):3560–3564. [DOI] [PubMed] [Google Scholar]
- 16.Kamoun WS, Ley CD, Farrar CT, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27(15):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. [DOI] [PubMed] [Google Scholar]
- 18.Preusser M, Lim M, Hafler DA, et al. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nature reviews. Neurology. 2015;11(9):504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama Y, Kimura Y, Enatsu R, et al. Advantages and disadvantages of combined chemotherapy with carmustine wafer and bevacizumab in patients with newly diagnosed glioblastoma: a single-institutional experience. World Neurosurg. 2018;113:e508–e514. [DOI] [PubMed] [Google Scholar]
- 21.Dai B, Qi N, Li J, et al. Temozolomide combined with PD-1 Antibody therapy for mouse orthotopic glioma model. Biochem Biophys Res Commun. 2018;501(4):871–876. [DOI] [PubMed] [Google Scholar]
- 22.Fleurence J, Bahri M, Fougeray S, et al. Impairing temozolomide resistance driven by glioma stem-like cells with adjuvant immunotherapy targeting O-acetyl GD2 ganglioside. Int J Cancer. 2020;146(2):424–438. [DOI] [PubMed] [Google Scholar]
- 23.Abbruzzese C, Matteoni S, Signore M, et al. Drug repurposing for the treatment of glioblastoma multiforme. J ExpClin Cancer Res. 2017;36(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan SK, Jermakowicz A, Mookhtiar AK, et al. Drug repositioning in glioblastoma: a pathway perspective. Front Pharmacol. 2018. March 16;9:218. PMID: 29615902; PMCID: PMC5864870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahmad HF, Elajami MK, El Zarif T, et al. Drug repurposing towards targeting cancer stem cells in pediatric brain tumors. Cancer Metastasis Rev. 2020;39(1):127–148. [DOI] [PubMed] [Google Scholar]
- 26.Daisy Precilla S, Kuduvalli SS, Thirugnanasambandhar Sivasubramanian A. Disentangling the therapeutic tactics in GBM: from bench to bedside and beyond. Cell Biol Int. 2021;45(1):18–53. [DOI] [PubMed] [Google Scholar]
- 27.Siegelin MD, Schneider E, Westhoff MA, et al. Current state and future perspective of drug repurposing in malignant glioma. Seminars in cancer biology; 68, 92–104; 2021. [DOI] [PubMed] [Google Scholar]
- 28.Hammoud H, Saker Z, Harati H, et al. Drug repurposing in medullo-blastoma: challenges and recommendations. Curr Treat Options Oncol. 2020;22(1):6. [DOI] [PubMed] [Google Scholar]
- 29.Peyvandipour A, Saberian N, Shafi A, et al. A novel computational approach for drug repurposing using systems biology. Bioinformatics. 2018;34(16):2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowak-Sliwinska P, Scapozza L, Ruiz I Altaba A. Drug repurposing in oncology: compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochimica et biophysicaacta. Rev Cancer. 2019;1871(2):434–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez JJ, Pryszlak M, Smith L, et al. Giving drugs a second chance: overcoming regulatory and financial hurdles in repurposing approved drugs as cancer therapeutics. Front Oncol. 2017;7:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wurth R, Pattarozzi A, Gatti M, et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: a role for metformin-induced inhibition of Akt. Cell Cycle. 2013;12 (1):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seliger C, Meyer A-L, Renner K, et al. Metformin inhibits proliferation and migration of glioblastoma cells independently of TGF-β2. Cell Cycle. 2016;15(13):1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Z, Zhao G, Xie G, et al. Metformin and temozolomide act synergistically to inhibit growth of glioma cells and glioma stem cells in vitro and in vivo. Oncotarget. 2015;6(32):32930–32943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stockhammer F, Misch M, Koch A, et al. Continuous low-dose temozolomide and celecoxib in recurrent glioblastoma. J Neurooncol. 2010;100(3):407–415. [DOI] [PubMed] [Google Scholar]
- 36.Reardon DA, Quinn JA, Vredenburgh J, et al. Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma. Cancer. 2005;103(2):329–338. [DOI] [PubMed] [Google Scholar]
- 37. Casaos J, Gorelick NL, Huq S, et al. The Use of Ribavirin as an Anticancer Therapeutic: will It Go Viral? Mol Cancer Ther. 2019;18 (7):1185–1194. •• Drug repurposing may hold particular interest for GBM treatment.
- 38.Volpin F, Casaos J, Sesen J, et al. Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma therapeutic. Oncogene. 2017;36 (21):3037–3047. [DOI] [PubMed] [Google Scholar]
- 39.Brennan CW, Verhaak RGW, McKenna A, TCGA Research Network, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golub D, Iyengar N, Dogra S, et al. Mutant isocitrate dehydrogenase inhibitors as targeted cancer therapeutics. Front Oncol. 2019;9:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buser DP, Ritz M-F, Moes S, et al. Quantitative proteomics reveals reduction of endocytic machinery components in gliomas. EBioMedicine. 2019;46:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dettling S, Stamova S, Warta R, et al. Identification of CRKII, CFL1, CNTN1, NME2, and TKT as novel and frequent T-Cell targets in human IDH-mutant glioma. Clin Cancer Res. 2018;24 (12):2951–2962. [DOI] [PubMed] [Google Scholar]
- 44.Heiland DH, Haaker G, Delev D, et al. Comprehensive analysis of PD-L1 expression in glioblastoma multiforme. Oncotarget. 2017;8 (26):42214–42225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shraibman B, Barnea E, Kadosh DM, et al. Identification of tumor antigens among the HLA peptidomes of glioblastoma tumors and plasma. Mol Cell Proteomics. 2019a;18(6):1255–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shraibman B, Kadosh DM, Barnea E, et al. Human Leukocyte Antigen (HLA) peptides derived from tumor antigens induced by inhibition of DNA methylation for development of drug-facilitated immunotherapy. Mol Cell Proteomics. 2016;15(9):3058–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain R, Atak A, Yeola A, et al. Proteomic level changes associated with S3I201 treated U87 glioma cells. J Proteomics. 2017;150:341–350. [DOI] [PubMed] [Google Scholar]
- 48.Rapp C, Warta R, Stamova S, et al. Identification of T cell target antigens in glioblastoma stem-like cells using an integrated proteomics-based approach in patient specimens. Acta Neuropathol. 2017;134(2):297–316. [DOI] [PubMed] [Google Scholar]
- 49.Graner MW, Alzate O, Dechkovskaia AM, et al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23 (5):1541–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho D-Y, Yang W-K, Lee H-C, et al. Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multi-forme: a phase ii clinical trial. World Neurosurg. 2012;77 (5):736–744. [DOI] [PubMed] [Google Scholar]
- 51. Land CA, Musich PR, Haydar D, et al. Chimeric antigen receptor T-cell therapy in glioblastoma: charging the T cells to fight. J Transl Med. 2020. November 11;18(1):428. PMID: 33176788; PMCID: PMC7659102. •• Comprehensive review of innovations in CAR design and choice of targets for GBM.
- 52.Daubon T, Hemadou A, Romero Garmendia I, et al. Glioblastoma immune landscape and the potential of new immunotherapies. Front Immunol. 2020;11:585616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Upreti D, Bakhshinyan D, Bloemberg D, et al. Strategies to enhance the efficacy of T-Cell therapy for central nervous system tumors. Front Immunol. 2020. November 12;11:599253. PMID: 33281826; PMCID: PMC7689359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie T, Chen X, Fang J, et al. Non-invasive monitoring of the kinetic infiltration and therapeutic efficacy of nanoparticle-labeled chimeric antigen receptor T cells in glioblastoma via 7.0-Tesla magnetic resonance imaging. Cytotherapy. 2020. December 14. DOI: 10.1016/j.jcyt.2020.10.006.S14653249(20)30931-2. Epub ahead of print. PMID: 33334686. [DOI] [PubMed] [Google Scholar]
- 55.Wang D, Prager BC, Gimple RC, et al. CRISPR screening of CAR T Cells and cancer stem cells reveals critical dependencies for cell-based therapies. Cancer Discov. 2020. December 16;CD-20–1243. Epub ahead of print. PMID: 33328215. DOI: 10.1158/2159-8290.CD-20-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacob F, Salinas RD, Zhang DY, et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020. January 9;180(1):188–204.e22. Epub 2019 Dec 26. PMID: 31883794; PMCID: PMC7556703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Zhao H-Y, Zhang F-C, et al. Dendritic cell-based vaccine for the treatment of malignant glioma: a systematic review. Cancer Invest. 2014;32(9):451–457. [DOI] [PubMed] [Google Scholar]
- 58.Liau LM, Ashkan K, Tran DD, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wick W, van den Bent MJ. First results on the DCVax phase III trial: raising more questions than providing answers. Neuro Oncol. 2018;20(10):1283–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel TR. Nanocarrier-based therapies for CNS tumors. CNS Oncol. 2014;3(2):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao J-Q, Lv Q, Li L-M, et al. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials. 2013;34(22):5628–5639. [DOI] [PubMed] [Google Scholar]
- 63.Grafals-Ruiz N, Rios-Vicil CI, Lozada-Delgado EL, et al. Brain targeted gold liposomes improve RNAi delivery for glioblastoma. Int J Nanomedicine. 2020;15:2809–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vangala V, Nimmu NV, Khalid S, et al. Combating glioblastoma by codelivering the small-molecule inhibitor of STAT3 and STAT3siRNA with a5β1 integrin receptor-selective liposomes. Mol Pharm. 2020;17(6):1859–1874. [DOI] [PubMed] [Google Scholar]
- 65.Krishnamoorthy B, Karanam V, Chellan VR, et al. Polymersomes as an effective drug delivery system for glioma—A review. J Drug Target. 2014;22(6):469–477. [DOI] [PubMed] [Google Scholar]
- 66.Fan Q, Liu Y, Cui G, et al. Brain delivery of Plk1 inhibitor via chimaeric polypeptide polymersomes for safe and superb treatment of orthotopic glioblastoma. J Control Release. 2020. DOI: 10.1016/j.jconrel.2020.10.043 [DOI] [PubMed] [Google Scholar]
- 67.Qin H, Jiang Y, Zhang J, et al. Oncoprotein inhibitor rigosertib loaded in ApoE-Targeted smart polymersomes reveals high safety and potency against human glioblastoma in mice. Mol Pharm. 2019;16(8):3711–3719. [DOI] [PubMed] [Google Scholar]
- 68.Alphandéry E. Bio-Synthesized Iron oxide nanoparticles for cancer treatment. Int J Pharm. 2020. August 30;586:119472. [DOI] [PubMed] [Google Scholar]
- 69. Alphandéry E. Applications of magnetotactic bacteria and magnetosome for cancer treatment: a review emphasizing on practical and mechanistic aspects. Drug Discov Today. 2020. August; 25 (8):1444–1452. •• An exciting new and promising treatment avenue for GBM.
- 70.Alphandéry E, Idbaih A, Adam C, et al. Biodegraded with reduced size and heating power maintain a persistent activity against intracranial U87-Luc Mouse GBM tumors. J Nanobiotechnol. 2019. December 23;17(1):126. DOI: 10.1186/s12951-019-0555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alphandéry E, Idbaih A, Adam C, et al. Development of non-pyrogenic magnetosome minerals coated with Poly-l-Lysine leading to full disappearance of intracranial U87-Luc glioblastoma in 100% of treated mice using magnetic hyperthermia. Biomaterials. 2017. October;141:210–222. [DOI] [PubMed] [Google Scholar]
- 72.Hamdous Y, Chebbi I, Mandawala C, et al. Biocompatible coated magnetosome minerals with various organization and cellular interaction properties induce cytotoxicity towards RG-2 and GL-261 glioma cells in the presence of an alternating magnetic field. J Nanobiotechnol. 2017;15(1). DOI: 10.1186/s12951-017-0293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]