Abstract
Therapeutic delivery to the central nervous system (CNS) continues to be a considerable challenge in the pharmacological treatment and management of neurological disorders. This is primarily due to the physiological and biochemical characteristics of brain barrier sites (i.e., blood–brain barrier (BBB), blood–cerebrospinal fluid barrier (BCSFB)). Drug uptake into brain tissue is highly restricted by expression of tight junction protein complexes and adherens junctions between brain microvascular endothelial cells and choroid plexus epithelial cells. Additionally, efflux transport proteins expressed at the plasma membrane of these same endothelial and epithelial cells act to limit CNS concentrations of centrally acting drugs. In contrast, facilitated diffusion via transporter proteins allows for substrate-specific flux of molecules across the plasma membrane, directing drug uptake into the CNS. Organic Cation Transporters (OCTs) and Novel Organic Cation Transporters (OCTNs) are two subfamilies of the solute carrier 22 (SLC22) family of proteins that have significant potential to mediate delivery of positively charged, zwitterionic, and uncharged therapeutics. While expression of these transporters has been well characterized in peripheral tissues, the functional expression of OCT and OCTN transporters at CNS barrier sites and their role in delivery of therapeutic drugs to molecular targets in the brain require more detailed analysis. In this chapter, we will review current knowledge on localization, function, and regulation of OCT and OCTN isoforms at the BBB and BCSFB with a particular emphasis on how these transporters can be utilized for CNS delivery of therapeutic agents.
Keywords: Blood–Brain Barrier (BBB), Blood–Cerebrospinal Fluid Barrier (BCSFB), Brain parenchymal transporters, CNS drug delivery, Organic cation transport
1. Introduction
Targeted drug delivery to the CNS requires overcoming anatomical barriers that restrict blood-to-brain transport of therapeutic molecules. The two principal barrier tissues that separate the peripheral circulation from brain parenchyma include the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCFSB) (Fig. 1). The BBB is composed of endothelial cells where the paracellular cleft between adjacent cells is “sealed” by tight junction protein complexes. These brain microvascular endothelial cells acquire a barrier phenotype through communication with glial cells (i.e., astrocytes, microglia), pericytes, and neurons as well as via protein and enzymatic components of the extracellular matrix, a concept referred to as the neurovascular unit (NVU). For example, expression of tight junction proteins and adherens junction constituents is regulated through trophic factors that are released from pericytes and astrocytes. Such mechanisms aid in maintenance of BBB functional integrity. Interactions between brain microvascular endothelial cells and other constituents of the NVU enable a rapid response to environmental changes by allowing cerebral blood flow to be matched with brain metabolic demands (Iadecola 2017). Indeed, current knowledge on brain barriers emphasizes the fact that NVU components work in concert to enable dynamic responses to pathological and pharmacological stressors including selective solute uptake from blood into brain tissue. Similarly, the BCSFB is comprised of a monolayer of choroid plexus epithelial cells that also possess tight junctions. Tight junctions at the BCSFB function in a manner similar to that of the BBB by limiting free passage of circulating substances from fenestrated capillaries into the cerebrospinal fluid (CSF) that is separated from brain tissue by a layer of ependymal cells (Hosoya and Tachikawa 2011). Transendothelial/transepithelial electrical resistance (TEER) values for the BCSFB are less than that of the BBB (i.e., approximately 150 Ω cm2 for the BCSFB versus 1,500–2,000 Ω cm2 for the BBB), which suggests that the BCSFB is somewhat leaky relative to the BBB (Redzic 2011; Lochhead et al. 2017). Nonetheless, paracellular transport across the BBB and BCSFB is limited to small molecular weight substances that can pass transcellularly through brain microvascular endothelial cells or choroid plexus epithelial cells (Liddelow 2015; Brzica et al. 2017). Passive diffusion across brain barrier cellular layers is a potential mechanism for uptake; however, drug physicochemical properties (i.e., molecular weight, hydrophilicity, pKa, number of hydrogen bond acceptors and donors) can limit the effectiveness of this therapeutic delivery route (Mikitsh and Chacko 2014). Additionally, drugs that are capable of partitioning into the plasma membrane are often substrates for ATP-dependent efflux transporters such as P-glycoprotein (P-gp), Multidrug Resistance Proteins (MRPs in humans; Mrps in rodents), and Breast Cancer Resistance Protein (BCRP in humans, Bcrp in rodents) (Chaves et al. 2014; Abdullahi et al. 2017; Yang et al. 2018). These transporters are involved in cellular extrusion of drugs and constitute a considerable biochemical barrier to effective brain delivery of therapeutic agents. In general, P-gp transports cationic or basic and neutral compounds while MRPs/Mrps are involved in cellular efflux of anionic drugs and their glucuronidated, sulfated, and glutathione-conjugated metabolites (Polli et al. 2009; Ronaldson and Davis 2015). BCRP/Bcrp has considerable substrate overlap with P-gp and is thought to function in synergy with P-gp to limit drug uptake into brain tissue (Polli et al. 2009; Ronaldson and Davis 2015; Williams et al. 2020). Clearly, the physical and biochemical characteristics of the BBB and BCSFB emphasize a need to consider novel approaches that can enable drugs to attain effective concentrations in the brain. As a result, there is considerable interest in facilitated transport mechanisms that can be exploited for selective delivery of therapeutics to the CNS for treatment of neurological diseases (Razzak and Florence 2019; Williams et al. 2020).
Fig. 1.

Structure of CNS barriers. (a) Blood–brain barrier composed of endothelial cells lining the systemic circulation expressing numerous tight junction protein complexes and adherens junctions (green) regulated by other cells within the neurovascular unit including the astrocytes (purple), neurons (yellow), microglia (blue), and pericytes (orange). (b) Blood–cerebrospinal fluid barrier composed of choroid plexus epithelial cells (blue) expressing tight junction protein complexes to regulated molecular passage from the fenestrated capillaries into the cerebrospinal fluid (Created with biorender.com)
The solute carrier (SLC) superfamily of transporters is responsible for blood-to-brain transport of circulating solutes into brain tissue (Abdullahi et al. 2017; Williams et al. 2020). The subfamily SLC22A is primarily involved in uptake transport of therapeutics from blood to brain and includes numerous antiporters, cotransporters and facilitated diffusion systems such as organic anion transporters (OATs in humans; Oats in rodents), organic cation transporters (OCTs in humans; Octs in rodents), and novel organic cation transporters (OCTNs in humans; Octns in rodents) (VanWert et al. 2010; Zhu et al. 2015). OCTs and OCTNs themselves are comprised of three main subtypes: facilitated diffusion transporters OCT1 (SLC22A1), OCT2 (SLC22A2), and OCT3 (SLC22A3); a cation and carnitine transporter OCTN1 (SLC22A4), a sodium carnitine or carnitine derivative cotransporters OCTN2 (SLC22A5) and OCTN3 (SLC22A21) (Gründemann et al. 1994; Okuda et al. 1996;Gründemann et al. 1998; Tamai et al. 1997; Wu et al. 1998). The membrane potential and concentration gradients of cationic substrates contribute to the driving force for OCT/Oct and OCTN1/Octn1 substrate flux across biological membranes with ion-independent electrogenic transport and predominantly coupled transport with either sodium or protons reported for OCTN2/Octn2- and OCTN3/Octn3-mediated transport (Tamai et al. 2001, 2004). While expression of OCTs and OCTNs has been well characterized in peripheral tissues, their localization and function within CNS barriers require more extensive research. To date, most information involving OCT- and OCTN-mediated transport at the CNS has been derived from non-human cell culture systems, OCT or OCTN-overexpressing cells or Xenopus oocytes, and proteoliposome experimentation (Friedrich et al. 2003). It has been shown, at the mRNA level, that all of the known OCT isoforms as well as OCTN2 are expressed in brain microvascular endothelial cells (Geier et al. 2013). Further studies have shown that OCT1–3 and OCTN2 proteins can be found within CNS barrier tissues, with greater expression of transporters observed at the BBB as compared to the BCSFB (Morris et al. 2017). Despite these expression data, the exact localization of OCTs and OCTNs in brain microvascular endothelial cells or choroid plexus epithelial cells has yet to be determined. Such information on localization and substrate specificity of OCT and OCTN transporters can aid in determining their role in facilitating drug delivery to the brain for treatment of neurological diseases. Furthermore, a detailed understanding of OCT and OCTN transport dynamics at brain barrier sites can inform discovery and development of novel centrally-acting therapeutics that display more efficient brain penetration due to selective CNS uptake mediated by cation transport mechanisms.
2. BBB Localization and Expression of OCTs and OCTNs
Central to targeting OCTs for CNS drug delivery is the understanding of their localization at brain barrier tissues. Several studies have shown that cultured brain endothelial cells or brain microvessels are highly enriched with OCT mRNA (Friedrich et al. 2003; Sung et al. 2005; Miecz et al. 2008; Wu et al. 2015). More recently, protein expression of OCT1/Oct1, OCT2/Oct2, and OCT3/Oct3 were reported in the murine brain endothelial cell line (bEND3) and in the human brain microvessel endothelial cell line (hCMEC/d3) (Sekhar et al. 2017). Interestingly, this same study localized Oct1 to both the luminal and abluminal plasma membrane in bEND3 cells; however, Oct1 expression was reported to be significantly higher at the luminal plasma membrane (Sekhar et al. 2017). This observation is consistent with a previous study by Lin and colleagues that also showed elevated luminal expression of Oct1 and Oct2 in cultured rat brain endothelial cells (Lin et al. 2010). Our laboratory has reported protein expression of Oct1 in intact microvessels isolated from rat brain (Brzica et al. 2017; Fig. 2). More recently, global proteomic analysis of human brain microvessels from healthy individuals revealed detectable quantities of OCT1 and OCT3 protein (Al-Majdoub et al. 2019). In contrast, quantitative targeted proteomics has failed to detect either OCT1 or OCT2 at the BBB in hippocampal Brodmann Areas 17 and 39 (Billington et al. 2019). When compared to the work of Al-Majdoub and colleagues, these data imply regional differences in BBB expression of OCTs; however, such results must be confirmed by detailed molecular studies and functional analyses. Although BBB expression of OCT and OCTN isoforms have been detected at the mRNA and/or protein level, there is insufficient evidence to determine the exact localization of these transporters at the apical or basolateral plasma membrane of brain microvessels in vivo. Such information is critical to advancing this family of transporters as a platform for drug delivery.
Fig. 2.
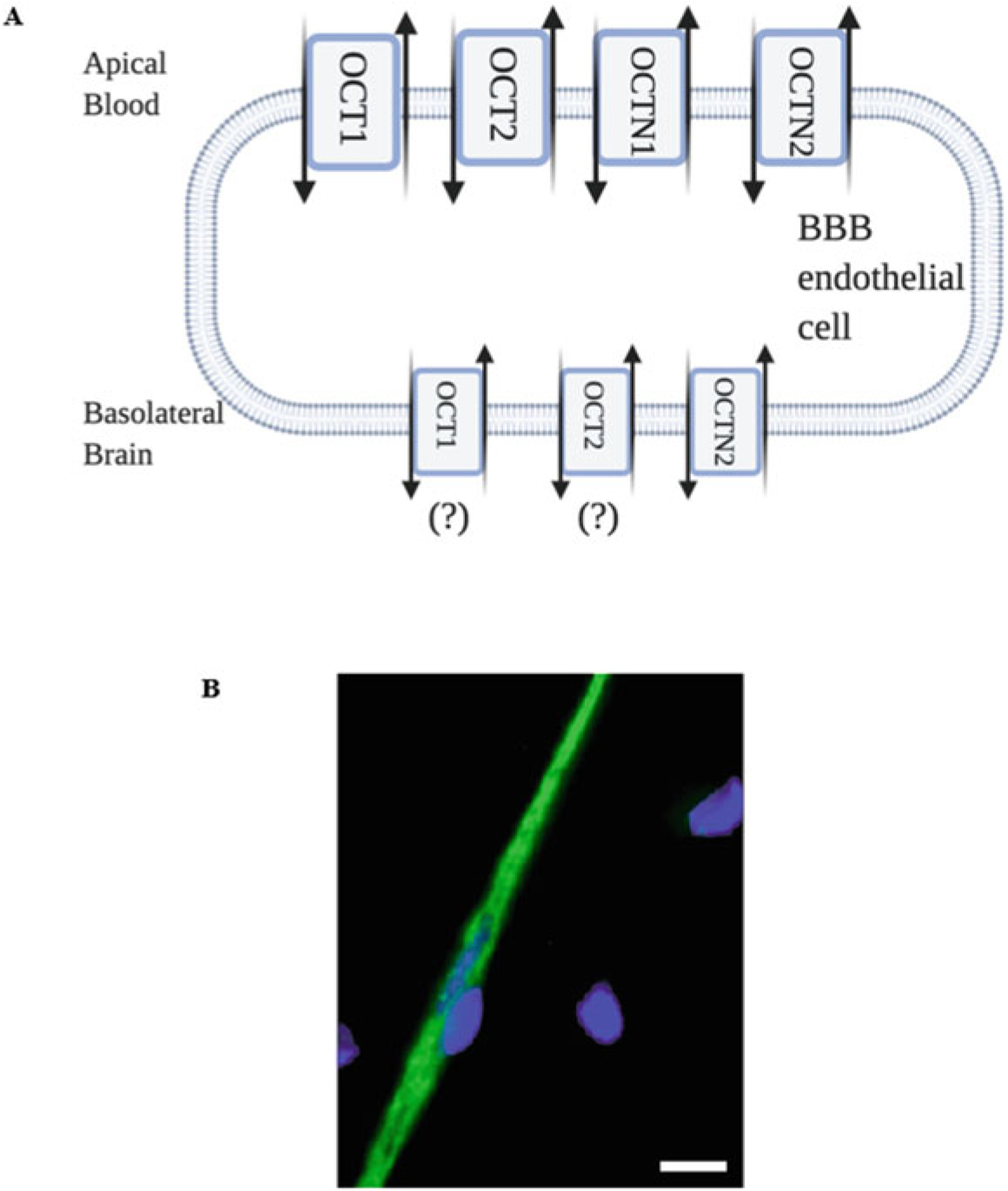
Proposed localization of OCT and OCTN transporters at the BBB. (a) Based on previous studies, various OCT and OCTN isoforms have been detected in brain microvessel endothelial cells (Created with biorender.com). (b) Fluorescence confocal microscopy data from our laboratory has shown that Oct1 is expressed in isolated microvessels from the brain of Sprague-Dawley rats. Green = Oct1; Blue = DAPI. Scale bar = 4 μm (Adapted from Brzica et al. J Cent Nerv Syst Dis. 9:1179573517693802, 2017)
Typically, translocation of cationic substrates across polarized epithelia involves a two-step process in which OCTs are involved in initial cellular uptake. A good example is organic cation secretion in the human kidney that involves both OCT2 and the multidrug and toxin extrusion transporter (MATE) 1 (Sandoval et al. 2018). In this context, MATE1 functions to ensure extrusion of cationic substrates into the urinary filtrate so that they can be efficiently excreted. At the BBB, evaluation of OCT/Oct localization has indicated that these transporters are preferentially expressed at the luminal plasma membrane of microvascular endothelial cells (Fig. 2). Indeed, functional expression of Octs has been demonstrated at the BBB as evidenced by OCT1/Oct1 and OCT2/Oct2-mediated uptake of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a substance that is able to access brain tissue and does not remain “trapped” within the endothelial cell (Lin et al. 2010). Taken together, these studies suggest that additional transporters such as MATEs may be required to ensure effective delivery of cationic solutes to the brain. Recently, protein expression of MATE1 and MATE2 was detected in hCMEC/d3 cells (Sekhar et al. 2019). Additionally, Mate1 and Mate2 protein expression was observed in microvessels isolated from C57BL6/129 mice (Sekhar et al. 2019) and Mate1 mRNA and protein expression was reported in brain capillaries from male ddY mice (Hiasa et al. 2006). More recently, Mate1 mRNA was shown to be expressed in brain microvessels from Swiss, FVB, and C57BL/6JRj mice (Chaves et al. 2020). Of particular significance, MATE1 and MATE2 protein was detected by western blot analysis in human frontal cortex, caudate nucleus, and putamen brain regions (Sekhar et al. 2019). These results are consistent with immunofluorescence staining of human brain microvessels, which demonstrated expression of MATE1 at the BBB (Geier et al. 2013). In contrast, Chaves and colleagues failed to detect MATE isoforms in brain microvessels isolated from human temporal lobe glioma specimens (Chaves et al. 2020). Since these data were derived from human tumor tissue, it is possible that transporters for organic cations such as MATEs were downregulated in response to cancer pathogenesis or pharmacotherapy. The variability in MATE/Mate expression data represented by these studies indicates the need to further clarify involvement of MATE isoforms as critical transporters that function in concert with OCTs/Octs to deliver cationic substances including drugs across polarized epithelial/endothelial cellular layers in the brain. This information is particularly critical to developing cation transporters as targets for CNS drug delivery given current data implying preferential expression of OCTs/Octs at the luminal plasma membrane of brain microvascular endothelial cells.
While it is generally accepted that neurons require efficient delivery of carnitine to allow for beta-oxidation of fatty acids, expression of OCTN isoforms at the BBB has not been well elucidated (Tracey et al. 2018). Some clarification of this critical issue has been provided in the scientific literature where mRNA and protein expression of OCTN2 has been confirmed within the cerebral microvasculature (Tsuji 2005; Okura et al. 2014). This is consistent with the known physiology for OCTN2, which has increased substrate specificity for carnitine derivatives and can facilitate delivery of these substances to brain parenchyma (Kido et al. 2001). More recently, Kurosawa and colleagues reported measurable expression of OCTN1 and OCTN2 mRNA in human brain microvessel endothelial cells derived from induced pluripotent stem cells as well as in the hCMEC/d3 cell line (Kurosawa et al. 2018). Functional studies that have evaluated substrate permeation across the BBB have allowed for more detailed identification of specific OCTN isoforms. For example, brain accumulation of ergothioneine, a specific OCTN1 substrate, provides functional evidence for OCTN1 expression at the level of the BBB (Tang et al. 2018). In studies using in vitro human brain endothelial cell culture systems, OCTN2 expression was shown to control transport of carnitine derivatives across the cell monolayer, suggesting that expression of this novel organic cation transporter is required for transcellular passage of large quantities of carnitine to promote neuronal homeostasis (Okura et al. 2014). Further evidence for functional OCTN2 expression in human brain endothelial cells was provided by Kurosawa and colleagues who showed that cellular uptake of L-carnitine (KM = 4.08 ± 2.61 μM; Vmax = 0.000797 ± 0.000256 nmol/mg protein/min) could be blocked by addition of an excess concentration of unlabeled carnitine (Kurosawa et al. 2018). In an in vitro mouse model, Octn2 has been detected at the protein level in cultured brain endothelial cells and was predominantly localized to the abluminal plasma membrane (Miecz et al. 2008). Overall, these studies provide evidence for localization and functional expression of OCTN isoforms at the BBB (Fig. 2) and suggest that these transporters can be targeted to facilitate drug delivery to the CNS.
3. BCSFB Localization and Expression of OCTs and OCTNs
In addition to the BBB, transporters that are localized to the BCSFB are known to highly regulate permeation of circulating molecules between the systemic circulation, the CSF, and brain interstitial space. The majority of transporters within the BCSFB are localized to choroid plexus epithelial cells that form a barrier between the blood and the CSF. Similar to the BBB, BCFSB epithelial cells express tight junction protein complexes, which limit paracellular diffusion of molecules. Although the BCSFB barrier is leakier in comparison with the BBB endothelium, it remains highly efficient in controlling molecular composition of the CSF through selective transcellular transport mechanisms. This includes transporters for cationic substances such as OCT/Oct and OCTN/Octn isoforms (Fig. 3). At the mRNA level, Oct1, Oct3, Octn1, and Octn2 have been detected in choroid plexus epithelium from Sprague-Dawley rats (Choudhuri et al. 2003). In contrast, Sweet and colleagues detected mRNA expression of Oct2 and Oct3, but not Oct1, at the adult rat choroid plexus (Sweet et al. 2001). In this particular study, transfection of GFP-tagged Oct2 was primarily localized to the apical membrane of choroid plexus epithelium (Sweet et al. 2001). Protein expression of Oct2 was exclusively detected in choroid plexus epithelial cells isolated from the third ventricle of pig brain (Uchida et al. 2020). Consistent with data of Sweet and colleagues, Oct2 was proposed to be localized to the apical membrane of choroid plexus epithelial cells and to function in concert with Mate1 to facilitate removal of cationic substances from the CSF (Uchida et al. 2020). Interestingly, this same study demonstrated Octn2 protein expression in choroid plexus isolated from all cerebral ventricles (Uchida et al. 2020). Additionally, the plasma membrane monoamine transporter (PMAT; Slc29a4), a novel identified multispecific organic cation transporter, has been identified at the apical membrane of choroid plexus epithelial cells (Engel et al. 2004; Duan and Wang 2013). The functional role of PMAT at the choroid plexus has been demonstrated in a Pmat knockout mouse model. Specifically, choroid plexus epithelial uptake of cationic substrates (i.e., dopamine, 5-HT, 1-methyl-4-phenylpyridinium (MPP+)) was reduced in Pmat(−/−) mice (Duan and Wang 2013). Furthermore, substrate uptake in these mice was insensitive to pharmacological Oct or neurotransmitter transporter inhibitors (Duan and Wang 2013), data that further confirms the functional importance of PMAT in the uptake of cationic substances into choroid plexus epithelial cells. High levels of Oct3 protein have been reported in choroid plexus epithelium isolated from male Wistar rats (Nakayama et al. 2007) and in mouse choroid plexus epithelium and ependymal cells (Vialou et al. 2004). Of particular note, antisense oligonucleotides targeting Oct3 and injected directly into the CSF increased brain concentrations of the Oct3 transport substrate methamphetamine, suggesting that this organic cation transporter is localized to the apical membrane of choroid plexus epithelial cells and can participate in extrusion of potentially toxic organic cations from the brain (Nakayama et al. 2007). In the context of drug delivery, further work needs to be done to confirm localization of OCT/Oct and/or OCTN/Octn isoforms to the basolateral plasma membrane of choroid plexus epithelial cells. Such information is critical in determining the therapeutic potential of targeting the BCSFB to enable therapeutic delivery directly into the CSF.
Fig. 3.

Proposed localization of OCT and OCTN transporters at the BCSFB (Created with biorender.com)
4. Localization of OCTs and OCTNs in Brain Parenchyma
Once cationic substrates have bypassed brain barrier tissues, they can also display cell-type specific uptake due to functional expression of OCTs/Octs and OCTNs/Octns within brain parenchyma cell types (Fig. 4). For example, neurons have been shown to express numerous OCT and OCTN isoforms that facilitate uptake of specific substrates (i.e., choline, carnitine, thiamine, ergothioneine) and neurotransmitters within the brain to mediate chemical signaling within neural networks (Busch et al. 1998; Duan and Wang 2010; Januszewicz et al. 2010). In neurons, expression of OCTN2 is required for uptake of carnitine to allow for beta-oxidation of fatty acids and subsequent increases in energy production and control of neurological functions (Pochini et al. 2019). Several OCT isoforms have substrate selectivity for precursor molecules required for neuronal synthesis of acetylcholine and dopamine. Indeed, expression of OCT2 and OCT3 within neuronal cells and OCT3 in glial cells illustrates the necessity of these transporters for adequate delivery of precursory subunits used in neurotransmitter synthesis (Blakely and Edwards 2012). Reuptake of neurotransmitters by neuronal and astrocytic OCT2 and OCT3 has also been identified as an essential regulatory mechanism for neurotransmission (Bacq et al. 2012; Nishijima and Tomiyama 2016). In studies performed using in vitro and murine models, brain parenchymal transport of dopamine, serotonin, epinephrine, norepinephrine, and histamine was shown to require functional expression of OCT2 while OCT3/Oct3 is involved in cellular uptake of dopamine and serotonin (Baganz et al. 2010; Kristufek et al. 2002; Yoshikawa et al. 2013). Immunohistochemical analysis of murine brain tissue confirmed localization of Oct2 expression primarily within neurons while Oct3 localization was detected in both neuronal and glial cells (Vialou et al. 2004). Localization of Oct3 in neurons is more controversial with some studies proposing expression of this transporter proximal to the synaptic cleft (Gasser et al. 2017) while others imply that Oct3 expression is exclusively found on astrocyte processes (Furihata and Anzai 2017). Such observations have led to the hypothesis that parenchymal Oct3 expression may vary depending upon brain region. In microglia, it has been proposed that OCTN1 expression and transport of homeostatic substrates such as ergothioneine can control pathological functions such as production of reactive oxygen species and release of proinflammatory cytokines (Ishimoto et al. 2018). In contrast, Oct2 or Oct3 mRNA was not detected in a mouse microglial cell line (BV2) (Fan et al. 2018). In terms of pharmacotherapy, numerous therapeutics (i.e., amphetamines, anti-convulsant agents, neuroprotective drugs) require selective uptake by OCT isoforms so that they can access molecular targets in the brain (Table 1). Therefore, consideration of OCT and OCTN localization and functional expression in brain tissue is critical to understanding drug distribution within the brain as well as the ability of centrally acting cationic drugs to access their molecular targets and elicit a therapeutic effect.
Fig. 4.

Proposed localization of OCT and OCTN transporters in neurons and in glial cells within brain parenchyma (Created with biorender.com)
Table 1.
Key centrally-acting transport substrates for OCTs and OCTNs (adapted from Koepsell 2020)
| Transporter | Endogenous substrates | Exogenous substrates |
|---|---|---|
| OCT1 | 5-HT, DA, NE, ACh, epinephrine, histamine, agmatine, salsolinol, tyramine, choline | Amantadine, amisulpride, berberine, butylscopolamine, O-desmethyltramadol, fluoxetine, haloperidol, ketamine, memantine, morphine, perphenazine, pramipexole, sumatriptan, sulpiride, varenicline, rizatriptan, naratriptan, sumatriptan, zolmitriptan |
| OCT2 | 5-HT, DA, NE, ACh, epinephrine, histamine, agmatine, salsolinol, choline, thiamine | Amisulpride, apomorphine, butylscopolamine, memantine, chlorprothixene, fampridine, ketamine, lappaconitine, methamphetamine, morphine, phenamil, selegiline, sulpiride, varenicline |
| OCT3 | 5-HT, DA, NE, ACh, epinephrine, histamine, agmatine, salsolinol, tyramine, thiamine | Amisulpride, butylscopolamine, ketamine, sulpiride |
| OCTN1 | Cholines, ergothioneine, stachydrine | Amisulpride, bupropion |
| OCTN2 | Cholines, acetyl-L-carnitine, D-carnitine, L-carnitine | Amisulpride |
5-HT 5-hydroxytryptamine (serotonin), DA dopamine, NE norepinephrine, ACh acetylcholine
5. Regulation of OCTs and OCTNs at Brain Barrier Sites
Targeting OCTs and OCTNs for optimized CNS delivery of centrally acting drugs requires an understanding of the “cellular machinery” involved in their regulation. Such pathways offer a unique opportunity to control OCT- and/or OCTN-mediated transport over a time course conducive to effective blood-to-brain drug uptake and, by extension, more efficacious pharmacotherapy. The regulation of OCT and OCTN isoforms that are expressed within the BBB and BCSFB is poorly understood; however, regulation in peripheral tissues (i.e., liver, kidney, and bronchial epithelial cells) can provide essential insights as to how these transporters may be regulated at brain barrier sites. Transcriptional regulation of OCTs/OCTNs involves binding of regulatory proteins to distinct binding sites in the gene promoter to modulate mRNA expression. Upstream binding factors that control de novo synthesis of these critical cation transporters include the upstream binding factor (USF)1, USF2, hepatic nuclear factor 4α (HNF-4α), and CCAAT/enhancer-binding proteins (Saborowski et al. 2006; Kajiwara et al. 2008; Rulcova et al. 2013). For example, treatment with dexamethasone, a glucocorticoid receptor agonist, has been shown to enhance cellular HNF-4α levels and subsequently increase expression of OCT1 within cultured human hepatocytes (Rulcova et al. 2013). HNF-4α has also been identified as a regulatory transcription factor in brain tissue (Wang et al. 2013; Niehof and Borlak 2009; Xu et al. 2011). Furthermore, evidence has been shown that regulation of numerous drug transporters at choroid plexus cells within the BCSFB is associated with HNF expression patterns (Wang et al. 2013; Niehof and Borlak 2009). Of particular note, cell-type specific and species-dependent variability of HNF-4α expression patterns can lead to variable OCT transporter expression (O’Brien et al. 2013; Lau et al. 2018). Indeed, upregulation of HNF-4α in brain microvessel endothelial cells and/or choroid plexus epithelial cells offers an opportunity to control OCT/Oct-mediated transport and optimize CNS disposition of centrally acting cationic drugs. Additionally, rodent Oct2 expression and transport activity is sensitive to exposure of sex-related hormones. Testosterone treatment has been shown to increase epithelial cell expression of Oct2 mRNA and to stimulate cellular uptake of the prototypical Oct transport substrate triethylammonium (TEA) (Urakami et al. 2000). In contrast, estradiol treatment caused an opposite response characterized by reduced Oct2 mRNA and decreased TEA transport (Urakami et al. 2000). Further evidence has implicated peroxisome proliferator-activated receptor (PPAR)-γ in the upregulation of OCTN2 expression within colonic epithelial cells and estrogen mediated upregulation within breast cancer cells (Maeda et al. 2008). PPAR-γ signaling pathways may prove to be an important regulatory target for cation transporters because they are expressed at brain barrier sites and have been implicated in the regulation of various transporters that are involved in determining tissue drug permeation (More et al. 2017; Stopa et al. 2018). While more detailed molecular studies must be completed to confirm regulation of Oct isoforms by HNF-4α or steroid pathways at brain barrier sites, these data do provide a framework for experiments that can improve our understanding of cation transporter regulation at the BBB and/or BCSFB. The future implication of this work is an ability to control cation transport in brain barrier tissues in an effort to provide more effective treatment of neurological diseases.
Aside from pathways stimulated by steroid hormones, multiple other intracellular pathways have been identified as causal factors in altered cation transporter expression and decreased mRNA levels. For example, functional changes to human embryonic kidney (HEK) cellular uptake of a fluorescent OCT1 transport substrate (i.e., 4-(4-(dimethylamino)styryl)-N-methylpyridinium (ASP)) have identified specific regulatory mechanisms for this transporter that involve decreased activation of protein kinase A (PKA) via inhibition of calmodulin, calcium dependent CaM-kinase II, or selective p56lck tyrosine kinase (Ciarimboli et al. 2004). At the functional level, decreased PKA activity resulted in a reduction in ASP uptake (Ciarimboli et al. 2004). Further post-translational downregulation of Oct isoforms has been identified in ischemic models. Of particular note, these studies have identified the role of ischemia/reperfusion inducible protein (IRIP), a regulatory protein (RS1) pathway that co-regulates the expression and membrane translocation of several transporters including OCT1 and MATE1 (Li et al. 2013). The work of Li and colleagues demonstrated that overexpression of IRIP in transfected HEK293 cells resulted in reduced transport of the prototypical organic cation MPP+ by both OCT1 and MATE1. IRIP-induced downregulation of OCT-mediated transport may result from altered trafficking of OCT1 from the Golgi to the cell membrane (Jiang et al. 2005). Of particular note, Jiang and colleagues showed that phosphorylation of the N-terminal RS1 domain on the SLC22A1 gene following activation of IRIP led to decreased exocytosis of OCT1 from Golgi vesicles (Jiang et al. 2005). Post-translational modification of OCT isoforms has also been shown to play a role in the regulation of these transporters. Specifically, regulation of OCT transporter activity was observed to occur via changes in the phosphorylation state of tyrosine residues localized to intracellular loop domains, thereby altering transport kinetics and reducing OCT-mediated transport (Sprowl et al. 2016).
Studies involving regulation of OCTN are less abundant in the scientific literature. Transcriptional regulation of OCTN1 has been shown to be modulated by the Runt-related transcription factor 1(RUNX1), which binds to the first intron of the OCTN1 (SLC22A4) gene (Tokuhiro et al. 2003). Heat-shock factors have also been identified as critical regulators of OCTN1 and OCTN2 expression as binding of heat-shock protein 70 (Hsp70) to the promoter of the SLC22A4 and SLC22A5 genes can modulate transport of the cationic substrates TEA and carnitine (Peltekova et al. 2004). More recent studies have pointed towards epigenetic modifications as critical regulators of OCT and OCTN functional expression. For example, hypermethylation to the promoter region of the SLC22A2 gene is associated with reduced OCT2 protein expression in human hepatocytes (Aoki et al. 2008). In contrast, the level of methylation of CpG sites in the SLC22A2 promoter was lower in the kidney, an organ known to have much higher expression of OCT2 relative to the liver (Aoki et al. 2008). Similarly, previous studies on OCT2 localization at the BBB and BCSFB have shown higher levels of this cation transporter relative to OCT1; however, epigenetic modulation of OCT isoforms at brain barrier sites has not yet been determined. Such regulation is an important avenue to consider in an effort to fully understand both tissue-specific expression of OCTs and OCTNs and molecular regulation of these critical transport proteins.
5.1. CNS Delivery by OCTs and/or OCTNs of Centrally Acting Drugs for Treatment of Neurological Diseases
Targeting OCTs and OCTNs offers a unique opportunity to optimize CNS delivery of cationic therapeutic agents. Indeed, the potential for utilizing this family of transporters to improve CNS pharmacotherapy is supported by previous studies demonstrating blood-to-brain uptake of MPTP by Oct1 and Oct2 (Lin et al. 2010; Wu et al. 2015) as well as brain delivery of acetyl-L-carnitine that is mediated by OCTN2 at the BBB (Inano et al. 2003). Interestingly, current knowledge on localization of OCTs and OCTNs at brain barrier sites suggests that blood-to-brain transport of cationic substrates may occur primarily at the BBB. In contrast, cation transporters expressed at the BCSFB appear to be primarily involved in extrusion of potentially toxic organic cations from the CSF and/or maintenance of CSF carnitine concentrations. Therefore, this section will describe opportunities for improving CNS drug delivery that will primarily focus on targeting OCTs and OCTNs at the BBB. Below, we provide examples of neurological diseases that can be treated using cationic drugs and insights as to how OCTs and/or OCTNs can be targeted for optimized CNS delivery of such compounds. Examples of therapeutic drugs that are known transport substrates for OCTs and/or OCTNs are presented in Table 1.
5.2. Ischemic Stroke
Stroke is a neurological and vascular disease resulting from impairment of blood flow to a specific brain region. This restriction in cerebral blood flow results in decreased nutrient (i.e., glucose, oxygen) delivery to neurons and other cell types within the brain parenchyma. Additionally, the BBB is disrupted in ischemic stroke, an effect that contributes to development of vasogenic edema and/or hemorrhagic transformation (An et al. 2017; Abdullahi et al. 2018). Stroke is the third leading cause of death within developed nations following coronary disease and cancers and it affects a large subset of the population with progressively increasing incidence rates over the past decade (Gorelick 2019). The severity of stroke symptoms and the potential for post-stroke neurological recovery greatly depends upon physical factors (i.e., the region of the brain that is impacted) as well as environmental factors (i.e., mood and motivation), social factors (i.e., family support), and therapeutic factors (i.e., early start of pharmacotherapy and rehabilitation). Clinical signs that are utilized to indicate acute stroke onset follow the FAST acronym and include facial droop (F), arm weakness (A), and impaired speech (S), which indicate that it is time (T) to go to the hospital (Musuka et al. 2015).
Central to the pathophysiology of ischemic stroke is the NVU. Deprivation of oxygen and glucose leads to an irreversibly damaged ischemic core and potentially salvageable surrounding tissue known as the penumbra (Liu et al. 2010; Abdullahi et al. 2018). This process causes neuronal cell death in the core and substantial neuronal injury in the penumbra. Additionally, cell–cell interactions and signaling occur in a coordinated manner between the multiple NVU cell types and matrix constituents, events that lead to BBB dysfunction and further CNS injury. Indeed, BBB permeabilization enables blood-borne substances that are normally peripherally restricted, such as excitatory amino acids, kinins, prostaglandins, metals, and proteins, to enter the CNS and accelerate cell death in ischemic brain tissue (Thompson and Ronaldson 2014). Oxidative stress injury secondary to reperfusion is a critical process that leads to BBB dysfunction. Such mechanisms are accelerated by reperfusion (i.e., recanalization) and restoration of oxygen supply, which results in production of reactive oxygen and nitrogen species, such as superoxide, nitric oxide, and peroxynitrite, within the endothelium (Heo et al. 2005; Garcia-Bonilla et al. 2014). Oxidative stress in excess of the antioxidant capacity of the endothelial cell leads to alterations in organization and localization of tight junction protein complexes and contributes to endothelial dysfunction and increased BBB permeability (Lochhead et al. 2010, 2012). Such endothelial dysfunction permits movement of water and circulating proteins into brain parenchyma (Heo et al. 2005; Sandoval and Witt 2008; Brouns et al. 2011). In an in vivo global hypoxia-reoxygenation system, which models a component of stroke, oxidative stress due to reoxygenation caused changes in both structure and localization of occludin oligomeric assemblies at the tight junction and increased permeability of the BBB to [14C]-sucrose (Lochhead et al. 2010; McCaffrey et al. 2009; Witt et al. 2003). Sucrose is a vascular marker that does not permeate the BBB under normal physiological conditions (Lochhead et al. 2020). Clinically, such BBB changes are evident in patients with stroke 3–4 h following stroke onset (Giraud et al. 2015). Vasogenic edema following ischemia/reperfusion is a consequence of BBB disruption due to phasic tight junction disruption and MMP-9 activity, which leads to extravasation of fluid and plasma proteins into brain parenchyma. When fluid is permitted to accumulate in the extracellular space, brain volume is increased in concordance with intracranial pressure due to vasogenic edema (Michinaga and Koyama 2015; Witt et al. 2008). Consequences of increased BBB permeability following ischemic stroke are not limited to fluid extravasation. Blood–brain barrier dysfunction can lead to uncontrolled leak of exogenous xenobiotics, including drugs, into brain parenchyma. Pharmacologic interventions aimed at BBB protection can prevent this exacerbation of brain tissue damage and promote stroke recovery. Preservation of BBB integrity is critical to maximize stroke recovery and to provide optimal CNS delivery of drugs with neuroprotective properties (Brzica et al. 2017; Williams et al. 2020).
As a stroke therapeutic, memantine functions as an antagonist of N-methyl-D-aspartate (NMDA) glutamate receptors. During ischemia, decreased CNS concentrations of oxygen and glucose can trigger increased neuronal calcium influx, a process that results in enhanced release of the excitatory neurotransmitter glutamate. Excessive synaptic accumulation of glutamate is associated with neuronal cell death, a pathological condition known as excitotoxicity. Pharmacological blockade of NMDA receptors is known to protect against such neuronal cell death and it is on this basis that memantine has been developed as a neurotherapeutic with efficacy in the setting of stroke. Memantine is a small molecule that can cross biological membranes by passive transcellular diffusion; however, it is also predominantly positively charged at physiological pH as demarcated by a pKa of 10.27 (Mehta et al. 2013). The consensus is that memantine requires a specific transport mechanism to be taken up into target tissues. At present, transport properties of memantine at the BBB have not been fully elucidated; however, memantine has been reported to be a substrate for proton-coupled transport systems, such as OCT1 and OCT2 (Mehta et al. 2013). Studies in Xenopus laevis oocytes transfected with OCT2 showed saturable uptake transport (Km = 34 ± 5 μM) for memantine (Busch et al. 1998). More recently, memantine uptake via in situ transcardiac perfusion in Swiss outbred mice was shown to be independent of transmembrane electrochemical potential (i.e., changes in K+ concentration in the perfusate), which is an established characteristic of OCT1–3 mediated transport (Mehta et al. 2013). Additionally, memantine uptake was increased in the presence of an enhanced outwardly directed proton gradient, commonly observed in OCTN1 mediated transport (Mehta et al. 2013). In contrast, in studies using an immortalized human brain endothelial cell line, uptake of memantine was not inhibited by ergothioneine, an OCTN1 substrate (Higuchi et al. 2015). Indeed, the exact mechanism of memantine transport across the BBB requires more extensive research; however, therapeutic targeting of memantine to the CNS via OCT-dependent drug delivery may prove to be an effective mechanism to enhance the utility of this neuroprotective drug in ischemic stroke therapy. Furthermore, targeting of novel neuroprotective drugs to OCT or OCTN isoforms at the BBB is a viable strategy to ensure effective therapeutic delivery of such compounds to the ischemic brain.
Similar to memantine, several natural products that have been shown to elicit neuroprotective or vascular protective effects within the brain are also substrates for OCTN1 (Zhang et al. 2020; Koh et al. 2020). In human brain microvascular endothelial cells, ergothioneine exhibited vascular protection as demarcated by suppression of NADPH-1 oxidase and increased activity of glutathione reductase, catalase, and superoxide dismutase (Li et al. 2017). These beneficial effects were abolished when human brain endothelial cells were treated with an siRNA probe that selectively targeted OCTN1, suggesting the requirement of this transporter to facilitate protective effects associated with ergothioneine (Li et al. 2017). Indeed, ergothioneine has been reported to be a high-affinity transport substrate for OCTN1 (Gründemann et al. 2005; Engelhart et al. 2020). Recently, stachydrine, a component of Japanese motherwort, was shown to protect against neurological deficits in male Sprague-Dawley rats subjected to experimental ischemic stroke (Li et al. 2020). Work by Grundemann and colleagues demonstrated that stachydrine is a transport substrate for OCTN1 (Gründemann et al. 2005), providing evidence for an endogenous mechanism that enables this natural product to traverse the BBB. As such, expression of OCTN1 in cerebral endothelial cells is a crucial mechanism that enables neuroprotective substrates to access brain parenchyma, thereby limiting pathology mediated degradation of neurons and, possibly, glial cells.
5.3. Parkinson’s Disease
Parkinson’s disease is a neurological disorder associated with progressive loss of voluntary motor control, increased muscle rigidity, and resting muscle tremors resulting from impaired striatal dopaminergic neurotransmission (DeMaagd and Philip 2015). Affecting more than 60,000 people per year, Parkinson’s disease has become the second most common neurodegenerative disease to Alzheimer’s disease with increased incidence rates observed in aging populations. The etiology of Parkinson’s disease is associated with both genetic (i.e., inherited Parkinson’s disease) and environmental factors (i.e., idiopathic or sporadic Parkinson’s disease), which both result in elevation of alpha-synuclein accumulation within brain tissue and selective loss of dopaminergic neurons (Ball et al. 2019). Anatomically, dopaminergic neuronal loss is reflected by loss of pigmentation within the substantia nigra, which is observed in post-mortem brain tissue collected from patients with a positive diagnosis of Parkinson’s disease. Evidence that dopaminergic cell loss is the root cause of Parkinson’s disease comes from observations that the cationic neurotoxin MPP+ induces symptoms of Parkinson’s disease in animal models (Kopin 1992). MPP+ is produced via a monoamine oxidase B (MAO-B)-mediated oxidation reaction of MPTP, a highly lipophilic substance that can readily cross the BBB. Additionally, MPP+ is a known transport substrate of OCT isoforms, a mechanism that can contribute to its ability to permeate the neuronal plasma membrane and inhibit regeneration of ATP by oxidative phosphorylation and trigger neuronal apoptosis (Langston 2017; Hörmann et al. 2020). While the exact process of selective MPP+ toxicity in dopaminergic neurons has yet to be fully elucidated, a previous in vitro study has discovered that disruption of intracellular dopamine homeostasis may be the “trigger” for cell death in this class of neurons (Choi et al. 2015). These results are critical to gaining a mechanistic understanding of neurodegeneration in Parkinson’s disease.
Oral delivery of the dopamine prodrug, Levodopa (L-DOPA), is the gold standard of care for patients with Parkinson’s disease; however, L-DOPA therapy is characterized by numerous side effects that require adjunct pharmacotherapy and/or limit its therapeutic use. As a result, multiple other drugs have been developed for treatment of Parkinson’s disease. Interestingly, several anti-Parkinson’s disease drugs including pramipexole (i.e., a dopamine D2 receptor agonist), selegiline (i.e., a selective MAO-B inhibitor), and amantadine (i.e., an antiviral drug that promotes dopamine release) are transport substrates for OCT1 and/or OCT2 (Goralski et al. 2002; Ishiguro et al. 2005; Hendrickx et al. 2013). Clinical evidence for OCT-mediated transport of anti-Parkinson’s disease drugs was provided by Becker and colleagues who showed that patients with a specific polymorphism in the SLC22A1 gene required higher doses in order to achieve a pharmacological effect (Becker et al. 2011). As noted in this paper, the minor polymorphic C allele at rs622342 in the SLC22A1 gene is associated with reduced transport function of OCT1 (Becker et al. 2011), an effect that may decrease brain uptake of substrate drugs. Additionally, both amantadine and selegiline have been reported to be OCT transport inhibitors (Lin et al. 2010). This is an important consideration given the multiple drugs commonly prescribed to patients with Parkinson’s disease. Specifically, interactions with OCTs by amantadine or selegiline can alter the pharmacokinetics and tissue disposition of co-administered OCT substrate drugs, an effect that can lead to unwanted pharmacological effects and/or toxicity.
5.4. Schizophrenia
Psychoses such as schizophrenia are mental disorders in which thought and emotions are so impaired that contact is lost with external reality. The lifetime prevalence of schizophrenia and related disorders in the USA is approximately 1% (Dixon et al. 2018). The predominant symptoms of schizophrenia include delusions, hallucinations, incoherent or nonsensical speech, and inappropriate behavior for specific social situations. Schizophrenia is a multifactorial psychosis that is affected by environmental, maturational, neurological, and genetic/epigenetic factors. The general etiology of schizophrenia involves dysfunction in neurotransmitter systems associated with dopamine, serotonin, or glutamate. Specifically, dopaminergic overactivity in the mesolimbic tract is associated with delusions and hallucinations while hypoactivity in the mesocortical tract resulting from overactivation of presynaptic D2 receptors is associated with cognitive and emotional deficits (Brisch et al. 2014). Serotonergic overactivity in the frontal cortex, which is mediated via 5-HT2A receptors, can also cause cognitive and emotional abnormalities in schizophrenia (Garcia-Bea et al. 2019; Puig and Gulledge 2011). Finally, reduced activity of NMDA glutamate receptors on inhibitory neurons leads to disinhibition of glutamatergic neurotransmission in the prefrontal cortex and subsequent cognitive and motor impairment (Stahl 2018). Indeed, neurotransmitter receptors associated with the pathogenesis of schizophrenia (i.e., D2 dopamine receptors, 5-HT2A serotonin receptors, NMDA glutamate receptors) can only be effectively modified by drugs capable of permeating brain barriers. This requirement suggests that transporters such as OCT isoforms constitute an effective mechanism to facilitate blood-to-brain delivery of antipsychotic drugs.
Pharmacological treatment of schizophrenia requires chronic administration of antipsychotic drugs in order to manage symptoms of this disease. In order to achieve optimal symptom control, it is critical that these drugs permeate the BBB and attain effective free concentrations at their site of action. Indeed, several antipsychotic drugs have been demonstrated to be OCT transport substrates. For example, studies in both a human brain endothelial cell line (hCMEC/d3) and a murine brain microvascular endothelial cell line (bEND.3) demonstrated that the commonly prescribed antipsychotic drugs (i.e., amisulpride, haloperidol) are transport substrates for OCT1/Oct1 (Sekhar et al. 2019). Involvement of OCT1 in the transport of both amisulpride and haloperidol was demonstrated using the OCT1/OCT3 inhibitor prazosin and the OCT1/OCT2 inhibitor amantadine (Sekhar et al. 2019). The atypical antipsychotic drug sulpiride has also been shown to be a transport substrate for both OCT1 and OCT2 (Dos Santos Pereira et al. 2014; Li et al. 2017; Takano et al. 2006). Of particular note, in vitro studies in transfected HEK293 cells (i.e., hOCT1-HEK293 and hOCT2-HEK293) demonstrated that sulpiride has greater affinity for OCT1 (KM = 2.6 μM) than OCT2 (KM = 68 μM) (Takano et al. 2006). Plasma concentrations of sulpiride following a pharmacological daily dose range between 70.1 ng/mL and 1,121.2 ng/mL (Tokunaga et al. 1997). These values correspond to molar concentrations of 0.21 μM and 3.28 μM, suggesting that OCT1 is more likely to contribute to CNS uptake of this compound. Since sulpiride is primarily charged at physiological pH 7.4, it is essential that a carrier-mediated transport process such as OCT1 be available at brain barrier sites to enable this compound to attain efficacious free concentrations at its site of action in the CNS (Li et al. 2017). Additional cation transporters that may be involved in sulpiride disposition include OCTN1, OCTN2, MATE1, and MATE2 (Watanabe et al. 2002; Li et al. 2017). Taken together, these studies provide strong evidence for the involvement of OCT isoforms in the delivery of antipsychotic drugs to therapeutic targets in the brain.
Polypharmacy in the treatment of schizophrenia implies a potential for transporter-mediated drug–drug interactions. Many such interactions can adversely affect delivery of drugs to molecular targets in the brain, thereby reducing the effectiveness of various treatment strategies. A good example is the interaction between lamotrigine, a commonly prescribed drug for treatment of bipolar disorder and seizures, and the antipsychotic drug quetiapine (Dickens et al. 2012). Lamotrigine is subject to active uptake in hCMEC/d3 cells that is mediated by OCT1 (Dickens et al. 2012). The KM for OCT1-mediated lamotrigine transport (i.e., 68 μM) is higher than pharmacologically relevant concentrations between 4 and 42 μM (Dickens et al. 2012), suggesting that this transporter is likely to play a key role in lamotrigine delivery to the brain. Additionally, quetiapine is a potent inhibitor of OCT1-mediated transport (Dickens et al. 2012, 2018). In fact, the in vitro IC50 for quetiapine inhibition of OCT1-mediated lamotrigine transport (i.e., 1.9 μM) is slightly higher than reported average plasma concentrations of 1–1.6 μM (DeVane and Nemeroff 2001; Dickens et al. 2012); however, a standard quetiapine dosing regimen of 250 mg three times daily yields a Cmax of 2.7 μM (Wong et al. 2001), indicating that concentrations necessary to inhibit OCT1-mediated lamotrigine transport can be achieved clinically. While this suggests a strong potential for a transporter-mediated drug–drug interaction, the effects of quetiapine-mediated inhibition of CNS delivery of lamotrigine require further study. Additionally, pharmacological inhibition of OCT-mediated antipsychotic drug transport at brain barrier sites can have profound implications on treatment of other disorders such as brain infections. For example, haloperidol has been shown to inhibit OCT-mediated transport of the antimicrobial agent pentamidine at the BBB (Sekhar et al. 2017). While this interaction suggests that elevated concentrations of pentamidine are required to treat CNS effects of infections such as Human African trypanosomiasis in individuals that require haloperidol therapy for management of schizophrenic symptoms (Sekhar et al. 2017), it emphasizes an overall need to consider transporter-mediated interactions that can affect CNS drug delivery in patients receiving multiple drugs that interact with OCT isoforms.
6. Summary and Conclusion
OCTs and OCTNs are a compelling class of membrane transporters. It has been suggested that such transporters for organic cations may represent therapeutic targets in the brain, particularly in the setting of clinical depression where OCT2 is known to participate in clearance of monoamines such as norepinephrine and serotonin (Bacq et al. 2012). This is particularly relevant for antidepressant drugs such as bupropion where pharmacological inhibition of OCT-mediated transport may account for a component of its mechanism of action (Haenisch et al. 2012; Sandoval et al. 2018; Han et al. 2019). For other centrally-acting cationic drugs, the ability of OCTs and OCTNs to facilitate delivery across brain barriers is a critical mechanism that determines therapeutic effectiveness. This is certainly the case for currently marketed drugs used for treatment of ischemic stroke, Parkinson’s disease, and schizophrenia. To date, localization, regulation, and functional expression of OCTs and OCTNs at the BBB and BCSFB have been understudied and many existing publications provide conflicting evidence of their mRNA and/or protein expression in these CNS barrier tissues. These discrepancies could be due to species differences, variable transporter expression in cell culture models, age-related expression differences, and/or environmental/epigenetic factors. Perhaps the strongest evidence for the role of cation transporters in CNS drug delivery comes from functional studies that have repeatedly shown selective uptake via OCTs and/or OCTNs of centrally acting cationic drugs into brain microvascular endothelial cells and/or choroid plexus epithelial cells as well as in animal models. Indeed, these studies highlight the potential for OCT- and/or OCTN-mediated drug delivery as an opportunity to optimize therapeutic effectiveness of novel compounds for treatment of neurological diseases. Central to this endeavor is knowledge derived from membrane topological studies of OCTs and OCTNs that have revealed specific amino acid residues responsible for substrate binding. Specifically, site-directed mutagenesis studies have determined key residues (i.e., Phe160, Trp218, Arg440, Leu447, and Asp475) as key contributors to substrate binding affinity, which have been demonstrated through modified cellular uptake of established transport substrates (Volk et al. 2009). Such information is crucial to rational design of novel brain penetrant cationic drugs. Indeed, optimized OCT- or OCTN-mediated transport of currently marketed drugs does not necessarily correlate to improved therapeutic effectiveness. A good example is the case of anti-migraine triptan drugs (i.e., rizatriptan, naratriptan, zolmitriptan). While these compounds are established substrates for OCTs (Matthaei et al. 2016), it is questionable as to whether their blood-to-brain transport is necessary to confer an optimal therapeutic effect (Ahn and Basbaum, 2005). Therefore, it is critical to consider the molecular target of a drug before determining that optimized therapeutic delivery by a BBB transporter is required for compound advancement or for development of improved treatment paradigms for neurological diseases. While further research is needed to enhance our understanding of OCT and/or OCTN-mediated transport at the BBB and BCSFB, current data provides strong evidence for the utility of these transporters as targets for CNS drug delivery. Furthermore, the observation that numerous OCT/OCTN substrate drugs that are specific to CNS pathologies are commonly prescribed provides a critical framework and link for future discovery of new centrally acting drugs that are OCT and/or OCTN substrates.
Acknowledgments
This work is funded by grants from the National Institutes of Neurological Diseases and Stroke (NINDS; R01 NS084941) and the American Heart Association (19TPA34910113) to PTR and by a grant from the National Institute on Drug Abuse (NIDA; R01 DA051812) to TPD and PTR.
References
- Abdullahi W, Davis TP, Ronaldson PT (2017) Functional expression of P-glycoprotein and organic anion transporting polypeptides at the blood-brain barrier: understanding transport mechanisms for improved CNS drug delivery? AAPS J 19(4):931–939. 10.1208/s12248-017-0081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullahi W, Tripathi D, Ronaldson PT (2018) Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol 315 (3):C343–C356. 10.1152/ajpcell.00095.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn AH, Basbaum AI (2005) Where do triptans act in the treatment of migraine? Pain 115 (1–2):1–4. 10.1016/j.pain.2005.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majdoub ZM, Al Feteisi H, Achour B et al. (2019) Proteomic quantification of human blood–brain barrier SLC and ABC transporters in healthy individuals and dementia patients. Mol Pharm 16(3):1220–1233. 10.1021/acs.molpharmaceut.8b01189 [DOI] [PubMed] [Google Scholar]
- An SJ, Kim TJ, Yoon B-W (2017) Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 19(1):3–10. 10.5853/jos.2016.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki M, Terada T, Kajiwara M et al. (2008) Kidney-specific expression of human organic cation transporter 2 (OCT2/SLC22A2) is regulated by DNA methylation. Am J Physiol Physiol 295 (1):F165–F170. 10.1152/ajprenal.90257.2008 [DOI] [PubMed] [Google Scholar]
- Bacq A, Balasse L, Biala G et al. (2012) Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol Psychiatry 17(9):926–939. 10.1038/mp.2011.87 [DOI] [PubMed] [Google Scholar]
- Baganz N, Horton R, Martin K, Holmes A, Daws LC (2010) Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci 30(45):15185–15195. 10.1523/JNEUROSCI.2740-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball N, Teo W-P, Chandra S, Chapman J (2019) Parkinson’s disease and the environment. Front Neurol 10:218. 10.3389/fneur.2019.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker ML, Visser LE, van Schaik RHN, Hofman A, Uitterlinden AG, BHC S (2011) OCT1 polymorphism is associated with response and survival time in anti-Parkinsonian drug users. Neurogenetics 12(1):79–82. 10.1007/s10048-010-0254-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington S, Salphati L, Hop CECA et al. (2019) Interindividual and regional variability in drug transporter abundance at the human blood–brain barrier measured by quantitative targeted proteomics. Clin Pharmacol Ther 106(1):228–237. 10.1002/cpt.1373 [DOI] [PubMed] [Google Scholar]
- Blakely RD, Edwards RH (2012) Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harb Perspect Biol 4(2):a005595. 10.1101/cshperspect.a005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisch R, Saniotis A, Wolf R et al. (2014) The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psych 5:47. 10.3389/fpsyt.2014.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns R, Wauters A, De Surgeloose D, Mariën P, De Deyn PP (2011) Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur Neurol 65(1):23–31. 10.1159/000321965 [DOI] [PubMed] [Google Scholar]
- Brzica H, Abdullahi W, Ibbotson K, Ronaldson PT (2017) Role of transporters in central nervous system drug delivery and blood-brain barrier protection: relevance to treatment of stroke. J Cent Nerv Syst Dis 9:1179573517693802. 10.1177/1179573517693802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch AE, Karbach U, Miska D et al. (1998) Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol 54(2):342–352. 10.1124/mol.54.2.342 [DOI] [PubMed] [Google Scholar]
- Chaves C, Shawahna R, Jacob A (2014) Declèves* J-MS and X. Human ABC transporters at blood-CNS interfaces as determinants of CNS drug penetration. Curr Pharm Des 20(10):1450–1462. 10.2174/13816128113199990466 [DOI] [PubMed] [Google Scholar]
- Chaves C, Campanelli F, Chapy H et al. (2020) An interspecies molecular and functional study of organic cation transporters at the blood-brain barrier: from rodents to humans. Pharmaceutics 12 (4). 10.3390/pharmaceutics12040308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Panhelainen A, Schmitz Y et al. (2015) Changes in neuronal dopamine homeostasis following 1-methyl-4-phenylpyridinium (MPP+) exposure. J Biol Chem 290(11):6799–6809. 10.1074/jbc.M114.631556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri S, Cherrington NJ, Li N, Klaassen CD (2003) Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metab Dispos 31(11):1337–1345. 10.1124/dmd.31.11.1337 [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Struwe K, Arndt P et al. (2004) Regulation of the human organic cation transporter hOCT1. J Cell Physiol 201(3):420–428. 10.1002/jcp.20081 [DOI] [PubMed] [Google Scholar]
- DeMaagd G, Philip A (2015) Parkinson’s disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P T 40(8):504–532. https://pubmed.ncbi.nlm.nih.gov/26236139 [PMC free article] [PubMed] [Google Scholar]
- DeVane CL, Nemeroff CB (2001) Clinical pharmacokinetics of quetiapine. Clin Pharmacokinet 40 (7):509–522. 10.2165/00003088-200140070-00003 [DOI] [PubMed] [Google Scholar]
- Dickens D, Owen A, Alfirevic A et al. (2012) Lamotrigine is a substrate for OCT1 in brain endothelial cells. Biochem Pharmacol 83(6):805–814. 10.1016/j.bcp.2011.12.032 [DOI] [PubMed] [Google Scholar]
- Dickens D, Rädisch S, Chiduza GN et al. (2018) Cellular uptake of the atypical antipsychotic clozapine is a carrier-mediated process. Mol Pharm 15(8):3557–3572. 10.1021/acs.molpharmaceut.8b00547 [DOI] [PubMed] [Google Scholar]
- Dixon LB, Goldman HH, Srihari VH, Kane JM (2018) Transforming the treatment of schizophrenia in the United States: the RAISE initiative. Annu Rev Clin Psychol 14(1):237–258. 10.1146/annurev-clinpsy-050817-084934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos Pereira JN, Tadjerpisheh S, Abu Abed M et al. (2014) The poorly membrane permeable antipsychotic drugs amisulpride and sulpiride are substrates of the organic cation transporters from the SLC22 family. AAPS J 16(6):1247–1258. 10.1208/s12248-014-9649-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J (2010) Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther 335 (3):743–753. 10.1124/jpet.110.170142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J (2013) Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene. J Biol Chem 288(5):3535–3544. 10.1074/jbc.M112.436972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J (2004) Identification and characterization of a novel monoamine transporter in human brain. J Biol Chem 279(48):50042–50049. 10.1074/jbc.M407913200 [DOI] [PubMed] [Google Scholar]
- Engelhart DC, Granados JC, Shi D et al. (2020) Systems biology analysis reveals eight SLC22 transporter subgroups, including OATs, OCTs, and OCTNs. Int J Mol Sci 21(5):1791. 10.3390/ijms21051791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Chen Z, Pathak JL, Carneiro AMD, Chung CY (2018) Differential regulation of adhesion and phagocytosis of resting and activated microglia by dopamine. Front Cell Neurosci 12:309. 10.3389/fncel.2018.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich A, Prasad PD, Freyer D, Ganapathy V, Brust P (2003) Molecular cloning and functional characterization of the OCTN2 transporter at the RBE4 cells, an in vitro model of the blood-brain barrier. Brain Res 968(1):69–79. 10.1016/s0006-8993(02)04271-3 [DOI] [PubMed] [Google Scholar]
- Furihata T, Anzai N (2017) Functional expression of organic ion transporters in astrocytes and their potential as a drug target in the treatment of central nervous system diseases. Biol Pharm Bull 40 (8):1153–1160. 10.1248/bpb.b17-00076 [DOI] [PubMed] [Google Scholar]
- Garcia-Bea A, Miranda-Azpiazu P, Muguruza C et al. (2019) Serotonin 5-HT2A receptor expression and functionality in postmortem frontal cortex of subjects with schizophrenia: selective biased agonism via Gαi1-proteins. Eur Neuropsychopharmacol 29(12):1453–1463. 10.1016/j/euroneuro.2019.10.013 [DOI] [PubMed] [Google Scholar]
- Garcia-Bonilla L, Moore JM, Racchumi G et al. (2014) Inducible nitric oxide synthase in neutrophils and endothelium contributes to ischemic brain injury in mice. J Immunol 193(5):2531–2537. 10.4049/jimmunol.1400918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Hurley MM, Chan J, Pickel VM (2017) Organic cation transporter 3 (OCT3) is localized to intracellular and surface membranes in select glial and neuronal cells within the basolateral amygdaloid complex of both rats and mice. Brain Struct Funct 222(4):1913–1928. 10.1007/s00429-016-1315-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier EG, Chen EC, Webb A et al. (2013) Profiling solute carrier transporters in the human blood–brain barrier. Clin Pharmacol Ther 94(6):636–639. 10.1038/clpt.2013.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud M, Cho T-H, Nighoghossian N et al. (2015) Early blood brain barrier changes in acute ischemic stroke: a sequential MRI study. J Neuroimaging 25(6):959–963. 10.1111/jon.12225 [DOI] [PubMed] [Google Scholar]
- Goralski KB, Lou G, Prowse MT et al. (2002) The cation transporters rOCT1 and rOCT2 interact with bicarbonate but play only a minor role for amantadine uptake into rat renal proximal tubules. J Pharmacol Exp Ther 303(3):959–968. 10.1124/jpet.102.038885 [DOI] [PubMed] [Google Scholar]
- Gorelick PB (2019) The global burden of stroke: persistent and disabling. Lancet Neurol 18 (5):417–418. 10.1016/S1474-4422(19)30030-4 [DOI] [PubMed] [Google Scholar]
- Gründemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H (1994) Drug excretion mediated by a new prototype of polyspecific transporter. Nature 372(6506):549–552. 10.1038/372549a0 [DOI] [PubMed] [Google Scholar]
- Gründemann D, Köster S, Kiefer N et al. (1998) Transport of monoamine transmitters by the organic cation transporter type 2, OCT2. J Biol Chem 273(47):30915–30920. 10.1074/jbc.273.47.30915 [DOI] [PubMed] [Google Scholar]
- Gründemann D, Harlfinger S, Golz S et al. (2005) Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A 102(14):5256–5261. 10.1073/pnas.0408624102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, Drescher E, Thiemer L et al. (2012) Interaction of antidepressant and antipsychotic drugs with the human organic cation transporters hOCT1, hOCT2 and hOCT3. Naunyn Schmiedebergs Arch Pharmacol 385(10):1017–1023. 10.1007/s00210-012-0781-8 [DOI] [PubMed] [Google Scholar]
- Han LW, Gao C, Zhang Y, Wang J, Mao Q (2019) Transport of bupropion and its metabolites by the model CHO and HEK293 cell lines. Drug Metab Lett 13(1):25–36. 10.2174/1872312813666181129101507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx R, Johansson JG, Lohmann C et al. (2013) Identification of novel substrates and structure–activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem 56(18):7232–7242. 10.1021/jm400966v [DOI] [PubMed] [Google Scholar]
- Heo JH, Han SW, Lee SK (2005) Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med 39(1):51–70. 10.1016/j.freeradbiomed.2005.03.035 [DOI] [PubMed] [Google Scholar]
- Hiasa M, Matsumoto T, Komatsu T, Moriyama Y (2006) Wide variety of locations for rodent MATE1, a transporter protein that mediates the final excretion step for toxic organic cations. Am J Physiol Physiol 291(4):C678–C686. 10.1152/ajpcell.00090.2006 [DOI] [PubMed] [Google Scholar]
- Higuchi K, Kitamura A, Okura T, Deguchi Y (2015) Memantine transport by a proton-coupled organic cation antiporter in hCMEC/D3 cells, an in vitro human blood-brain barrier model. Drug Metab Pharmacokinet 30(2):182–187. 10.1016/j.dmpk.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Hörmann S, Gai Z, Kullak-Ublick GA, Visentin M (2020) Plasma membrane cholesterol regulates the allosteric binding of 1-methyl-4-phenylpyridinium to organic cation transporter 2 (SLC22A2). J Pharmacol Exp Ther 372(1):46–53. 10.1124/jpet.119.260877 [DOI] [PubMed] [Google Scholar]
- Hosoya K, Tachikawa M (2011) Roles of organic anion/cation transporters at the blood–brain and blood–cerebrospinal fluid barriers involving uremic toxins. Clin Exp Nephrol 15(4):478–485. 10.1007/s10157-011-0460-y [DOI] [PubMed] [Google Scholar]
- Iadecola C (2017) The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96(1):17–42. 10.1016/j.neuron.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano A, Sai Y, Nikaido H et al. (2003) Acetyl-L-carnitine permeability across the blood–brain barrier and involvement of carnitine transporter OCTN2. Biopharm Drug Dispos 24 (8):357–365. 10.1002/bdd.371 [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Saito A, Yokoyama K, Morikawa M, Igarashi T, Tamai I (2005) Transport of the dopamine D2; agonist pramipexole by rat organic cation transporters OCT1 and OCT2 in kidney. Drug Metab Dispos 33(4):495–499. 10.1124/dmd.104.002519 [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Nakamichi N, Nishijima H, Masuo Y, Kato Y (2018) Carnitine/organic cation transporter OCTN1 negatively regulates activation in murine cultured microglial cells. Neurochem Res 43(1):116–128. 10.1007/s11064-017-2350-5 [DOI] [PubMed] [Google Scholar]
- Januszewicz E, Bekisz M, Mozrzymas JW, Nałęcz KA (2010) High affinity carnitine transporters from OCTN family in neural cells. Neurochem Res 35(5):743–748. 10.1007/s11064-010-0131-5 [DOI] [PubMed] [Google Scholar]
- Jiang W, Prokopenko O, Wong L, Inouye M, Mirochnitchenko O (2005) IRIP, a new ischemia/reperfusion-inducible protein that participates in the regulation of transporter activity. Mol Cell Biol 25(15):6496–6508. 10.1128/MCB.25.15.6496-6508.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara M, Terada T, Asaka J et al. (2008) Regulation of basal core promoter activity of human organic cation transporter 1 (OCT1/SLC22A1). Am J Physiol Liver Physiol 295(6):G1211–G1216. 10.1152/ajpgi.90360.2008 [DOI] [PubMed] [Google Scholar]
- Kido Y, Tamai I, Ohnari A et al. (2001) Functional relevance of carnitine transporter OCTN2 to brain distribution of l-carnitine and acetyl-l-carnitine across the blood–brain barrier. J Neurochem 79(5):959–969. 10.1046/j.1471-4159.2001.00621.x [DOI] [PubMed] [Google Scholar]
- Koepsell H (2020) Organic cation transporters in health and disease. Pharmacol Rev 72 (1):253–319. 10.1124/pr.118.015578 [DOI] [PubMed] [Google Scholar]
- Koh SS, Ooi SC-Y, Lui NM-Y et al. (2020) Effect of ergothioneine on 7-ketocholesterol-induced endothelial injury. Neuromolecular Med. 10.1007/s12017-020-08620-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ (1992) Features of the dopaminergic neurotoxin MPTP. Ann N Y Acad Sci 648:96–104. 10.1111/j.1749-6632.1992.tb24527.x [DOI] [PubMed] [Google Scholar]
- Kristufek D, Rudorfer W, Pifl C, Huck S (2002) Organic cation transporter mRNA and function in the rat superior cervical ganglion. J Physiol 543(Pt 1):117–134. 10.1113/jphysiol.2002.021170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa T, Tega Y, Higuchi K et al. (2018) Expression and functional characterization of drug transporters in brain microvascular endothelial cells derived from human induced pluripotent stem cells. Mol Pharm 15(12):5546–5555. 10.1021/acs.molpharmaceut.8b00697 [DOI] [PubMed] [Google Scholar]
- Langston JW (2017) The MPTP story. J Parkinsons Dis 7(s1):S11–S19. 10.3233/JPD-179006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HH, Ng NHJ, Loo LSW, Jasmen JB, Teo AKK (2018) The molecular functions of hepatocyte nuclear factors - in and beyond the liver. J Hepatol 68(5):1033–1048. 10.1016/j.jhep.2017.11.026 [DOI] [PubMed] [Google Scholar]
- Li Q, Yang H, Peng X et al. (2013) Ischemia/reperfusion-inducible protein modulates the function of organic cation transporter 1 and multidrug and toxin extrusion 1. Mol Pharm 10(7):2578–2587. 10.1021/mp400013t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Weng Y, Wang W et al. (2017) Multiple organic cation transporters contribute to the renal transport of sulpiride. Biopharm Drug Dispos 38(9):526–534. 10.1002/bdd.2104 [DOI] [PubMed] [Google Scholar]
- Li L, Sun L, Qiu Y, Zhu W, Hu K, Mao J (2020) Protective effect of Stachydrine against cerebral ischemia-reperfusion injury by reducing inflammation and apoptosis through P65 and JAK2/STAT3 signaling pathway. Front Pharmacol 11:64. 10.3389/fphar.2020.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA (2015) Development of the choroid plexus and blood-CSF barrier. Front Neurosci 9:32. 10.3389/fnins.2015.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-J, Tai Y, Huang M-T et al. (2010) Cellular localization of the organic cation transporters, OCT1 and OCT2, in brain microvessel endothelial cells and its implication for MPTP transport across the blood-brain barrier and MPTP-induced dopaminergic toxicity in rodents. J Neurochem 114(3):717–727. 10.1111/j.1471-4159.2010.06801.x [DOI] [PubMed] [Google Scholar]
- Liu S, Levine SR, Winn HR (2010) Targeting ischemic penumbra: part I - from pathophysiology to therapeutic strategy. J Exp Stroke Transl Med 3(1):47–55. 10.6030/1939-067x-3.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE et al. (2010) Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 30(9):1625–1636. 10.1038/jcbfm.2010.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Sanchez-Covarrubias L et al. (2012) Tempol modulates changes in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory pain. Am J Physiol Heart Circ Physiol 302(3):H582–H593. 10.1152/ajpheart.00889.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, Ronaldson PT, Davis TP (2017) Hypoxic stress and inflammatory pain disrupt blood-brain barrier tight junctions: implications for drug delivery to the central nervous system. AAPS J 19(4):910–920. 10.1208/s12248-017-0076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, Yang J, Ronaldson PT, Davis TP (2020) Structure, function, and regulation of the blood-brain barrier tight junction in central nervous system disorders. Front Physiol 11:914. 10.3389/fphys.2020.00914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Wakasawa T, Funabashi M et al. (2008) Regulation of Octn2 transporter (SLC22A5) by peroxisome proliferator activated receptor alpha. Biol Pharm Bull 31(6):1230–1236. 10.1248/bpb.31.1230 [DOI] [PubMed] [Google Scholar]
- Matthaei J, Kuron D, Faltraco F et al. (2016) OCT1 mediates hepatic uptake of sumatriptan and loss-of-function OCT1 polymorphisms affect sumatriptan pharmacokinetics. Clin Pharmacol Ther 99(6):633–641. 10.1002/cpt.317 [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Willis CL, Staatz WD et al. (2009) Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem 110 (1):58–71. 10.1111/j.1471-4159.2009.06113.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta DC, Short JL, Nicolazzo JA (2013) Memantine transport across the mouse blood–brain barrier is mediated by a cationic influx H+ antiporter. Mol Pharm 10(12):4491–4498. 10.1021/mp400316e [DOI] [PubMed] [Google Scholar]
- Michinaga S, Koyama Y (2015) Pathogenesis of brain edema and investigation into anti-edema drugs. Int J Mol Sci 16(5):9949–9975. 10.3390/ijms16059949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miecz D, Januszewicz E, Czeredys M et al. (2008) Localization of organic cation/carnitine transporter (OCTN2) in cells forming the blood–brain barrier. J Neurochem 104(1):113–123. 10.1111/j.1471-4159.2007.05024.x [DOI] [PubMed] [Google Scholar]
- Mikitsh JL, Chacko A-M (2014) Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect Med Chem 6:11–24. 10.4137/PMC.S13384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More VR, Campos CR, Evans RA et al. (2017) PPAR-α, a lipid-sensing transcription factor, regulates blood-brain barrier efflux transporter expression. J Cereb Blood Flow Metab 37 (4):1199–1212. 10.1177/0271678X16650216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Rodriguez-Cruz V, Felmlee MA (2017) SLC and ABC transporters: expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. AAPS J 19(5):1317–1331. 10.1208/s12248-017-0110-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musuka TD, Wilton SB, Traboulsi M, Hill MD (2015) Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ 187(12):887–893. 10.1503/cmaj.140355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Kitaichi K, Ito Y et al. (2007) The role of organic cation transporter-3 in methamphetamine disposition and its behavioral response in rats. Brain Res 1184:260–269. 10.1016/j.brainres.2007.09.072 [DOI] [PubMed] [Google Scholar]
- Niehof M, Borlak J (2009) Expression of HNF4alpha in the human and rat choroid plexus: implications for drug transport across the blood-cerebrospinal-fluid (CSF) barrier. BMC Mol Biol 10:68. 10.1186/1471-2199-10-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima H, Tomiyama M (2016) What mechanisms are responsible for the reuptake of levodopa-derived dopamine in parkinsonian striatum? Front Neurosci 10:575. 10.3389/fnins.2016.00575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien VP, Bokelmann K, Ramírez J et al. (2013) Hepatocyte nuclear factor 1 regulates the expression of the organic cation transporter 1 via binding to an evolutionary conserved region in intron 1 of the OCT1 gene. J Pharmacol Exp Ther 347(1):181–192. 10.1124/jpet.113.206359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda M, Saito H, Urakami Y, Takano M, Inui K (1996) cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem Biophys Res Commun 224 (2):500–507. 10.1006/bbrc.1996.1056 [DOI] [PubMed] [Google Scholar]
- Okura T, Kato S, Deguchi Y (2014) Functional expression of organic cation/carnitine transporter 2 (OCTN2/SLC22A5) in human brain capillary endothelial cell line hCMEC/D3, a human blood-brain barrier model. Drug Metab Pharmacokinet 29(1):69–74. 10.2133/dmpk.dmpk-13-rg-058 [DOI] [PubMed] [Google Scholar]
- Peltekova VD, Wintle RF, Rubin LA et al. (2004) Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet 36(5):471–475. 10.1038/ng1339 [DOI] [PubMed] [Google Scholar]
- Pochini L, Galluccio M, Scalise M, Console L, Indiveri C (2019) OCTN: a small transporter subfamily with great relevance to human pathophysiology, drug discovery, and diagnostics. SLAS Discov Adv Life Sci R D 24(2):89–110. 10.1177/2472555218812821 [DOI] [PubMed] [Google Scholar]
- Polli JW, Olson KL, Chism JP et al. (2009) An unexpected synergist role of p-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor Lapatinib. Drug Metab Dispos 37(2):439–442. 10.1124/dmd.108.024646 [DOI] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT (2011) Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol 44(3):449–464. 10.1007/s12035-011-8214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzak AR, Florence GJ (2019) Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int J Mol Sci 20(12):3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzic Z (2011) Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 8(1):3. 10.1186/2045-8118-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP (2015) Targeting transporters: promoting blood-brain barrier repair in response to oxidative stress injury. Brain Res 1623:39–52. 10.1016/j.brainres.2015.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulcova A, Krausova L, Smutny T et al. (2013) Glucocorticoid receptor regulates organic cation transporter 1 (OCT1, SLC22A1) expression via HNF4α upregulation in primary human hepatocytes. Pharmacol Rep 65(5):1322–1335. 10.1016/s1734-1140(13)71491-9 [DOI] [PubMed] [Google Scholar]
- Saborowski M, Kullak-Ublick GA, Eloranta JJ (2006) The human organic cation transporter-1 gene is transactivated by hepatocyte nuclear factor-4alpha. J Pharmacol Exp Ther 317(2):778–785. 10.1124/jpet.105.099929 [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA (2008) Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 32(2):200–219. 10.1016/j.nbd.2008.08.005 [DOI] [PubMed] [Google Scholar]
- Sandoval PJ, Zorn KM, Clark AM, Ekins S, Wright SH (2018) Assessment of substrate-dependent ligand interactions at the organic cation transporter OCT2 using six model substrates. Mol Pharmacol 94(3):1057–1068. 10.1124/mol.117.111443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhar GN, Georgian AR, Sanderson L et al. (2017) Organic cation transporter 1 (OCT1) is involved in pentamidine transport at the human and mouse blood-brain barrier (BBB). PLoS One 12(3):e0173474. 10.1371/journal.pone.0173474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhar GN, Fleckney AL, Boyanova ST et al. (2019) Region-specific blood–brain barrier transporter changes leads to increased sensitivity to amisulpride in Alzheimer’s disease. Fluids Barriers CNS 16(1):38. 10.1186/s12987-019-0158-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowl JA, Ong SS, Gibson AA et al. (2016) A phosphotyrosine switch regulates organic cation transporters. Nat Commun 7(1):10880. 10.1038/ncomms10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S (2018) Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr 23(3):187–191. 10.1017/S1092852918001013 [DOI] [PubMed] [Google Scholar]
- Stopa EG, Tanis KQ, Miller MC et al. (2018) Comparative transcriptomics of choroid plexus in Alzheimer’s disease, frontotemporal dementia and Huntington’s disease: implications for CSF homeostasis. Fluids Barriers CNS 15(1):18. 10.1186/s12987-018-0102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J-H, Yu K-H, Park J-S et al. (2005) Saturable distribution of Tacrine into the striatal extracellular fluid of the rat: evidence of involvement of multiple organic cation transporters in the transport. Drug Metab Dispos 33(3):440–448. 10.1124/dmd.104.002220 [DOI] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB (2001) Ventricular choline transport: a role for organic cation transporter 2 expressed in choroid plexus. J Biol Chem 276(45):41611–41619. 10.1074/jbc.M108472200 [DOI] [PubMed] [Google Scholar]
- Takano A, Suhara T, Yasuno F et al. (2006) The antipsychotic sultopride is overdosed--a PET study of drug-induced receptor occupancy in comparison with sulpiride. Int J Neuropsychopharmacol 9(5):539–545. 10.1017/S1461145705006103 [DOI] [PubMed] [Google Scholar]
- Tamai I, Yabuuchi H, Nezu J et al. (1997) Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett 419(1):107–111. 10.1016/s0014-5793(97)01441-5 [DOI] [PubMed] [Google Scholar]
- Tamai I, China K, Sai Y et al. (2001) Na(+)-coupled transport of L-carnitine via high-affinity carnitine transporter OCTN2 and its subcellular localization in kidney. Biochim Biophys Acta 1512(2):273–284. 10.1016/s0005-2736(01)00328-5 [DOI] [PubMed] [Google Scholar]
- Tamai I, Nakanishi T, Kobayashi D et al. (2004) Involvement of OCTN1 (SLC22A4) in pH-dependent transport of organic cations. Mol Pharm 1(1):57–66. 10.1021/mp0340082 [DOI] [PubMed] [Google Scholar]
- Tang RMY, Cheah IK-M, Yew TSK, Halliwell B (2018) Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci Rep 8(1):1601. 10.1038/s41598-018-20021-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Ronaldson PT (2014) Drug delivery to the ischemic brain. Adv Pharmacol 71:165–202. 10.1016/bs.apha.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhiro S, Yamada R, Chang X et al. (2003) An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet 35(4):341–348. 10.1038/ng1267 [DOI] [PubMed] [Google Scholar]
- Tokunaga H, Kudo K, Jitsufuchi N, Ohtsuka Y, Imamura T (1997) Sensitive determination of sulpiride in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 691(1):203–207. 10.1016/s0378-4347(96)00428-8 [DOI] [PubMed] [Google Scholar]
- Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST (2018) Neuronal lipid metabolism: multiple pathways driving functional outcomes in health and disease. Front Mol Neurosci 11:10. 10.3389/fnmol.2018.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A (2005) Influx transporters and drug targeting: application of peptide and cation transporters. Int Congr Ser 1277:75–84. 10.1016/j.ics.2005.02.013 [DOI] [Google Scholar]
- Uchida Y, Goto R, Takeuchi H et al. (2020) Abundant expression of OCT2, MATE1, OAT1, OAT3, PEPT2, BCRP, MDR1 and xCT transporters in blood-arachnoid barrier of pig, and polarized localizations at CSF- and blood-facing plasma membranes. Drug Metab Dispos 48(2):135–145. 10.1124/dmd.119.089516 [DOI] [PubMed] [Google Scholar]
- Urakami Y, Okuda M, Saito H, Inui K (2000) Hormonal regulation of organic cation transporter OCT2 expression in rat kidney. FEBS Lett 473(2):173–176. 10.1016/S0014-5793(00)01525-8 [DOI] [PubMed] [Google Scholar]
- VanWert AL, Gionfriddo MR, Sweet DH (2010) Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos 31(1):1–71. 10.1002/bdd.693 [DOI] [PubMed] [Google Scholar]
- Vialou V, Amphoux A, Zwart R, Giros B, Gautron S (2004) Organic cation transporter 3 (Slc22a3) is implicated in salt-intake regulation. J Neurosci 24(11):2846–2851. 10.1523/JNEUROSCI.5147-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk C, Gorboulev V, Kotzsch A, Müller TD, Koepsell H (2009) Five amino acids in the innermost cavity of the substrate binding cleft of organic cation transporter 1 interact with extracellular and intracellular corticosterone. Mol Pharmacol 76(2):275–289. 10.1124/mol.109.054783 [DOI] [PubMed] [Google Scholar]
- Wang J, Cheng H, Li X, Lu W, Wang K, Wen T (2013) Regulation of neural stem cell differentiation by transcription factors HNF4–1 and MAZ-1. Mol Neurobiol 47(1):228–240. 10.1007/s12035-012-8335-0 [DOI] [PubMed] [Google Scholar]
- Watanabe K, Sawano T, Endo T, Sakata M, Sato J (2002) Studies on intestinal absorption of Sulpiride (2): Transepithelial transport of Sulpiride across the human intestinal cell line Caco-2. Biol Pharm Bull 25(10):1345–1350. 10.1248/bpb.25.1345 [DOI] [PubMed] [Google Scholar]
- Williams EI, Betterton RD, Davis TP, Ronaldson PT (2020) Transporter-mediated delivery of small molecule drugs to the brain: a critical mechanism that can advance therapeutic development for ischemic stroke. Pharmaceutics 12(2):154. 10.3390/pharmaceutics12020154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Hom S, Davis TP (2003) Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Circ Physiol 285(6): H2820–H2831. 10.1152/ajpheart.00589.2003 [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Sandoval KE, Davis TP (2008) Reoxygenation stress on blood–brain barrier paracellular permeability and edema in the rat. Microvasc Res 75(1):91–96. 10.1016/j.mvr.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YWJ, Yeh C, Thyrum PT (2001) The effects of concomitant phenytoin administration on the steady-state pharmacokinetics of quetiapine. J Clin Psychopharmacol 21(1). https://journals.lww.com/psychopharmacology/Fulltext/2001/02000/The_Effects_of_Concomitant_Phenytoin.16.aspx [DOI] [PubMed] [Google Scholar]
- Wu X, Prasad PD, Leibach FH, Ganapathy V (1998) cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochem Biophys Res Commun 246(3):589–595. 10.1006/bbrc.1998.8669 [DOI] [PubMed] [Google Scholar]
- Wu K-C, Lu Y-H, Peng Y-H et al. (2015) Decreased expression of organic cation transporters, Oct1 and Oct2, in brain microvessels and its implication to MPTP-induced dopaminergic toxicity in aged mice. J Cereb Blood Flow Metab 35(1):37–47. 10.1038/jcbfm.2014.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Lu A, Sharp FR (2011) Regional genome transcriptional response of adult mouse brain to hypoxia. BMC Genomics 12(1):499. 10.1186/1471-2164-12-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Reilly BG, Davis TP, Ronaldson PT (2018) Modulation of opioid transport at the blood-brain barrier by altered ATP-binding cassette (ABC) transporter expression and activity. Pharmaceutics 10(4):192. 10.3390/pharmaceutics10040192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Naganuma F, Iida T et al. (2013) Molecular mechanism of histamine clearance by primary human astrocytes. Glia 61(6):905–916. 10.1002/glia.22484 [DOI] [PubMed] [Google Scholar]
- Zhang L, Li F, Hou C et al. (2020) Design, synthesis, and biological evaluation of novel stachydrine derivatives as potent neuroprotective agents for cerebral ischemic stroke. Naunyn Schmiedebergs Arch Pharmacol 393(12):2529–2542. 10.1007/s00210-020-01868-4 [DOI] [PubMed] [Google Scholar]
- Zhu C, Nigam KB, Date RC et al. (2015) Evolutionary analysis and classification of OATs, OCTs, OCTNs, and other SLC22 transporters: structure-function implications and analysis of sequence motifs. PLoS One 10(11):e0140569. 10.1371/journal.pone.0140569. [DOI] [PMC free article] [PubMed] [Google Scholar]


