Abstract
Twenty-eight Borrelia burgdorferi isolates from the Charleston, S.C., area are described. This represents the first report and characterization of the Lyme disease spirochete from that state. The isolates were obtained from December 1994 through December 1995 from the tick Ixodes scapularis, collected from vegetation, and from the rodents Peromyscus gossypinus (cotton mouse), Neotoma floridana (eastern wood rat), and Sigmodon hispidus (cotton rat). All isolates were screened immunologically by indirect immunofluorescence with monoclonal antibodies to B. burgdorferi-specific outer surface protein A (OspA) (antibodies H5332 and H3TS) and B. burgdorferi-specific OspB (antibodies H6831 and H614), a Borrelia (genus)-specific antiflagellin antibody (H9724), Borrelia hermsii-specific antibodies (H9826 and H4825), and two polyclonal antibodies (one to Borrelia species and another to B. burgdorferi). Six of the isolates were analyzed by exposing Western blots to monoclonal antibodies H5332, H3TS, H6831, and H9724. All isolates were also analyzed by PCR with five pairs of primers known to amplify selected DNA target sequences specifically reported to be present in the reference strain, B. burgdorferi B-31. The protein profiles of six of the isolates (two from ticks, one from a cotton mouse, two from wood rats, and one from a cotton rat) also were compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. We conclude that the 28 Charleston isolates are B. burgdorferi sensu stricto based on their similarities to the B. burgdorferi B-31 reference strain.
Human cases of Lyme disease (LD) have been reported in 46 of the 48 contiguous states of the United States, with most cases recorded in the mid-Atlantic and northeastern areas, followed by the north central and northern California coastal regions (5, 20). More than 13,000 cases of LD in 43 states were reported to the Centers for Disease Control and Prevention (CDC) in 1994 (16); more than 15,000 cases were reported in 1998 (CDC, personal communication). Nevertheless, controversy as to whether LD occurs in the southern United States (4, 8, 22, 23) exists.
Clinical cases of LD in South Carolina have been reported (34, 35), but there is disagreement as to whether they were true LD cases (7). In the absence of isolates of Borrelia burgdorferi, the etiologic agent of LD, from humans in South Carolina, epidemiologic evidence assumes great importance. Ixodes scapularis, the main tick vector of B. burgdorferi in most regions of the United States, is widely distributed in South Carolina (10, 11, 18, 26) and infests various vertebrates there (18), including humans (13). Serologic surveys for antibodies against B. burgdorferi in South Carolina rodents indicated a prevalence of 38% (11 of 29) in cotton mice (Peromyscus gossypinus) in the eastern counties of Marion and Dillon (21). Moreover, putative B. burgdorferi isolates have been cultured from birds, rodents, and ticks in South Carolina (12, 25; J. H. Oliver, Jr., unpublished data). If these isolates are definitively confirmed to be B. burgdorferi, it would indicate that this pathogen is endemic and cycling enzootically in South Carolina. This would greatly strengthen the epidemiologic arguments for the likely presence of human cases of LD in that state.
Here we report the first isolation, cultivation, and characterization of 28 isolates of B. burgdorferi from ticks and rodents in the area of Charleston, S.C. These 28 spirochetal isolates are among 146 that we have obtained from ticks, rodents, and birds from seven geographic areas within five counties in South Carolina, encompassing sites in the Piedmont, Sandhills, Coastal Plain, and Coastal Zone regions of the state.
MATERIALS AND METHODS
Tick and rodent collections and spirochete isolation.
Males and females of I. scapularis were collected in December 1994 and January, February, and December 1995 by drag sampling in the Mt. Pleasant area of Charleston County, a suburb of the city of Charleston, S.C. Ticks were removed from the cloth and identified by us (identification was confirmed by the associate curator of the U.S. National Tick Collection). Rodents were livetrapped from the same area from February through December 1995, excluding March, June, August, and November. A sample of ticks was surface sterilized, triturated, and inoculated into Barbour-Stoenner-Kelly (BSK) II medium (3) as described previously (27). Cultures were incubated at 34°C and then examined for spirochetes by dark-field microscopy twice weekly for the first 2 weeks and weekly thereafter for 6 weeks. The urinary bladders and ear clip tissues from cotton mice (P. gossypinus), eastern wood rats (Neotoma floridana), and cotton rats (Sigmodon hispidus) were also inoculated into BSK II medium. The ear clips consisted of small triangular pieces of tissue from the peripheral tip of the external pinna of each animal. Prior to inoculation, rodent ears (the external pinna of each animal) were cleaned with 95% ethanol and tissues were sliced into smaller pieces. The ear tissues were washed again in 95% ethanol, followed by a rinse in a 1:1 mixture of 10% Clorox and 95% ethanol (26).
Indirect immunofluorescence.
Spirochetal isolates were analyzed immunologically by indirect immunofluorescence with several monoclonal antibodies (MAbs) and polyclonal antibodies (see Table 1). They included two B. burgdorferi-specific anti-outer surface protein A (OspA) MAbs (H5332 and H3TS), two B. burgdorferi-specific anti-outer surface protein B (OspB) MAbs (H614 and H6831), a polyclonal B. burgdorferi-specific antibody, two Borrelia (genus)-specific antiflagellin MAbs (H9724 and H605), a polyclonal Borrelia (genus)-specific antibody, and two Borrelia hermsii-specific MAbs (H9826 and H4825).
TABLE 1.
Immunofluorescent reactivities of spirochetal isolates from Charleston, S.C., to antibodies
| Isolate | Host | Immunofluorescent reactivity of isolate toa:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OspA MAbs
|
OspB MAbs
|
Borrelia sp. (fla) antibodies
|
Anti- B. burgdorferi PAb | Anti-B. hermsii MAbs
|
|||||||
| H5332 | H3TS | H614 | H6831 | H605 | H9724 | PAb | H4825 | H9826 | |||
| SCCH-1 | I. scapularis (male) | 4 | 4 | — | 4 | — | |||||
| SCCH-2 | I. scapularis (male) | 4 | 4 | — | 4 | — | |||||
| SCCH-3 | I. scapularis (male) | 4 | 4 | — | 4 | — | |||||
| SCCH-4 | N. floridana | 4 | 4 | — | 4 | — | |||||
| SCCH-6 | P. gossypinus | 4 | 4 | — | 4 | — | |||||
| SCCH-7 | P. gossypinus | 4 | 4 | — | 4 | — | |||||
| SCCH-8 | P. gossypinus | 4 | 4 | 4 | — | 4 | 4 | 4 | 4 | — | — |
| SCCH-9 | P. gossypinus | 4 | 4 | 4 | — | 3 | 4 | 4 | 4 | — | — |
| SCCH-10 | P. gossypinus | 4 | 4 | — | — | 3 | 4 | 4 | 4 | — | — |
| SCCH-11 | N. floridana | 4 | 4 | — | — | 3 | 4 | 4 | 4 | — | — |
| SCCH-12 | N. floridana | 4 | 4 | — | — | 4 | 4 | 4 | 4 | — | — |
| SCCH-13 | P. gossypinus | 4 | 4 | 4 | — | 4 | 4 | 4 | 4 | — | — |
| SCCH-14 | P. gossypinus | 4 | 4 | — | — | 3 | 4 | 4 | 4 | — | |
| SCCH-15 | N. floridana | 4 | 4 | — | — | 3 | 4 | 4 | 4 | — | |
| SCCH-16 | N. floridana | 4 | 4 | — | — | 3 | 4 | 4 | 4 | — | |
| SCCH-17 | S. hispidus | 4 | 4 | — | — | 4 | 4 | 4 | 4 | — | — |
| SCCH-19 | P. gossypinus | 4 | 4 | 4 | — | 4 | 4 | 4 | 4 | — | |
| SCCH-23 | S. hispidus | 4 | 4 | — | — | 3 | 4 | 4 | 4 | — | |
| SCCH-24 | S. hispidus | 4 | 4 | 4 | — | 3 | 4 | 4 | 4 | — | |
| SCCH-25 | S. hispidus | 4 | 4 | 4 | — | 3 | 4 | 4 | 4 | — | |
| SCCH-26 | S. hispidus | 4 | 4 | — | — | 3 | 4 | 4 | 4 | — | |
| SCCH-28 | S. hispidus | 4 | 4 | 4 | — | 3 | 4 | 4 | 3 | — | |
| SCCH-30 | P. gossypinus | 4 | 4 | 4 | — | 3 | 4 | 4 | 4 | — | |
| SCCH-31 | P. gossypinus | 4 | 4 | 4 | — | 3 | 4 | 4 | 4 | — | |
| SCCH-32 | N. floridana | 4 | 4 | — | — | 3 | 4 | 4 | 4 | — | |
| SCCH-33 | N. floridana | 4 | 4 | ±4b | — | 4 | 4 | 4 | 4 | — | |
| SCCH-34 | I. scapularis (male) | 4 | 4 | 4 | — | 3 | 4 | 4 | 4 | — | |
Numbers indicate intensity of reactivity (4 is maximum). Dashes indicate that there was no reactivity. Blanks indicate that the isolate was not tested. PAb, polyclonal antibody.
A few spirochetes reacted to MAb H614, but most did not.
Western blots.
Spirochetal isolates were analyzed immunologically by Western blotting with several MAbs (see Fig. 1 to 4). Western blottings were carried out by electrotransferring the proteins from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels to nitrocellulose membranes (Bio-Rad). Membranes were blocked by immersion in 5% dry milk for 1 h at room temperature. Whole membranes were exposed to MAbs H5332 (1:800), H3TS (1:3,200), H6831 (1:100), and H9724 (1:100) for 1 h at room temperature. The membranes were then washed in Tris-buffered saline with 0.1% Tween 20 three times for 5 min each at room temperature. They were subsequently incubated in horseradish peroxidase-labeled anti-mouse secondary antibody (Kirkegaard & Perry Laboratories, Inc.) at a 1:1,000 dilution for 1 h at room temperature. The membranes were then washed three times in Tris-buffered saline with 0.1% Tween 20 and once in distilled water for 5 min per washing. The membranes were incubated in 3,3′,5,5′-tetramethylbenzidine substrate solution at room temperature. When a suitable color intensity was observed, the reactions were stopped by immersing the membranes in distilled water for 10 to 20 s.
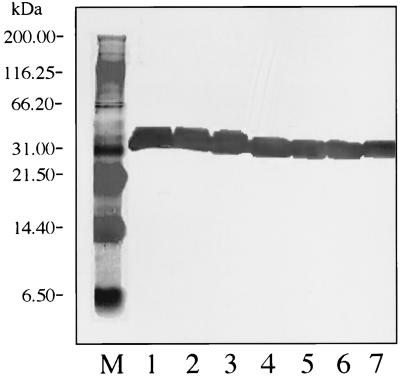
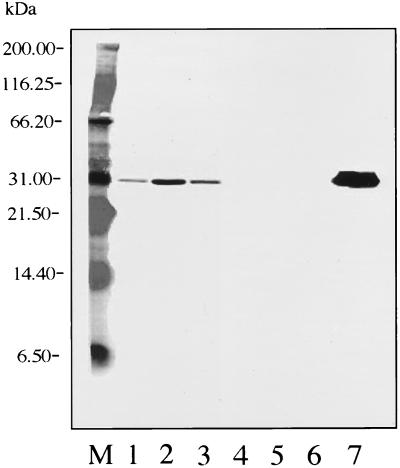
FIG. 1.
Western blot with MAb 5332 (against OspA). Lane M, molecular mass standards; lane 1, SCCH-17; lane 2, SCCH-12; lane 3, SCCH-11; lane 4, SCCH-8; lane 5, SCCH-2; lane 6, SCCH-1; lane 7, B. burgdorferi B-31 (reference strain).
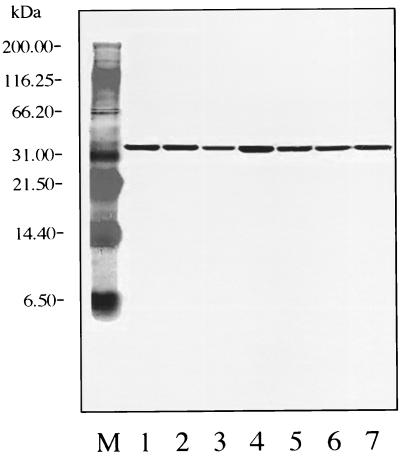
FIG. 4.
Western blot with MAb H9724 (against Fla). Lane M, molecular mass standards; lane 1, B. burgdorferi B-31 (reference strain); lane 2, SCCH-1; lane 3, SCCH-2; lane 4, SCCH-8; lane 5, SCCH-11; lane 6, SCCH-12; lane 7, SCCH-17.
PCR.
PCR was used to screen the Charleston (Mt. Pleasant) spirochetal isolates for five known DNA target sequences specifically found in B. burgdorferi reference strain B-31. Details of the five primer pairs and the parameters involved have been published (28), as has the protocol followed (27). Briefly, three pairs of primers (149 and 319, 149 and 459, and 3′ and 5′) amplified 170-, 310-, and 879-bp sequences, respectively, within the B. burgdorferi B-31 strain's outer surface protein A (ospA) gene (31). Another set of primers (245 and 855) amplified a 610-bp target sequence present in the flagellin (fla) gene of the B-31 strain (17). A fifth pair of primers (147 and 520) was specific for a 373-bp chromosomal sequence present in 17 of 18 verified strains of B. burgdorferi globally (33). The positive control for each PCR assay was pure genomic DNA (5 ng) of B. burgdorferi B-31, and the negative control was sterile distilled water. The PCR-amplified products were electrophoresed in 2% agarose gels, stained with ethidium bromide, and visualized by UV transilluminated light. The DNA bands were compared to known standards and documented for permanent record with photographs (Polaroid, Cambridge, Mass.) of UV-transilluminated gels.
SDS-PAGE.
Characterization by SDS-PAGE was begun by preparing whole-cell spirochetal lysates from each of the six BSK II culture isolates selected for analysis (see Fig. 5). The protocol followed was a standard one used in our laboratory, details of which have been published elsewhere (26–29). The isolates selected for SDS-PAGE analysis represented two samples (SCCH-1 and SCCH-2) from I. scapularis ticks, one (SCCH-8) from a cotton mouse (P. gossypinus), two (SCCH-11 and SCCH-12) from eastern wood rats (N. floridana), and one (SCCH-17) from a cotton rat (S. hispidus).
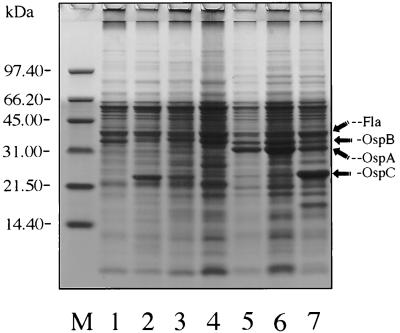
FIG. 5.
Coomassie blue-stained SDS-PAGE gel of whole spirochetal lysates. Lane M, molecular mass standards; lane 1, B. burgdorferi B-31 (reference strain); lanes 2 and 3, SCCH-1 and SCCH-2 isolates from I. scapularis, respectively; lane 4, SCCH-8 isolate from a cotton mouse; lanes 5 and 6, SCCH-11 and SCCH-12 isolates from wood rats, respectively; lane 7, SCCH-17 isolate from a cotton rat.
RESULTS
Tick and rodent collections and spirochete isolations.
Adults of I. scapularis actively seek hosts during the cooler parts of the year, from October through March, especially October through February in the South Carolina Coastal Zone, and may attach to animals or objects that come in contact with them (data not shown). After the ticks were identified and their tissues were inoculated into BSK medium, spirochetal isolates were obtained from four male and one female I. scapularis tick. The four isolates from the males included SCCH-1, SCCH-2, SCCH-3, and SCCH-34; SCCH-5 was obtained from a female I. scapularis tick. Two of 37 (5.4%) ticks from one area were culture positive and 3 of 209 (1.4%) from another area were culture positive.
The 25 isolates obtained from rodents included 10 from P. gossypinus (SCCH-6, SCCH-7, SCCH-8, SCCH-9, SCCH-10, SCCH-13, SCCH-14, SCCH-19, SCCH-30, and SCCH-31), 7 from N. floridana (SCCH-4, SCCH-11, SCCH-12, SCCH-15, SCCH-16, SCCH-32, and SCCH-33), and 8 from S. hispidus (SCCH-17, SCCH-23, SCCH-24, SCCH-25, SCCH-26, SCCH-27, SCCH-28, and SCCH-29). All of the isolates from rodents were from ear tissues; isolates from the urinary bladders of three of the rodents also yielded cultures (SCCH-4 from N. floridana and SCCH-6 and SCCH-19 from P. gossypinus). Two of the isolates could not be maintained in culture and thus were not analyzed.
Indirect immunofluorescence.
All of the Charleston area isolates tested reacted positively to the Borrelia (genus)-specific MAbs (H9724 and H605) and to a Borrelia polyclonal antibody but failed to react to the B. hermsii-specific antibodies (H9826 and H4825) (Table 1). All of the isolates also reacted positively to the two OspA MAbs (H5332 and H3TS) but negatively to an OspB MAb (H6831). Reactivity to another OspB antibody (H614) varied depending on the isolate tested. The only tick-derived isolate exposed to H614 reacted positively, six of the eight isolates from P. gossypinus reacted positively, and three of the six isolates from S. hispidus were positive. None of the five isolates from N. floridana reacted with antibody H614. However, a sixth isolate from N. floridana (SCCH-33) contained some spirochetes that reacted and others that did not.
Western blots.
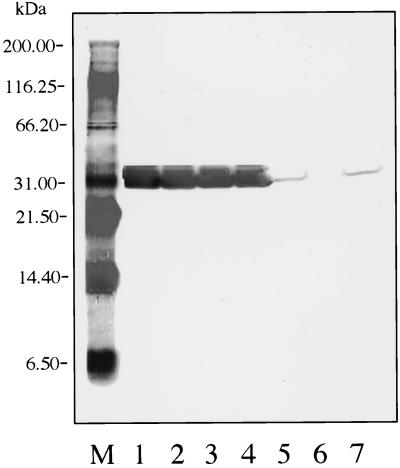
Western blot analysis of OspA for all Charleston isolates and the B-31 reference strain with MAb 5332 revealed uniform reactivities. However, isolates SCCH-11, -12, and -17 expressed OspA at 31.8 kDa, and isolates SCCH-1, -2, and -8 and B-31 expressed it at 31 kDa (Fig. 1). An analysis of OspA for all strains with B. burgdorferi sensu stricto species-specific MAb H3TS indicated that all isolates reacted with it; however, B-31 and SCCH-1, -2, and -8 expressed it at 31 kDa and had a much stronger reaction than the weak reactions of strains SCCH-11, -12, and -17, which expressed it at 31.8 kDa (Fig. 2).
FIG. 2.
Western blot with MAb H3TS (against OspA). Lane M, molecular mass standards; lane 1, B. burgdorferi B-31 (reference strain); lane 2, SCCH-1; lane 3, SCCH-2; lane 4, SCCH-8; lane 5, SCCH-11; lane 6, SCCH-12; lane 7, SCCH-17.
Western blots of OspB with MAb H6831 revealed that the B-31 reference strain had a very strong reaction. Strains SCCH-11, -12, and -17 also reacted, but less strongly than B-31; strains SCCH-1, -2, and -8 did not react (Fig. 3).
FIG. 3.
Western blot with MAb H6831 (against OspB). Lane M, molecular mass standards; lane 1, SCCH-17; lane 2, SCCH-12; lane 3, SCCH-11; lane 4, SCCH-8; lane 5, SCCH-2; lane 6, SCCH-1; lane 7, B. burgdorferi B-31 (reference strain).
Western blot analysis for Fla with MAb H9724 revealed that B-31 and the six Charleston isolates uniformly reacted to the antibody (Fig. 4).
PCR.
All 28 isolates analyzed by PCR were consistently positive when each of the five primer pairs were used (three ospA, one fla, and one chromosomal), regardless of the tick or animal species from which they were isolated. These isolates included 5 from I. scapularis, 10 from P. gossypinus, 7 from N. floridana, and 5 from S. hispidus.
SDS-PAGE.
Major proteins of the B. burgdorferi B-31 reference strain, including the 31-kDa OspA, the 34-kDa OspB, and the 41-kDa flagellin proteins, were clearly resolved in a Coomassie brilliant blue-stained 14% polyacrylamide gel. The 22 to 23-kDa OspC protein also was recognized in the six Charleston isolates (Fig. 5). Each of the six Charleston strains showed some heterogeneity in some of the banding patterns; however, compared to that of B-31, the 41-kDa flagellin protein was identical. The composition of the OspA (≈31-kDa) protein of the Charleston isolates was also similar to that of the OspA protein of the B-31 reference strain; however, SCCH-11, -12, and -17 expressed the protein more abundantly than did the other three isolates and B-31, and at 31.8 kDa; strains SCCH-1, -2, and -8 and B31-expressed the protein relatively less abundantly, and at 31 kDa. In strains SCCH-11, -12, and -17 there was a shift in OspB proteins to slightly lower molecular masses. All Charleston isolates contained recognizable low-molecular-mass protein OspC (≈23 to 24 kDa), which was especially abundant in SCCH-1 and SCCH-17 (Fig. 5).
DISCUSSION
Ninety clinical cases of LD, 34 serologically confirmed, were reported during a 1988 survey of 1,331 physicians concerning tick-borne diseases in South Carolina (34). A subsequent survey in 1990 of 2,224 physicians (42.3% response rate) reported 334 clinical cases in the state (35). A total of 89 cases were reported in South Carolina for 1998 (CDC, personal communication). LD was reported in North Carolina as early as 1982 (30), and from 1992 to 1997 an average of about 58 cases of LD per year was reported to the CDC from that state; 66 cases were reported during 1996 (CDC, personal communication) and 61 cases were reported during 1998 (9). Cases of LD also have been reported in Georgia and reached a high of 715 in 1989 (1), but they have been declining since then (CDC, personal communication). In spite of these reports of LD in South Carolina and adjacent states, some scientists are not convinced that true LD occurs in the southern United States. They suggest that the cases reported are due to a Lyme-like disease (4, 8, 19). There is a report of a noncultivable Borrelia species in the lone star tick, Amblyomma americanum (6). Although this species of tick has not been demonstrated to transmit B. burgdorferi, it has been epidemiologically associated with LD or Lyme-like disease in Missouri (22, 23) and North Carolina (19). A recent study of 21 patients from Georgia and South Carolina exhibiting classic erythema migrans lesions provides evidence that some of the patients were infected with B. burgdorferi while others were not, and there is suspicion that several of the patients were bitten by A. americanum (14). Perhaps some patients in the South with erythema migrans rashes are infected with B. burgdorferi while others are infected with a closely related but different Borrelia species. B. burgdorferi sensu stricto, prevalent in the regions in the North where LD is hyperendemic, occur in the South; however, strains of B. burgdorferi sensu lato also occur in the South (24). Some of the latter strains are genetically similar to the DN127 group of strains from California, which have recently been described as a new species, Borrelia bissettii (32). Although strains in the DN127/25015 group from North America have not been shown to cause LD, samples from nine patients in Slovenia with disseminated LD yielded cultural isolates in the DN127/25015 group (36). Strain 25015, from New York, was thought to be infectious but nonpathogenic in a mouse model (2); however, a later study with a different murine system reported it to be mildly arthritogenic (15). The clinical presentations of the patients from Slovenia varied considerably; four patients appeared to have a relatively benign course of illness, but three other patients were severely affected and two others were not as severely affected. In addition, some patients had variable and unpredictable serologic responses, including an apparent lack of antibody response despite disseminated disease. Some LD or Lyme-like disease patients from the southern United States also lack a serologic response to antigens derived from B. burgdorferi sensu stricto (14).
Preliminary data (T. Lin, J. H. Oliver, Jr., T. M. Kollars, Jr., and K. L. Clark, VIII Internatl. Conf. Lyme Borreliosis Tick-Borne Dis., p. 6, 1999) indicate that considerable genetic variation exists among spirochetal isolates from several species of ticks, rodents, and birds from South Carolina. Nevertheless, at the present stage of characterization of the Charleston isolates reported in this paper, the isolates appear to be very similar to the B-31 reference strain of B. burgdorferi sensu stricto and therefore may be capable of causing LD similar to that found in the northern United States. Interestingly, analysis of the six Charleston isolates by SDS-PAGE and Western blotting suggests that although they all are probably B. burgdorferi sensu stricto, they can be divided into two groups. One group (SCCH-1, -2, and -8) expressed OspA at 31 kDa, reacted strongly with MAb H3TS, and did not react with MAb H6831, whereas the other three strains (SCCH-11, -12, and -17) expressed OspA at 31.8 kDa, reacted weakly with MAb H3TS, and reacted with MAb H6831. The less discriminatory immunofluorescence screening analyses (Table 1) indicated that none of the isolates reacted to H6831.
Based on serologic evidence that 38% of the P. gossypinus mice from South Carolina that were tested had antibodies to B. burgdorferi (21), the cultivation of 146 isolates of B. burgdorferi sensu lato from birds, rodents, and ticks from seven geographic sites within five counties in South Carolina (including Charleston County) (12, 25); J. H. Oliver, Jr., unpublished data), the widespread distribution of I. scapularis in South Carolina (10, 11, 18, 26) and its proclivity to feed on various vertebrates (18, 20) (including humans [13]), the reports of physician-diagnosed LD in the state (34, 35), and the characterization of 28 isolates as B. burgdorferi sensu stricto in this study, we conclude that B. burgdorferi is cycling enzootically in the state and speculate that humans are probably being infected with the spirochete.
ACKNOWLEDGMENTS
We thank Peggy Kollars for help with antibody screening. We are grateful to Barbara Johnson, CDC, Ft. Collins, Colo.; David Persing, Mayo Clinic, Rochester, Minn.; and Patricia Rosa, Rocky Mountain Laboratories, Hamilton, Mont., for supplying primers for genetic analyses and pure genomic DNA of B. burgdorferi B-31. We are grateful to Thomas G. Schwan, Rocky Mountain Laboratories, for providing a series of MAbs.
The research was supported in part by National Institutes of Health grant R 37A1-24899 to Georgia Southern University and CDC cooperative agreement U50/CCU410281 to Georgia Southern University.
REFERENCES
- 1.Alley J W, Sikes R K, Rochat R, McKinsley T W, Smith J D, Roberts J J. Tickborne diseases…Georgia, 1989. Ga Epidemiol Rep. 1990;6:2–4. [Google Scholar]
- 2.Anderson J F, Barthold S W, Magnarelli L A. Infectious but nonpathogenic isolate of Borrelia burgdorferi. J Clin Microbiol. 1990;28:2693–2699. doi: 10.1128/jcm.28.12.2693-2699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G. Does Lyme disease occur in the South?: a survey of emerging tick-borne infections in the region. Am J Med Sci. 1996;311:34–40. doi: 10.1097/00000441-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A G. Immunochemical analysis of Lyme disease spirochetes. Yale J Biol Med. 1984;57:581–586. [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour A G, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 6.Barbour A G, Maupin G O, Teltow G J, Carter C J, Piesman J. Identification of an uncultivable Borrelia sp. in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 7.Bryan C S. Lyme disease: how common in South Carolina? J S C Med Assoc. 1991;87:438–439. [PubMed] [Google Scholar]
- 8.Campbell G L, Paul W S, Schriefer M E, Craven R B, Robbins K E, Dennis D T. Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990–1993. J Infect Dis. 1995;172:470–480. doi: 10.1093/infdis/172.2.470. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Notifiable diseases/deaths in selected cities: weekly information. Morbid Mortal Weekly Rep. 1999;47:1119–1124. [Google Scholar]
- 10.Clark K L, Oliver J H, Jr, McKechnie D B, Williams D C. Distribution, abundance, and seasonal activities of ticks collected from rodents and vegetation in South Carolina. J Vector Ecol. 1998;23:89–105. [PubMed] [Google Scholar]
- 11.Dennis D T, Nekomoto T S, Victor J C, Paul W S, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- 12.Durden L A, McLean R G, Oliver J H, Jr, Ubico S R, James A M. Ticks, Lyme disease spirochetes, trypanosomes and antibody to encephalitis viruses in wild birds from coastal Georgia and South Carolina. J Parasitol. 1997;83:1178–1182. [PubMed] [Google Scholar]
- 13.Felz M W, Durden L A, Oliver J H., Jr Ticks parasitizing humans in Georgia and South Carolina. J Parasitol. 1996;82:505–508. [PubMed] [Google Scholar]
- 14.Felz, M. W., F. W. Chandler, Jr., J. H. Oliver, Jr., D. W. Rahn, and M. E. Schriefer. Erythema migrans in Georgia and South Carolina. Arch. Dermatol., in press. [DOI] [PubMed]
- 15.Fikrig E, Barthold S W, Persing D H, Sun X, Kantor F S, Flavell R A. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992;148:2256–2260. [PubMed] [Google Scholar]
- 16.Herrington J E., Jr An update on Lyme disease. Health Environ Digest. 1995;9:29–32. [Google Scholar]
- 17.Johnson B J B, Happ C M, Mayer L W, Piesman J. Detection of Borrelia burgdorferi in ticks by species-specific amplification of the flagellin gene. Am J Trop Med Hyg. 1992;47:730–741. doi: 10.4269/ajtmh.1992.47.730. [DOI] [PubMed] [Google Scholar]
- 18.Keirans J E, Hutcheson H J, Durden L A, Klompen J S H. Ixodes (Ixodes) scapularis (Acari: Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. 1996;33:297–318. doi: 10.1093/jmedent/33.3.297. [DOI] [PubMed] [Google Scholar]
- 19.Kirkland K B, Klimko T B, Meriwether R A, Schriefer M, Levin M, Levine J, Mackenzie W R, Dennis D T. Erythema migrans-like rash illness at a camp in North Carolina: a new tick-borne disease? Arch Intern Med. 1997;157:2635–2641. [PubMed] [Google Scholar]
- 20.Lane R S, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 21.Magnarelli L A, Oliver J H, Jr, Hutcheson H J, Boone J L, Anderson J F. Antibodies to Borrelia burgdorferi in rodents in the eastern and southern United States. J Clin Microbiol. 1992;30:1449–1452. doi: 10.1128/jcm.30.6.1449-1452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masters E J, Donnell H D. Lyme and/or Lyme-like disease in Missouri. Mo Med. 1995;92:346–353. [PubMed] [Google Scholar]
- 23.Masters E J, Donnell H D, Fobbs M. Missouri Lyme disease: 1989 through 1992. J Spirochet Tickborne Dis. 1994;1:12–17. [Google Scholar]
- 24.Mathiesen D A, Oliver J H, Jr, Kolbert C P, Tullson E D, Johnson B J B, Campbell G L, Mitchell P D, Reed K D, Telford III S R, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 25.Oliver J H., Jr Lyme borreliosis in the southern United States: a review. J Parasitol. 1996;82:926–935. [PubMed] [Google Scholar]
- 26.Oliver J H, Jr, Chandler F W, Jr, Luttrell M P, James A M, Stallknecht D E, McGuire B S, Hutcheson H J, Cummins G A, Lane R S. Isolation and transmission of the Lyme disease spirochete from the southeastern United States. Proc Natl Acad Sci USA. 1993;90:7371–7375. doi: 10.1073/pnas.90.15.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliver J H, Jr, Kollars T M, Jr, Chandler F W, Jr, James A M, Masters E J, Lane R S, Huey L O. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1–5. doi: 10.1128/jcm.36.1.1-5.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliver J H, Jr, Chandler F W, Jr, James A M, Huey L O, Vogel G N, Sanders F H., Jr Unusual strain of Borrelia burgdorferi isolated from Ixodes dentatus in central Georgia. J Parasitol. 1996;82:936–940. [PubMed] [Google Scholar]
- 29.Oliver J H, Jr, Chandler F W, Jr, James A M, Sanders F H, Jr, Hutcheson H J, Huey L O, McGuire B S, Lane R S. Natural occurrence and characterization of the Lyme disease spirochete, Borrelia burgdorferi in cotton rats (Sigmodon hispidus) from Georgia and Florida. J Parasitol. 1995;81:30–36. [PubMed] [Google Scholar]
- 30.Pegram P A, Jr, Sessler C N, London W L. Lyme disease in North Carolina. South Med J. 1983;76:740–742. doi: 10.1097/00007611-198306000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Persing D H, Telford III S R, Spielman A, Barthold S W. Detection of Borrelia burgdorferi infection in Ixodes dammini ticks with the polymerase chain reaction. J Clin Microbiol. 1990;28:566–572. doi: 10.1128/jcm.28.3.566-572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postic D, Marti Ras N, Lane R S, Hendson M, Baranton G. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127) J Clin Microbiol. 1998;36:3497–3504. doi: 10.1128/jcm.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosa P A, Schwan T G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989;160:1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- 34.Schuman S H, Caldwell S T. Lyme and other tick-borne diseases acquired in South Carolina in 1988: a survey of 1,331 physicians. J S C Med Assoc. 1989;85:311–314. [PubMed] [Google Scholar]
- 35.Schuman S H, Caldwell S T. 1990 South Carolina physician survey of tick, spider and fire ant morbidity. J S C Med Assoc. 1991;87:429–432. [PubMed] [Google Scholar]
- 36.Strle F, Picken R N, Cheng Y, Cimperman J, Maraspin V, Lotrie-Furian S, Ruzic-Sabljic E, Picken M M. Clinical findings for patients with Lyme borreliosis caused by Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities to strain 25015. Clin Infect Dis. 1997;25:273–280. doi: 10.1086/514551. [DOI] [PubMed] [Google Scholar]