Abstract
Cell membrane fusion and multinucleation in macrophages are associated with physiologic homeostasis as well as disease. Osteoclasts are multinucleated macrophages that resorb bone through increased metabolic activity resulting from cell fusion. Fusion of macrophages also generates multinucleated giant cells (MGCs) in white adipose tissue (WAT) of obese individuals. For years, our knowledge of MGCs in WAT has been limited to their description as part of crown-like structures (CLS) surrounding damaged adipocytes. However, recent evidence indicates that these cells can phagocytose oversized lipid remnants, suggesting that, as in osteoclasts, cell fusion and multinucleation are required for specialized catabolic functions. We thus reason that WAT MGCs can be viewed as functionally analogous to osteoclasts and refer to them in this article as adipoclasts. We first review current knowledge on adipoclasts and their described functions. In view of recent advances in single cell genomics, we describe WAT macrophages from a ‘fusion perspective’ and speculate on the ontogeny of adipoclasts. Specifically, we highlight the role of CD9 and TREM2, two plasma membrane markers of lipid-associated macrophages in WAT, which have been previously described as regulators of fusion and multinucleation in osteoclasts and MGCs. Finally, we consider whether strategies aiming to target WAT macrophages can be more selectively directed against adipoclasts.
Macrophages have a unique potential to fuse with themselves to form multinucleated giant cells (MGCs) [1]. During homeostasis, the majority of macrophages fuse infrequently and reside in tissues as mononuclear cells. The exception to this rule is the osteoclast of bone, a multinucleated monocyte/macrophage [2] that originated from embryonic erythro-myeloid progenitors and is responsible for the resorption of mineralized bone [3]. The multinucleation capability of the osteoclast correlates with its resorptive activity, suggesting that cell fusion confers a specialized stage of differentiation lacking in the mononuclear state [1]. The concept of a cellular gain of function as a result of fusion/multinucleation is supported by a recent discovery showing that multinucleated osteoclasts can undergo fission to form osteomorphs, daughter cells transcriptionally distinct from osteoclasts [4]. While osteoclasts regulate bone mass, pathological macrophage fusion can be an immune response to infectious pathogens (e.g. Mycobacterium tuberculosis) or foreign materials. MGCs are derived from monocyte progenitors [4] but their precise role within the granuloma is not yet clear. On the other hand, foreign-body giant cells (FBGCs) can be involved in the uptake of larger particles [5], an observation confirmed in vitro [6]. These observations suggest that enhanced phagocytic clearance of large particulates is an adaptive phenomenon resulting from macrophage fusion and multinucleation.
The adipose tissue contains macrophages and during obesity, their number increases significantly (up to 50% of all cells) to correlate with metabolic dysfunction characterized by inflammation, fibrosis and insulin resistance [7–11]. Their histological description as crown-like structures (CLS) refers to MGCs associated with necrotic adipocytes [12]; however, recent evidence demonstrated that these MGCs can phagocytose lipid remnants more efficiently when compared to unfused WAT macrophages [13].
There is an intriguing association between lipids and macrophage fusion. Cholesterol-rich MGCs have been reported as a frequent and non-specific histological feature in lung biopsies [14]. Historically, the Touton giant cell, which is frequently found in lesions containing high levels of cholesterol and lipid deposits, has been described as a product of fusion between macrophage-derived foam cells [15]. Multinucleated foam cells have been indeed observed as a result of high-fat diet in inflammatory sites such as the synovium [16]. Recent evidence shows that common monocyte progenitors accumulate cholesterol and lipids, which are required for MGC formation [17]. These studies suggest that a lipid rich microenvironment such as the white adipose tissue (WAT) can be ‘fusogenic’ for resident macrophages. Based on recent findings published by Braune and colleagues [13], and the existing literature on osteoclasts and MGCs, we postulate that macrophage fusion and multinucleation in the WAT may initiate a ‘gain of function’ to clear increasingly stressed adipocytes under metabolically challenging conditions such as obesity. Thus, in this review, we refer to MGCs of crown-like structures (CLS) as adipoclasts, the ‘fat-resorbing osteoclasts’ of the white adipose tissue (Fig. 1A, B). The term adipoclast does not differentiate between the MGCs with different nuclei numbers (binuclear, 2–4, > 4) and differs from the designation lipid-associated macrophage (LAM) by its unique multinucleated feature. The choice of this term is based on the (i) wide description of the CLS histologically in the white adipose tissue, (ii) their recent functional annotation as catabolic cells following fusion/multinucleation [13], and (iii) the functional analogy with osteoclasts—hence the suffix ‘clast’.
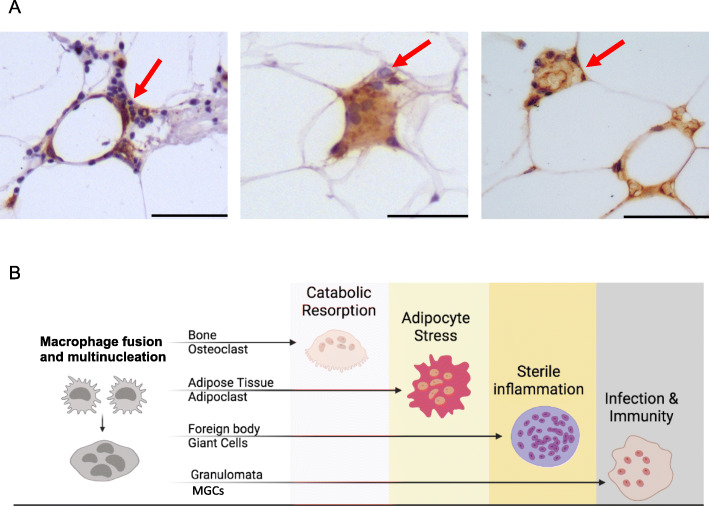
Fig. 1.
A Representative CD68 immunohistochemistry (brown) showing multinucleated adipoclasts (red arrows) in the white adipose tissue of obese patients undergoing bariatric surgery; scale bar, 50μm. B Macrophage fusion and multinucleation in health and disease. In addition to osteoclasts, foreign body giant cells and MGCs, adipoclasts contribute to the clearance of stressed adipocytes in the white adipose tissue
We first describe the current knowledge on CLS and their proposed function. We then review recent advances in WAT single cell transcriptomics, with a specific focus on TREM2 and CD9, membrane receptors that have been previously described in macrophage fusion and multinucleation. We highlight the respective roles of TREM2 and CD9 in osteoclasts, in order to speculate on the adipoclasts’ origin and function. Finally, we discuss whether recent macrophage-targeting therapies in the fat may be beneficial or fine-tuned in targeting adipoclasts in obesity. The review does not cover the polarization of macrophages in adipose tissue nor the significance of WAT inflammation in insulin resistance and metabolic disorders in general—an area that is amply covered by excellent reviews (some examples include [10, 18–23])
Crown-like structures are adipoclasts
The infiltration of immune cells in the obese adipose tissue was shown in the 1960s [24, 25] and then overlooked for almost four decades, except for an in vitro study showing that insulin resistance in adipocytes can be caused by a macrophage-derived mediator [26]. The presence of macrophages in human and mice adipose tissue was shown by several groups and while some reported their tissue localization adjacent to adipocytes, others highlighted their morphological appearance as MGCs arising from cell fusion [11, 12, 27, 28]. Clement et al. isolated CD14+ cells from the stromal vascular fraction (SVF) of human subcutaneous WAT, using CD14-coupled magnetic microbeads and confirmed the presence of macrophages in adipose tissue by immunohistochemistry [27]. Two contemporaneous studies reported the existence of macrophage syncytia (or MGCs) in the WAT of genetically obese mice (ob/ob) [11, 28]. From a histological point of view, Cinti et al. were the first to designate the WAT multinucleated macrophages as crown-like structures (CLS) [12], surrounding necrotic or lipolytic adipocytes [12, 28]. Today it is well-established that adipose tissue CLS contain multinucleated macrophages (i.e. adipoclasts; Fig. 1A) and increase in frequency with obesity. The origin of this augmented macrophage infiltration in the WAT is thought to be blood monocytes [29] and the literature on CLS has long assumed that these cells are implicated in efferocytosis of dead adipocytes because of their histological localization around dead adipocytes. A recent study brought definitive evidence by live imaging the WAT MGCs (i.e. adipoclasts) in mice, showing that these cells can take up lipid remnants which were not ingestible by mononuclear macrophages in the WAT [13]. A bead phagocytosis assay confirmed these findings and showed that, like MGCs [6], adipoclasts can phagocytose large particles [13]. Interestingly, confirming the previous associations between MGCs and lipids, adipoclasts display a relatively high lipid content [13] and this is not surprising given the fusogenic properties of the long-chain fatty acid binding scavenger receptor CD36 in macrophages [30].
In summary, while it is well-accepted that adipoclasts are specialized in efferocytosis of damaged adipocytes, many questions remain regarding the mechanisms underlying this process, as well as the other advantages that cell fusion and multinucleation may confer in the context of prolonged obesity. Furthermore, given the presence of mononucleated, often foamy macrophages in WAT, it is necessary to consider more trophic functions and crosstalk between macrophages and adipocytes [31] including the role of CD36 and other macrophage scavenger receptors [32], as well as clearance functions.
The complexity behind adipoclast function
During prolonged obesity, adipose tissue remodelling is a well-described phenomenon that consists in depot-dependent adipocyte death associated with macrophage infiltration [33, 34]. Our limited understanding of adipoclast function is due to the complex aspects of the evolution of adipocyte cell state under metabolically impaired conditions (see review [35]). During obesity, adipocytes can undergo various forms of death [36]—apoptotic [37], necrotic [12], and pyroptotic [38]. In addition, pre-adipocytes (i.e. the precursor of adipocytes) have been described to undergo senescence through different mechanisms during obesity [39, 40]. On the other hand, the macrophage clearance mechanisms of damaged adipocytes were reported to be through lysosomal exocytosis [41], in addition to phagocytosis [13]. By live-imaging, a recent report showed the requirement of a size threshold for efferocytosis of lipid remnants [42]. Adipocyte death induces a metabolically activated and pro-inflammatory macrophage phenotype [42]. Paradoxically, the clearance of dead adipocytes by CLS was also linked to preadipocyte proliferation [43], suggesting an adipogenic role for adipoclasts. Adding to this complexity, different fat depots (visceral vs. subcutaneous) can display different prevalence in adipocyte cell death. It was reported that CLS were widespread in visceral compared with subcutaneous fat in genetically obese mice (db/db and ob/ob) [44]. In keeping with this, adipoclast infiltrates may differ between murine and human WAT. In mice, a prolonged high-fat diet of 24 weeks is required to observe the adipoclasts histologically [13], suggesting that prolonged obesity is a prerequisite for multinucleation of these cells.
Hence adipoclasts have been linked to adipocytes in different cellular states that describe broadly cellular stress and ultimately death. This raises the question of whether adipoclasts can ‘sense’ a particular adipocyte state and whether their fusion from mononuclear macrophages is triggered through adipocyte-derived markers of stress. For instance, using a co-culture setup, it was shown that adipocyte death triggers MGC formation in vitro [13]. Further experiments will be crucial in order to establish the exact mechanisms underlying this process.
Adipoclasts and/or their precursors display multinucleation markers
While it is accepted that obesity is associated with a shift toward pro-inflammatory macrophage function [45–47], WAT macrophages have a unique polarization state (metabolically activated macrophages [48]) and paradoxically, crown-like structures contain the M2-like marker CD206 (mannose receptor) and CD11c expressing macrophages [49]. Recent single cell transcriptomics studies revealed the different subtypes of adipose tissue macrophages and their evolution upon obesogenic conditions [50–54]. Two markers of white adipose tissue macrophages of particular interest include TREM2 and CD9. Jaitin et al. were first to describe a TREM2-expressing lipid-associated macrophage (LAM) subset in human WAT [53], later confirmed by a separate study [51]. Similarly, CD9, another marker of LAMs [53], was found to colocalize with the pan-macrophage marker CD68 in human WAT [54]. Notably, TREM2+ and CD9+ LAMs were found to be part of CLS [52, 53] and their frequency increased with obesity in mice and humans [50, 51] with a shift toward a pro-inflammatory polarization characterized by IL-1β and TNF production [51].
None of the single cell RNA-seq studies in the WAT distinguished multinucleated macrophages (i.e. adipoclasts) from other macrophage subsets. Although technically challenging, this could have been attempted by sorting LAMs with > 2 nuclei. The advantage of such an approach would have been the identification of potential precursors of adipoclasts, in order to make a distinction between ‘fusion-competent’ LAMs and adipoclasts, as well as the polarization state of each cell type. Nevertheless, the recent single cell transcriptomic approaches in human WAT suggest that adipoclasts and/or adipoclast precursors express TREM2 and CD9 [51–54].
TREM2 and CD9: a parallel between adipoclasts and osteoclasts
The existence of CD9+ and TREM2+ adipoclasts is worth highlighting from a macrophage fusion perspective, especially given the relevance of these two membrane proteins in osteoclast and MGC fusion and multinucleation.
Besides its widely studied role in microglial phagocytosis [55] and neurodegeneration [56, 57], TREM2 (the triggering receptor expressed on myeloid cells 2) is essential for macrophage multinucleation as part of a signalling pathway that includes DAP12 and Syk [58]. TREM2 regulates osteoclast formation [59–61] and a recent report shows its regulatory role in granuloma formation through recruitment of mycobacterium-permissive macrophages [62]. Furthermore, deletions or loss-of-function mutations in either DAP12 or TREM2 are causally associated with Nasu–Hakola disease, a dementia associated with bone cystic lesions [63, 64]. Importantly, mutations in TREM2 and DAP12 induce defective multinucleation in osteoclasts, resulting in impaired bone resorption [60]. Trem2 is a trans-acting genetic regulator of a macrophage multinucleation gene co-expression network [65, 66], which also includes genes belonging to the Pi3K-mTORC1 pathway that controls osteoclast multinucleation and bone mass [66]. The TREM2-PI3K-mTOR axis is indeed well-defined in microglia [67] and the activation of PI3K signalling is a common feature of osteoclasts and MGCs [58, 68].
Jaitin et al. identified TREM2, not only as a marker, but also as a driver of the LAM cell molecular program as lipid uptake and storage were abrogated in the absence of Trem2 [53]. Interestingly, apolipoprotein E (ApoE) is a Trem2 ligand [69, 70] and both Trem2 and ApoE are expressed by a subpopulation of tumour-associated macrophages [71]. Macrophages can fuse with tumour cells and contribute to tumour heterogeneity [72], but a potential role of Trem2 in this process is yet to be found. The lipid sensing role of TREM2 has been shown as part of the microglia response [73] but also during infection, as TREM2 is capable of recognizing mycobacterial cell-wall mycolic acid (MA)-containing lipids [62]. This raises the possibility of a lipid uptake through TREM2 that can be a prerequisite mechanism for macrophage fusion and multinucleation. Local lipid changes are principal regulators of adipose tissue macrophage recruitment [74]. Interestingly, single cell RNA-sequencing analysis of aortic CD45+ cells from atherosclerotic high-fat diet-fed (Ldlr-/-) mice identified macrophages with high Trem2 expression, specialized in lipid metabolism/catabolism and enriched in the osteoclast gene signature [75]. If one extrapolates these findings to the WAT, it is plausible that Trem2 expressing macrophages accumulate lipids and become fusogenic, giving rise to adipoclast precursors and adipoclasts. Fusion and multinucleation could be considered as the final differentiation step of these precursors. However, the exact Trem2-dependent and lipid-related mechanisms allowing the transition from fusion-competent adipoclast precursors to adipoclasts remain to be identified, and in that sense, some parallels drawn from knowledge on osteoclast lipid metabolism may be of relevance. Cholesterol is indispensable for membrane fusion and osteoclast v-ATPase activity [76, 77] and Ldlr-/- mice have defective osteoclast fusion [78]. Since osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins [79], adipoclast integrity and function may also be under the influence of a cholesterol-rich environment in the adipose tissue. Similarly, saturated fatty acids enhance osteoclast survival [80] and palmitic acid increases RANKL-mediated osteoclast differentiation [81]. On the other hand, short-chain fatty acids such as propionate and butyrate induce metabolic reprogramming of osteoclasts and downregulate essential osteoclast genes [82]. This suggests that individual lipid species may have opposing roles on osteoclast differentiation and fusion and therefore the lipid dynamics in the WAT during obesity may determine the formation of adipoclasts. In this regard, it has been shown that ablation of fat cells in adult mice can induce massive bone gain [83]. As the diet and microbiome significantly contribute to the reserve and processing of fatty acids, the lipid composition of WAT under obesogenic conditions [84] can be a pivotal factor in determining adipoclast formation and function.
Tetraspanins are a superfamily of membrane proteins, and among them, CD9 and CD81 are closely related and known to control cell–cell fusion as they negatively regulate fusion of osteoclasts and MGCs [85, 86]. These proteins facilitate the organization of integrins and influence macrophage motility [87]. CD9/CD81 double-null mice spontaneously develop MGCs in the lung, showing enhanced osteoclastogenesis in the bone and signs of accelerated ageing with atrophy of adipose tissue [86, 88]. Interestingly, while CD9 has been robustly linked to WAT macrophages [51, 53, 54], CD81 has been recently described as a beige adipocyte progenitor cell marker and regulator of de novo beige fat biogenesis following cold exposure [89]. The potential involvement of CD81 in adipoclast differentiation and function remains to be identified. Given that CLS have been described to be an adipogenic niche for adipocyte progenitor cells [43], CD81 may be involved in a possible adipocyte progenitor-adipoclast/adipoclast-precursor interaction. Notably, tetraspanins are the only inhibitors of fusion that have been so far identified. Because their downregulation induces membrane fusion [1, 90], CD9 and CD81 may be expressed in adipoclast precursors and undergo down-regulation when fusion occurs. Hence, the transcriptomic characterization of CD9+ mononucleated and multinucleated cells in the WAT can confirm the precise role of tetraspanins in adipoclast formation.
In summary, the presence of TREM2+CD9+ adipoclasts or adipoclasts precursors seems to correlate with WAT inflammation and the severity of obesity-related pathologies (Fig. 2). In support of the pathogenic role of adipoclasts, a scar-associated and pro-fibrotic TREM2+CD9+ subpopulation of macrophages was identified in cirrhotic human liver [91]. These scar-associated macrophages were conserved in mice and express osteopontin (SPP1) [91], a protein that regulates FBGC formation [92] and osteoclast fusion and resorption [93]. Whether the scar-associated macrophages can fuse with each other remains to be confirmed. In non-alcoholic steatohepatitis (NASH), a specific macrophage population is characterized by high levels of expression of Trem2 [94] and other lipid-associated macrophage markers, forming hepatic CLS [95]. A NASH diet causes a partial loss of Kupffer cell identity, induction of Trem2 and Cd9 expression, and cell death in mice [96]. Interestingly, the expression of Trem2 and Cd9 is a result of substantial reprogramming of the Kupffer cell regulatory landscape due to the prolonged exposure to the NASH diet [96]. Hence, an interesting parallel can be made with TREM2+CD9+ adipoclasts, which may form as a result of chronic obesogenic conditions, whereby membrane fusion and multinucleation are likely to induce changes in the transcriptomic/epigenetic landscape, allowing phagocytosis of damaged adipocytes. In addition to metabolic tissues, TREM2+CD9+ microglia in the brain may play a pathogenic role. It is intriguing that lipid-droplet-accumulating microglia (a subgroup presumably distinct from the disease-associated microglia expressing TREM2 and CD9 [97]), represent a dysfunctional and proinflammatory state in the ageing brain [98].
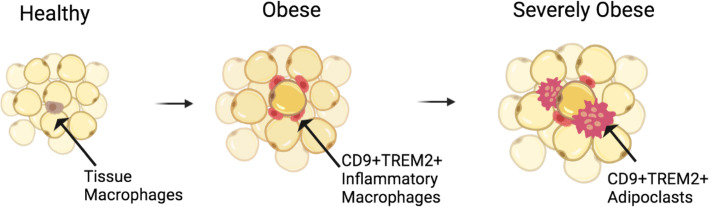
Fig. 2.
The transition from obese to severely obese state is characterized by increased macrophage infiltration and the formation of TREM2 and CD9 expressing pro-inflammatory macrophages that eventually give rise to multinucleated adipoclasts surrounding stressed adipocytes. How fusion/multinucleation affects the expression TREM2/CD9 and whether this causes de novo expression of adipoclasts markers is yet to be determined
Targeting macrophages and/or adipoclasts in obesity?
To date, it is well-accepted that obesity triggers the recruitment of monocytes into adipose tissue to promote inflammation, which itself may cause ectopic fat deposition in the liver and insulin resistance [99, 100]. The discovery of adipose tissue TNF [101, 102] and a decade later the monocyte-chemoattractant protein 1 (MCP-1) [103, 104], proved the importance of WAT inflammation and its indisputable macrophage component in the metabolic syndrome. Logically, this has seen the emergence of macrophage-targeting therapies that were initially aiming to inhibit the recruitment of these cells [105–107]. With the increasing recognition of macrophage metabolism in the regulation of its immune function [108], novel initiatives target mitochondrial function in macrophages [109, 110], given the relevance of mitochondrial oxidative phosphorylation in diet-induced obesity [111]. Drug delivery approaches, including nanomaterial-based ones targeting macrophages, hold promise [112]. Furthermore, in addition to their professional phagocytic activity and plasticity [113], tissue macrophages have unique features that differentiate them from surrounding cells. For instance, their enhanced sensitivity to changes in intracellular potassium levels and inflammasome activation [114], makes them attractive targets for Na+/K+-ATPase blockers such as ouabain [115]. A recent study exemplifies the strategic relevance of macrophage-targeted pharmacological interventions in obesity: macrophage-derived PDGFcc production is regulated by diet and increases lipid storage by white adipocytes [116].
When considering macrophage-targeted treatments in adipose tissue, it is crucial to keep in mind the heterogeneity and master regulatory role of macrophages in the development and homeostatic function of adipose tissue. It has become evident that macrophages express organ-specific genes in addition to canonical macrophage genes, a phenomenon referred to as niche-specific programming [96, 117]. The recently identified sympathetic neuron-associated macrophages increase with obesity and can be targeted for the browning of white fat [118]. This shows the heterogeneity of adipose tissue macrophages, which should be taken into account in any pharmacological approach aiming to reduce obesity-related complications. During homeostasis, many aspects of the mature function of macrophages are controlled by CSF1 and IL-34, which both bind CSF1R, a receptor restricted to cells of the myeloid lineage. Furthermore, Trib1, an adaptor protein involved in protein degradation, is critical for the differentiation of tissue-resident macrophages [119], while receptors known to be preferentially expressed by mononuclear phagocytes such as TREM2 [55, 120] and MARCO [121, 122], regulate an array of tissue-resident macrophage function including efferocytosis (TREM2) and scavenging (MARCO). The genetic deletion of Csf1r in rats and Trib1 in mice reduces adipose tissue mass [119, 123], while Trem2-/- and Marco-/- LAMs lose their efficacy in lipid buffering [53, 124]. Of note, CSF1R on microglial cells can control hypothalamic control of energy homeostasis in mice [125, 126] which suggests that CSF1R may be responsible for local and systemic control of adiposity. When considering macrophage-targeted therapies, a possible non-myeloid expression of some markers (e.g. Trem2) should be taken into consideration as it may influence metabolic health [127]. Altogether, these studies suggest that healthy macrophage differentiation and function is an unconditional part of adipose tissue homeostasis and therapeutic approaches must differentiate between optimal macrophage presence and pathological infiltration and accumulation of these cells.
Based on current knowledge, adipoclasts are likely to form when relatively high numbers of macrophages infiltrate the adipose tissue due to prolonged obesity. It is still not clear whether adipoclasts are only homokaryons or whether they can also form by fusion of mononucleated macrophages and adipocytes. Here we argue that inhibiting adipoclast formation may improve insulin sensitivity. Rather than global approaches aiming to target adipose tissue macrophages, one can envisage inhibition of adipoclast formation. However, such therapies require a better understanding of adipoclast formation and the identification of novel markers that differentiate mononucleated precursors from multinucleated fused cells. Integrating transcriptomic, epigenetic and metabolic events that accompany cell fusion and multinucleation in the WAT will fine-tune cell-based therapies in obesity and metabolic syndrome.
Acknowledgements
Not applicable.
Authors’ contributions
J.B. and S.G. conceptualized the review article. J.B. wrote the manuscript with contributions from A.O., S.M., C.H., F.O.M. and S.G. The figures were designed by J.B., T.O. and F.O.M. All authors have read and agreed on the content.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pereira M, Petretto E, Gordon S, Bassett JHD, Williams GR, Behmoaras J. Common signalling pathways in macrophage and osteoclast multinucleation. J Cell Sci. 2018;131(11) 10.1242/jcs.216267. [DOI] [PubMed]
- 2.Feng X, Teitelbaum SL. Osteoclasts: new insights. Bone Res. 2013;1:11–26. doi: 10.4248/BR201301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, Eitler J, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568:541–545. doi: 10.1038/s41586-019-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021;184(5):1330–1347. doi: 10.1016/j.cell.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milde R, Ritter J, Tennent GA, Loesch A, Martinez FO, Gordon S, Pepys MB, Verschoor A, Helming L. Multinucleated giant cells are specialized for complement-mediated phagocytosis and large target destruction. Cell Rep. 2015;13(9):1937–1948. doi: 10.1016/j.celrep.2015.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrante AW., Jr The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15(Suppl 3):34–38. doi: 10.1111/dom.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23:11–24. doi: 10.1210/me.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olona A, Terra X, Ko JH, Grau-Bove C, Pinent M, Ardevol A, et al. Epoxygenase inactivation exacerbates diet and aging-associated metabolic dysfunction resulting from impaired adipogenesis. Mol Metab. 2018;11:18–32. doi: 10.1016/j.molmet.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Braune J, Lindhorst A, Froba J, Hobusch C, Kovacs P, Bluher M, et al. Multinucleated giant cells in adipose tissue are specialized in adipocyte degradation. Diabetes. 2021;70:538–548. doi: 10.2337/db20-0293. [DOI] [PubMed] [Google Scholar]
- 14.Rossi G, Cavazza A, Spagnolo P, Bellafiore S, Kuhn E, Carassai P, et al. The role of macrophages in interstitial lung diseases: number 3 in the Series “Pathology for the clinician” Edited by Peter Dorfmuller and Alberto Cavazza. Eur Respir Rev. 2017;26 10.1183/16000617.0009-2017. [DOI] [PMC free article] [PubMed]
- 15.Dayan D, Buchner A, Garlick J. Touton-like giant cells in periapical granulomas. J Endod. 1989;15:210–211. doi: 10.1016/S0099-2399(89)80237-7. [DOI] [PubMed] [Google Scholar]
- 16.Prieto-Potin I, Roman-Blas JA, Martinez-Calatrava MJ, Gomez R, Largo R, Herrero-Beaumont G. Hypercholesterolemia boosts joint destruction in chronic arthritis. An experimental model aggravated by foam macrophage infiltration. Arthritis Res Ther. 2013;15:R81. doi: 10.1186/ar4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Losslein AK, Lohrmann F, Scheuermann L, Gharun K, Neuber J, Kolter J, et al. Monocyte progenitors give rise to multinucleated giant cells. Nat Commun. 2021;12:2027. doi: 10.1038/s41467-021-22103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127:74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 20.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 22.Ruggiero AD, Key CC, Kavanagh K. Adipose tissue macrophage polarization in healthy and unhealthy obesity. Front Nutr. 2021;8:625331. doi: 10.3389/fnut.2021.625331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Eijk M, Aerts J. The Unique Phenotype of Lipid-Laden Macrophages. Int J Mol Sci. 2021;22(8) 10.3390/ijms22084039. [DOI] [PMC free article] [PubMed]
- 24.Hausberger FX. Pathological changes in adipose tissue of obese mice. Anat Rec. 1966;154:651–660. doi: 10.1002/ar.1091540311. [DOI] [PubMed] [Google Scholar]
- 25.Hellman B. Studies in obese-hyperglycemic mice. Ann N Y Acad Sci. 1965;131:541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- 26.Pekala P, Kawakami M, Vine W, Lane MD, Cerami A. Studies of insulin resistance in adipocytes induced by macrophage mediator. J Exp Med. 1983;157:1360–1365. doi: 10.1084/jem.157.4.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, Bouloumie A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53(5):1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 30.Helming L, Winter J, Gordon S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J Cell Sci. 2009;122:453–459. doi: 10.1242/jcs.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014;37:365–371. doi: 10.14348/molcells.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai L, Wang Z, Ji A, Meyer JM, van der Westhuyzen DR. Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PLoS One. 2012;7(5):e36785. doi: 10.1371/journal.pone.0036785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 34.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda M, Sakaue H. Adipocyte death and chronic inflammation in obesity. J Med Invest. 2017;64(3.4):193–196. doi: 10.2152/jmi.64.193. [DOI] [PubMed] [Google Scholar]
- 36.Atkin-Smith GK. Phagocytic clearance of apoptotic, necrotic, necroptotic and pyroptotic cells. Biochem Soc Trans. 2021;49(2):793–804. doi: 10.1042/BST20200696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428–3438. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I, et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54:2423–2436. doi: 10.1194/jlr.M038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith U, Li Q, Ryden M, Spalding KL. Cellular senescence and its role in white adipose tissue. Int J Obes (Lond). 2021;45:934–943. doi: 10.1038/s41366-021-00757-x. [DOI] [PubMed] [Google Scholar]
- 40.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A, Zhou G, Fernandez S, Zhai L, Hall BA, Haka AS, Shah AM, Reardon CA, Brady MJ, Rhodes CJ, Maxfield FR, Becker L. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017;20(13):3149–3161. doi: 10.1016/j.celrep.2017.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindhorst A, Raulien N, Wieghofer P, Eilers J, Rossi FMV, Bechmann I, et al. Adipocyte death triggers a pro-inflammatory response and induces metabolic activation of resident macrophages. Cell Death Dis. 2021;12:579. doi: 10.1038/s41419-021-03872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18(3):355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 48.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O'Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harasymowicz NS, Rashidi N, Savadipour A, Wu CL, Tang R, Bramley J, et al. Single-cell RNA sequencing reveals the induction of novel myeloid and myeloid-associated cell populations in visceral fat with long-term obesity. FASEB J. 2021;35:e21417. doi: 10.1096/fj.202001970R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hildreth AD, Ma F, Wong YY, Sun R, Pellegrini M, O’Sullivan TE. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat Immunol. 2021;22:639–653. doi: 10.1038/s41590-021-00922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A. 2018;115:E5096–EE105. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178:686–698. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vijay J, Gauthier MF, Biswell RL, Louiselle DA, Johnston JJ, Cheung WA, et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2:97–109. doi: 10.1038/s42255-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulland TK, Colonna M. TREM2 - a key player in microglial biology and Alzheimer disease. Nat Rev Neurol. 2018;14:667–675. doi: 10.1038/s41582-018-0072-1. [DOI] [PubMed] [Google Scholar]
- 57.Yeh FL, Hansen DV, Sheng M. TREM2, Microglia, and neurodegenerative diseases. Trends Mol Med. 2017;23(6):512–533. doi: 10.1016/j.molmed.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Helming L, Tomasello E, Kyriakides TR, Martinez FO, Takai T, Gordon S, et al. Essential role of DAP12 signaling in macrophage programming into a fusion-competent state. Sci Signal. 2008;1:ra11. doi: 10.1126/scisignal.1159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Humphrey MB, Ogasawara K, Yao W, Spusta SC, Daws MR, Lane NE, Lanier LL, Nakamura MC. The signaling adapter protein DAP12 regulates multinucleation during osteoclast development. J Bone Miner Res. 2004;19(2):224–234. doi: 10.1359/JBMR.0301234. [DOI] [PubMed] [Google Scholar]
- 61.Otero K, Shinohara M, Zhao H, Cella M, Gilfillan S, Colucci A, Faccio R, Ross FP, Teitelbaum SL, Takayanagi H, Colonna M. TREM2 and beta-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J Immunol. 2012;188(6):2612–2621. doi: 10.4049/jimmunol.1102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iizasa E, Chuma Y, Uematsu T, Kubota M, Kawaguchi H, Umemura M, et al. TREM2 is a receptor for non-glycosylated mycolic acids of mycobacteria that limits anti-mycobacterial macrophage activation. Nat Commun. 2021;12:2299. doi: 10.1038/s41467-021-22620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 64.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang H, Kerloc’h A, Rotival M, Xu X, Zhang Q, D’Souza Z, Kim M, Scholz JC, Ko JH, Srivastava PK, Genzen JR, Cui W, Aitman TJ, Game L, Melvin JE, Hanidu A, Dimock J, Zheng J, Souza D, Behera AK, Nabozny G, Cook HT, Bassett JHD, Williams GR, Li J, Vignery A, Petretto E, Behmoaras J. Kcnn4 is a regulator of macrophage multinucleation in bone homeostasis and inflammatory disease. Cell Rep. 2014;8(4):1210–1224. doi: 10.1016/j.celrep.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pereira M, Ko JH, Logan J, Protheroe H, Kim KB, Tan ALM, et al. A trans-eQTL network regulates osteoclast multinucleation and bone mass. Elife. 2020;9 10.7554/eLife.55549. [DOI] [PMC free article] [PubMed]
- 67.Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell. 2017;170:649–663. doi: 10.1016/j.cell.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, Li X, Rademakers R, Kang SS, Xu H, Younkin S, Das P, Fryer JD, Bu G. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) J Biol Chem. 2015;290(43):26043–26050. doi: 10.1074/jbc.M115.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey CC, DeVaux LB, Farzan M. The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J Biol Chem. 2015;290:26033–26042. doi: 10.1074/jbc.M115.677286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Obradovic A, Chowdhury N, Haake SM, Ager C, Wang V, Vlahos L, et al. Single-cell protein activity analysis identifies recurrence-associated renal tumor macrophages. Cell. 2021; 10.1016/j.cell.2021.04.038. [DOI] [PMC free article] [PubMed]
- 72.Gast CE, Silk AD, Zarour L, Riegler L, Burkhart JG, Gustafson KT, et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci Adv. 2018;4:eaat7828. doi: 10.1126/sciadv.aat7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160(6):1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, Zernecke A. Single-Cell RNA-Seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122(12):1661–1674. doi: 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- 76.Ryu J, Kim H, Chang EJ, Kim HJ, Lee Y, Kim HH. Proteomic analysis of osteoclast lipid rafts: the role of the integrity of lipid rafts on V-ATPase activity in osteoclasts. J Bone Miner Metab. 2010;28:410–417. doi: 10.1007/s00774-009-0150-y. [DOI] [PubMed] [Google Scholar]
- 77.Sato T, Morita I, Murota S. Involvement of cholesterol in osteoclast-like cell formation via cellular fusion. Bone. 1998;23(2):135–140. doi: 10.1016/s8756-3282(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 78.Okayasu M, Nakayachi M, Hayashida C, Ito J, Kaneda T, Masuhara M, Suda N, Sato T, Hakeda Y. Low-density lipoprotein receptor deficiency causes impaired osteoclastogenesis and increased bone mass in mice because of defect in osteoclastic cell-cell fusion. J Biol Chem. 2012;287(23):19229–19241. doi: 10.1074/jbc.M111.323600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luegmayr E, Glantschnig H, Wesolowski GA, Gentile MA, Fisher JE, Rodan GA, et al. Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death Differ. 2004;11(Suppl 1):S108–S118. doi: 10.1038/sj.cdd.4401399. [DOI] [PubMed] [Google Scholar]
- 80.Oh SR, Sul OJ, Kim YY, Kim HJ, Yu R, Suh JH, et al. Saturated fatty acids enhance osteoclast survival. J Lipid Res. 2010;51:892–899. doi: 10.1194/jlr.M800626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drosatos-Tampakaki Z, Drosatos K, Siegelin Y, Gong S, Khan S, Van Dyke T, et al. Palmitic acid and DGAT1 deficiency enhance osteoclastogenesis, while oleic acid-induced triglyceride formation prevents it. J Bone Miner Res. 2014;29:1183–1195. doi: 10.1002/jbmr.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lucas S, Omata Y, Hofmann J, Bottcher M, Iljazovic A, Sarter K, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9(1):55. doi: 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zou W, Rohatgi N, Brestoff JR, Li Y, Barve RA, Tycksen E, et al. Ablation of fat cells in adult mice induces massive bone gain. Cell Metab. 2020;32:801–813. doi: 10.1016/j.cmet.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leiria LO, Tseng YH. Lipidomics of brown and white adipose tissue: implications for energy metabolism. Biochim Biophys Acta Mol Cell Biol Lipids. 1865;2020:158788. doi: 10.1016/j.bbalip.2020.158788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parthasarathy V, Martin F, Higginbottom A, Murray H, Moseley GW, Read RC, Mal G, Hulme R, Monk PN, Partridge LJ. Distinct roles for tetraspanins CD9, CD63 and CD81 in the formation of multinucleated giant cells. Immunology. 2009;127(2):237–248. doi: 10.1111/j.1365-2567.2008.02945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeda Y, Tachibana I, Miyado K, Kobayashi M, Miyazaki T, Funakoshi T, et al. Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol. 2003;161:945–956. doi: 10.1083/jcb.200212031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takeda Y, He P, Tachibana I, Zhou B, Miyado K, Kaneko H, et al. Double deficiency of tetraspanins CD9 and CD81 alters cell motility and protease production of macrophages and causes chronic obstructive pulmonary disease-like phenotype in mice. J Biol Chem. 2008;283:26089–26097. doi: 10.1074/jbc.M801902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin Y, Takeda Y, Kondo Y, Tripathi LP, Kang S, Takeshita H, et al. Double deletion of tetraspanins CD9 and CD81 in mice leads to a syndrome resembling accelerated aging. Sci Rep. 2018;8:5145. doi: 10.1038/s41598-018-23338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oguri Y, Shinoda K, Kim H, Alba DL, Bolus WR, Wang Q, et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell. 2020;182:563–577. doi: 10.1016/j.cell.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aguilar PS, Baylies MK, Fleissner A, Helming L, Inoue N, Podbilewicz B, et al. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013;29:427–437. doi: 10.1016/j.tig.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, Portman JR, Matchett KP, Brice M, Marwick JA, Taylor RS, Efremova M, Vento-Tormo R, Carragher NO, Kendall TJ, Fallowfield JA, Harrison EM, Mole DJ, Wigmore SJ, Newsome PN, Weston CJ, Iredale JP, Tacke F, Pollard JW, Ponting CP, Marioni JC, Teichmann SA, Henderson NC. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575(7783):512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsai AT, Rice J, Scatena M, Liaw L, Ratner BD, Giachelli CM. The role of osteopontin in foreign body giant cell formation. Biomaterials. 2005;26(29):5835–5843. doi: 10.1016/j.biomaterials.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki K, Zhu B, Rittling SR, Denhardt DT, Goldberg HA, McCulloch CA, et al. Colocalization of intracellular osteopontin with CD44 is associated with migration, cell fusion, and resorption in osteoclasts. J Bone Miner Res. 2002;17(8):1486–1497. doi: 10.1359/jbmr.2002.17.8.1486. [DOI] [PubMed] [Google Scholar]
- 94.Xiong X, Kuang H, Ansari S, Liu T, Gong J, Wang S, Zhao XY, Ji Y, Li C, Guo L, Zhou L, Chen Z, Leon-Mimila P, Chung MT, Kurabayashi K, Opp J, Campos-Pérez F, Villamil-Ramírez H, Canizales-Quinteros S, Lyons R, Lumeng CN, Zhou B, Qi L, Huertas-Vazquez A, Lusis AJ, Xu XZS, Li S, Yu Y, Li JZ, Lin JD. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol Cell. 2019;75(3):644–660. doi: 10.1016/j.molcel.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Daemen S, Gainullina A, Kalugotla G, He L, Chan MM, Beals JW, Liss KH, Klein S, Feldstein AE, Finck BN, Artyomov MN, Schilling JD. Dynamic shifts in the composition of resident and recruited macrophages influence tissue remodeling in NASH. Cell Rep. 2021;34(2):108626. doi: 10.1016/j.celrep.2020.108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seidman JS, Troutman TD, Sakai M, Gola A, Spann NJ, Bennett H, Bruni CM, Ouyang Z, Li RZ, Sun X, Vu BCT, Pasillas MP, Ego KM, Gosselin D, Link VM, Chong LW, Evans RM, Thompson BM, McDonald JG, Hosseini M, Witztum JL, Germain RN, Glass CK. Niche-specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity. 2020;52(6):1057–1074. doi: 10.1016/j.immuni.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169(7):1276–1290. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 98.Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, Kim J, Tevini J, Felder TK, Wolinski H, Bertozzi CR, Bassik MC, Aigner L, Wyss-Coray T. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020;23(2):194–208. doi: 10.1038/s41593-019-0566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 100.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 103.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281(36):26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 104.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim J, Chung K, Choi C, Beloor J, Ullah I, Kim N, Lee KY, Lee SK, Kumar P. Silencing CCR2 in macrophages alleviates adipose tissue inflammation and the associated metabolic syndrome in dietary obese mice. Mol Ther Nucleic Acids. 2016;5:e280. doi: 10.1038/mtna.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sullivan TJ, Miao Z, Zhao BN, Ertl LS, Wang Y, Krasinski A, et al. Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metabolism. 2013;62:1623–1632. doi: 10.1016/j.metabol.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 107.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Teijlingen BN, Pearce EJ. Cell-intrinsic metabolic regulation of mononuclear phagocyte activation: findings from the tip of the iceberg. Immunol Rev. 2020;295(1):54–67. doi: 10.1111/imr.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jung SB, Choi MJ, Ryu D, Yi HS, Lee SE, Chang JY, et al. Reduced oxidative capacity in macrophages results in systemic insulin resistance. Nat Commun. 2018;9:1551. doi: 10.1038/s41467-018-03998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y, Tang B, Long L, Luo P, Xiang W, Li X, et al. Improvement of obesity-associated disorders by a small-molecule drug targeting mitochondria of adipose tissue macrophages. Nat Commun. 2021;12:102. doi: 10.1038/s41467-020-20315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y, Sun C, Liu X, Jefferson LS, Xiong J, LaNoue KF, Chang Z, Lynch CJ, Wang H, Shi Y. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010;12(2):154–165. doi: 10.1016/j.cmet.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma L, Liu TW, Wallig MA, Dobrucki IT, Dobrucki LW, Nelson ER, et al. Efficient Targeting of Adipose Tissue Macrophages in Obesity with Polysaccharide Nanocarriers. ACS Nano. 2016;10:6952–6962. doi: 10.1021/acsnano.6b02878. [DOI] [PubMed] [Google Scholar]
- 113.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guermonprez P, Helft J. Inflammasome activation: a monocyte lineage privilege. Nat Immunol. 2019;20:383–385. doi: 10.1038/s41590-019-0348-7. [DOI] [PubMed] [Google Scholar]
- 115.Olona A, Hateley C, Guerrero A, Ko JH, Johnson MR, Anand PK, et al. Cardiac glycosides cause cytotoxicity in human macrophages and ameliorate white adipose tissue homeostasis. Br J Pharmacol. 2021; 10.1111/bph.15423. [DOI] [PubMed]
- 116.Cox N, Crozet L, Holtman IR, Loyher PL, Lazarov T, White JB, et al. Diet-regulated production of PDGFcc by macrophages controls energy storage. Science. 2021;373(6550) 10.1126/science.abe9383. [DOI] [PMC free article] [PubMed]
- 117.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sanchez NM, Mahu I, et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med. 2017;23:1309–1318. doi: 10.1038/nm.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Satoh T, Kidoya H, Naito H, Yamamoto M, Takemura N, Nakagawa K, et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495:524–528. doi: 10.1038/nature11930. [DOI] [PubMed] [Google Scholar]
- 120.Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194(8):1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kraal G, van der Laan LJ, Elomaa O, Tryggvason K. The macrophage receptor MARCO. Microbes Infect. 2000;2(3):313–316. doi: 10.1016/s1286-4579(00)00296-3. [DOI] [PubMed] [Google Scholar]
- 122.Mukhopadhyay S, Chen Y, Sankala M, Peiser L, Pikkarainen T, Kraal G, et al. MARCO, an innate activation marker of macrophages, is a class A scavenger receptor for Neisseria meningitidis. Eur J Immunol. 2006;36:940–949. doi: 10.1002/eji.200535389. [DOI] [PubMed] [Google Scholar]
- 123.Pridans C, Raper A, Davis GM, Alves J, Sauter KA, Lefevre L, et al. Pleiotropic impacts of macrophage and microglial deficiency on development in rats with targeted mutation of the Csf1r Locus. J Immunol. 2018;201:2683–2699. doi: 10.4049/jimmunol.1701783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brunner JS, Vogel A, Lercher A, Caldera M, Korosec A, Puhringer M, et al. The PI3K pathway preserves metabolic health through MARCO-dependent lipid uptake by adipose tissue macrophages. Nat Metab. 2020;2(12):1427–1442. doi: 10.1038/s42255-020-00311-5. [DOI] [PubMed] [Google Scholar]
- 125.Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017;26:185–197. doi: 10.1016/j.cmet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9(6):2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharif O, Brunner JS, Korosec A, Martins R, Jais A, Snijder B, et al. Beneficial metabolic effects of TREM2 in obesity are uncoupled from its expression on macrophages. Diabetes. 2021;70:2042–2057. doi: 10.2337/db20-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.