Abstract
Background
Reliable noninvasive predictors of the top three causes of death [cardiovascular diseases (CVDs), malignancies, and liver-related events in patients with non-alcoholic fatty liver disease (NAFLD)] have not yet been determined.
Methods
We retrospectively investigated the incidence of three complications [CVDs, malignancy (except for liver cancer), and liver-related events] in 477 Japanese patients with histo-pathologically confirmed NAFLD for a median follow-up of 5.9 years. In addition to histological findings, we also investigated noninvasive predictors.
Results
A score of ≥ 2.67 for the noninvasive diagnosis of stage 4 fibrosis based on the Fibrosis-4 (FIB-4) index indicated a high level area under the receiver operating characteristic (AUROC) curve (0.90), sensitivity (82.9%), specificity (86.4%), and negative predictive value [(NPV) of 98.5%]. The yearly incidence rates of CVDs, malignancies, and liver-related events were found to be 1.04%, 0.83%, and 0.30%, respectively. Multivariate analysis identified a FIB-4 index ≥ 2.67 score as a significant and independent, noninvasive predictor of these three complications. Furthermore, the cumulative incidence rates of CVDs were significantly different among the three genotypes of PNPLA3. PNPLA3 genotype CC, chronic kidney disease (CKD), and FIB-4 index ≥ 2.67 was could be attributed to these three significant CVD risk factors. The rates of CVDs were significantly different among the three subgroups based on the combination of risk factors. In malignancy (except for liver cancer), the incidence rate of colon cancer was 25.0%; in particular, the rate in females was 53.8%.
Conclusions
Our results highlighted the importance of the PNPLA3 genotype and FIB-4 index ≥ 2.67 on the incidence of complications in Japanese patients with NAFLD, especially the incidence of CVDs. Early diagnosis, based on the presence of one or more risk factors, and early treatment might improve the prognosis for NAFLD patients.
Keywords: Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, Cardiovascular diseases, Malignancies, Liver-related events, FIB-4 index, PNPLA3
Background
The most common worldwide liver disease is non-alcoholic fatty liver disease (NAFLD) [1–6]. Its pathology ranges from the typically benign non-alcoholic fatty liver to non-alcoholic steatohepatitis (NASH), which may progress to liver cirrhosis, liver cancer, and finally, to liver failure [7].
The American Association for the Study of Liver Diseases reported that the most common cause of death in patients with NAFLD is related to cardiovascular diseases (CVDs) independent of other metabolic comorbidities. Liver-related mortality was reported to be the second or third cause of death, and cancer-related mortality was among the top three causes of death [8]. In Asia, the incidence rates of CVDs, malignancies, and liver-related events in patients with histo-pathologically confirmed NAFLD still remains unclear.
Previous studies have suggested that the stage of fibrosis is a more reliable predictor of liver-specific mortality than the NAFLD activity score (NAS) [9]. The stage of fibrosis, exclusive of other histopathological features of steatohepatitis, has been reported to be an independent and significant predictor of overall mortality, need for liver transplantation, and liver-related events [10]. Furthermore, the fibrosis stage was also associated with the CVD incidence [11, 12]. However, reliable, non-histological, and noninvasive predictors of the top three causes of death have not yet been found.
The purpose of the present study was to determine the incidence rates of three complications [CVDs, malignancies (except for liver cancer), and liver-related events as the top three causes of death] and noninvasive predictors of these complications in patients with NAFLD by retrospectively analyzing the outcome of 477 Japanese patients with histo-pathologically confirmed NAFLD.
Methods
Patients
This study was designed as a retrospective cohort study of patients with histo-pathologically-confirmed NAFLD. Between 1976 and 2021, liver biopsies were performed at our hospital for patients with liver dysfunction and/or fatty liver diagnosed by abdominal ultrasonography. Of those conditions, the diagnosis of NAFLD was confirmed in 477 patients based on histopathology. Patient characteristics at the time of histopathological diagnosis of NAFLD are summarized in Table 1.
Table 1.
Patient characteristics at the time of histological diagnosis of non-alcoholic fatty acid liver disease (NAFLD)
| Demographic data | |
| Numbers of patients | 477 |
| Gender, male/female, n | 282/195 |
| Age, year* | 53 (20–87) |
| Body mass index, kg/m2* | 26.3 (16.6–42.4) |
| Waist circumference, cm* | 91.3 (68.2–132.1) |
| Smoking, absence/presence, n | 385 /92 |
| Previous or current events | |
| Cardiovascular diseases, absence/presence/unknown, n | 444/30/3 |
| Malignancies, except for liver cancer, absence/presence/unknown, n | 426/47/4 |
| Liver-related events, absence/presence/unknown, n | 432/42/3 |
| Liver cancer, absence/presence/unknown, n | 446/30/1 |
| Ascites, absence/presence/unknown, n | 472/2/3 |
| Hepatic encephalopathy, absence/presence/unknown, n | 474/0/3 |
| Jaundice, absence/presence/unknown, n | 471/3/3 |
| Esophago-gastric varices, absence/presence/unknown, n | 463/11/3 |
| Comorbid diseases | |
| Type 2 diabetes mellitus, absence/presence, n | 321/156 |
| Hypertension, absence/presence, n | 256/221 |
| Dyslipidemia, absence/presence, n | 125/352 |
| Hyperuricemia, absence/presence, n | 420/57 |
| Chronic kidney disease, absence/presence, n | 445/32 |
| Histological findings | |
| Steatosis, 5–33%/ > 33–66%/ > 66%, n | 174/175/126 |
| Lobular inflammation | |
| No foci/< 2 foci/2–4 foci/> 4 foci per 200 × field, n | 28/263/167/17 |
| Ballooning, none/few cells/many cells, n | 42/298/135 |
| Fibrosis stage, 0/1/2/3/4, n | 55/189/77/121/35 |
| NAFLD activity score, ≤ 2/3/4/ ≥ 5, n | 38/197/242 |
| Genetic factors | |
| PNPLA3 rs738409 (CC/CG/GG/Not determined) | 57/137/135/148 |
| TM6SF2 rs58542926 (CC/CT/TT/Not determined) | 248/74/7/148 |
| ALDH2 rs671 (GG/GA/AA/Not determined) | 158/142/29/148 |
| HSD17B13 rs6834314 (AA/AG/GG/Not determined) | 162/132/25/158 |
| Laaboratory dataa | |
| Serum aspartate aminotransferase (AST), U/L | 44 (3–378) |
| Serum alanine aminotransferase (ALT), U/L | 69 (13–783) |
| Gamma-glutamyl transpeptidase, U/L | 71 (11–1,135) |
| Platelet count, × 103/mm3 | 212 (40–471) |
| Albumin | 4.1 (2.8–6.9) |
| Serum ferritin, μg/L | 227 (< 10–2.067) |
| High sensitive C-reactive protein, mg/dL | 0.097 (0.004–2.240) |
| FIB-4 index | 1.36 (0.19–14.8) |
Data represent number of patients, except those denoted by a, which represent the median (range) values
Cardiovascular diseases included coronary artery disease, heart valve disease, arrhythmia, heart failure, hypertension, orthostatic hypotension, shock, endocarditis, diseases of the aorta and its branches, disorders of the peripheral vascular system, and stroke
Based on the practice guidance from the American Association for the Study of Liver Diseases [8], NAFLD diagnosis was based on the liver histopathological findings of steatosis in ≥ 5% of hepatocytes after excluding other liver diseases (such as autoimmune hepatitis, primary biliary cholangitis, viral hepatitis, drug-induced liver disease, biliary obstruction, hemochromatosis, Wilson disease, and α-1-antitrypsin deficiency-associated liver disease). Of these 447 patients, none of them who consumed more than 20 g of alcohol per day.
The Human Ethics Review Committee at Toranomon Hospital approved the protocol of the study (number 953), and a signed informed consent form was obtained from each of the patients at the time of liver histological diagnosis. The study complied with the International Conference on Harmonization Guidelines for Good Clinical Practice (E6), as well as the 2013 Declaration of Helsinki.
Diagnosis and follow-up
Liver-related events were evaluated as jaundice, ascites, esophago-gastric varices, hepatic encephalopathy, and liver cancer. CVDs included heart failure, coronary artery disease, hypertension, orthostatic hypotension, shock, heart valve disease, endocarditis, arrhythmia, disorders of the peripheral vascular system, diseases of the aorta and its branches, and stroke [6]. After the NAFLD diagnosis, biochemical and hematological data were collected at least twice yearly. At least once annually, abdominal ultrasonography (US), computed tomography (CT), or magnetic resonance imaging (MRI) studies were performed [6]. In all of 477 patients, the median duration of follow-up from diagnosis to the last visit or death was 5.9 years (range 0.0–44.9 years).
Liver histopathology
Liver specimens were obtained with a 14-gauge modified Vim Silverman needle (Tohoku University style, Kakinuma Factory, Tokyo, Japan), a 16-gauge core tissue biopsy needle (Bard Peripheral Vascular Inc., Tempe, AZ) or surgical resection. Specimen was fixed in 10% formalin, and the prepared sections were stained with hematoxylin–eosin, Masson trichrome, silver impregnation, or periodic acid-Schiff after diastase digestion. An adequate liver biopsy sample was defined as a specimen longer than 1.5 cm and/or containing more than 11 portal tracts [6]. Four pathologists (K.K., F.K., T.F., and T.F.), who were blinded to the clinical findings, evaluated each specimen, and the final assessment was reported by consensus [6].
Steatosis grades 0, 1, 2, and 3 corresponded to hepatocyte steatosis levels of < 5%, ≥ 5– < 33%, ≥ 33– < 66%, and ≥ 66%, respectively. Lobular inflammation scores of 0, 1, 2, and 3 corresponded to no, < 2, 2–4, and ≥ 4 foci per 200 × field, respectively. Hepatocyte ballooning scores of 0, 1, and 2 corresponded to none, few, and many cells, respectively. The sum of the steatosis, lobular inflammation, and hepatocyte ballooning scores (range 0–8 points) is termed the NAS [13]. Fibrosis stage was defined as 0, 1, 2, 3, or 4 [13, 14].
Clinical parameters
The fibrosis-4 (FIB-4) index has been used as a parameter for fibrosis progression [15]. The FIB-4 index is useful for excluding NAFLD with advanced fibrosis based on values < 1.30, which are considered to represent non-advanced fibrosis. and a FIB-4 index ≥ 2.67 is suggested before performing a liver biopsy to identify advanced fibrosis [5]. Chronic kidney disease (CKD) was defined as persistent positive proteinuria and/or estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 for more than three months [16]. Normal waist circumferences were defined as 85 and 90 cm for men and women, respectively.
The TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA, USA) was used for genotyping PNPLA3 rs738409, TM6SF2 rs58542926, ALDH2 rs671, and HSD17B13 rs6834314.
Statistical analysis
The incidence of each event was analyzed during the period from the time of histopathological NAFLD diagnosis until the last visit or occurrence of event. Stepwise Cox regression analysis was used to determine independent predictive factors associated with the incidence of CVDs, malignancies (except for liver cancer), and liver-related events. The hazard ratio and 95% confidence interval (HR and 95% CI, respectively) were also calculated. The potential predictive factors included several noninvasive variables, except for some confounding factors and histological findings: (1) demographic data (except for age), (2) previous and/or current events, (3) comorbid diseases, (4) genetic factors, and (5) laboratory data [except for aspartate and alanine transaminases (AST and ALT, respectively) and platelet count] as shown in Table 1. Variables that were statistically significant based on univariate analysis were tested using a multivariate analysis to identify significant independent factors after being converted into categorical data consisting of two simple ordinal numbers. Significance was set at P value < 0.05 by the two-tailed test. Statistical comparisons were performed with the SPSS software (SPSS Inc., Chicago, IL, USA). The area under the receiver-operating characteristic curve (AUROC), sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively) were calculated to determine the diagnostic performance of FIB-4 index for liver fibrosis detection.
Results
Diagnostic performance of FIB-4 index score for detection of liver fibrosis in NAFLD
Data from 477 patients were used for analysis of the diagnostic performance of FIB-4 index for the detection of liver fibrosis in NAFLD patients. The areas under the AUROC of the FIB-4 index for detection of stages ≥ 1, ≥ 2, ≥ 3, and 4 were 0.75 (95% CI 0.69–0.81), 0.84 (95% CI 0.80–0.87), 0.85 (95% CI 0.81–0.88), and 0.90 (95% CI 0.87–0.94), respectively. Sensitivity, of FIB-4 index ≥ 1.30 for detection of stages ≥ 1, ≥ 2, ≥ 3, and 4 were 56.4%, 78.2%, 95.2%, and 18.9%, respectively. Specificity values were 79.8%, 73.8%, 74.4%, and 79.3%, respectively; PPV values were 88.5%, 65.1%, 55.2%, and 92.1, respectively while NPV values were 100%, 51.4%, 14.0%, and 100%, respectively. Sensitivity results of the FIB-4 index ≥ 2.67 for detection of stages ≥ 1, ≥ 2, ≥ 3, and 4 were 20.9%, 98.2%, 98.9%, and 13.9%, respectively. Specificity values were 36.1%, 98.0%, 94.4%, and 61.6%, respectively. PPVs were 46.2%, 94.7%, 80.9%, and 78.4%, respectively while NPV values were 82.9%, 86.4%, 32.6%, and 98.5%, respectively. Thus, a FIB-4 index ≥ 2.67 for the noninvasive diagnosis of stage 4 indicated high AUROC, sensitivity, specificity, and NPV values (Table 2).
Table 2.
Diagnostic performance of Fibrosis-4 (FIB-4) index for the detection of liver fibrosis
| Overall (n = 477) | AUROC | (95%CI) | Fib-4 cut off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Fibrosis stage 1–4 (n = 422) versus Fibrosis stage 0 (n = 55) | 0.75 | (0.69–0.81) | 1.30 | 56.4 | 78.2 | 95.2 | 18.9 |
| 2.67 | 20.9 | 98.2 | 98.9 | 13.9 | |||
| Fibrosis stage 2–4 (n = 233) versus Fibrosis stage 0–1 (n = 244) | 0.84 | (0.80–0.87) | 1.30 | 79.8 | 73.8 | 74.4 | 79.3 |
| 2.67 | 36.1 | 98.0 | 94.4 | 61.6 | |||
| Fibrosis stage 3–4 (n = 156) versus Fibrosis stage 0–2 (n = 321) | 0.85 | (0.81–0.88) | 1.30 | 88.5 | 65.1 | 55.2 | 92.1 |
| 2.67 | 46.2 | 94.7 | 80.9 | 78.4 | |||
| Fibrosis stage 4 (n = 35) versus Fibrosis stage 0–3 (n = 442) | 0.90 | (0.87–0.94) | 1.30 | 100 | 51.4 | 14.0 | 100 |
| 2.67 | 82.9 | 86.4 | 32.6 | 98.5 |
AUROC, area under the receiver-operating characteristic curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value
Incidence and noninvasive predictors of CVDs in NAFLD
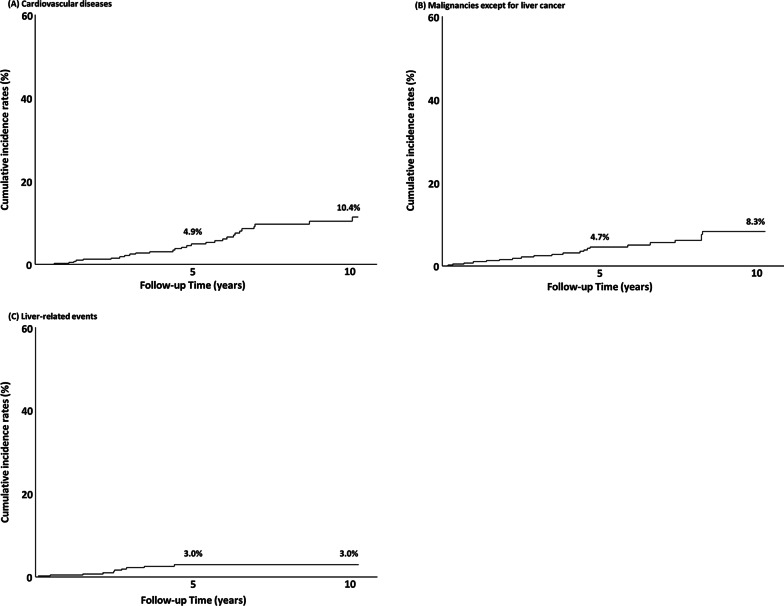
Characteristics of the 444 patients who were confirmed to have no previous or current CVDs at their NAFLD diagnoses were evaluated for the rate of CVD development. During the follow-up period, 43 patients (9.7%) developed CVDs. The cumulative incidence rates were 4.9% and 10.4% at the end of 5 and 10 years, respectively. The yearly incidence rate of CVDs over the 10-year period under study was 1.04% (Fig. 1a).
Fig. 1.
Cumulative incidence rates of three complications in Japanese patients with non-alcoholic fatty liver disease (NAFLD). A The yearly incidence of cardiovascular diseases (CVDs) was 1.04%. B The yearly incidence of malignancies except for liver cancer was 0.83%. C The yearly incidence of liver-related events was 0.30%
Univariate analysis identified six noninvasive parameters that significantly correlated with the incidence of CVDs: (1) body mass index (BMI), (2) previous or current malignancies (except for liver cancer), (3) CKD, (4) comorbid hypertension, (5) PNPLA3 genotype, and (6) FIB-4 index. These factors were entered into a multivariate analysis, which identified three noninvasive factors that significantly and independently influenced the incidence of CVDs: (1) PNPLA3 genotype (CC type; HR 3.66, 95% CI = 1.63–8.35; P = 0.002), (2) CKD (Presence; HR 3.62, 95% CI = 1.18–11.2; P = 0.025), and (3) FIB-4 index (≥ 2.67; HR 2.73, 95% CI = 1.21–6.14; P = 0.016) as shown in Table 3.
Table 3.
Non-invasive predictors associated with the incidence of cardiovascular diseases (CVDs)
| Factor | Category | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | (95% CI) | P value* | Hazard ratio | (95% CI) | P value* | ||
| PNPLA3 rs738409 | CG, GG | 1 | 1 | ||||
| CC | 2.97 | (1.35–6.53) | 0.007 | 3.66 | (1.61–8.35) | 0.002 | |
| Chronic kidney disease** | Absence | 1 | 1 | ||||
| Presence | 4.44 | (1.69–11.7) | 0.002 | 3.62 | (1.18–11.2) | 0.025 | |
| FIB-4 index | < 2.67 | 1 | 1 | ||||
| ≥ 2.67 | 3.14 | (1.58–6.23) | 0.001 | 2.73 | (1.21–6.14) | 0.016 | |
| Body mass index | < 25.0 kg/m2 | 1 | |||||
| ≥ 25.0 kg/m2 | 2.32 | (1.16–4.64) | 0.017 | ||||
| Previous or current malignancies, except for liver cancer | Absence | 1 | |||||
| Presence | 2.67 | (1.11–6.42) | 0.029 | ||||
| Hypertension | Absence | 1 | |||||
| Presence | 2.20 | (1.18–4.10) | 0.013 | ||||
*Significance was determined using the Cox proportional hazard model. Variables that were statistically significant on univariate analysis were entered into multivariate analysis to identify significant independent factors
**Chronic kidney disease was defined as persistent positive proteinuria and/or eGFR < 60 ml/min/1.73 m2 for more than 3 months. Characteristics of the 444 patients who were confirmed to have no previous or current CVDs at their NAFLD diagnoses were evaluated for the rate of CVD development. During the follow-up period, 43 patients developed CVDs. CVDs included coronary artery disease, heart valve disease, arrhythmia, heart failure, hypertension, orthostatic hypotension, shock, endocarditis, diseases of the aorta and its branches, disorders of the peripheral vascular system, and stroke.
Cumulative incidence rates of CVDs to a combination of risk factors
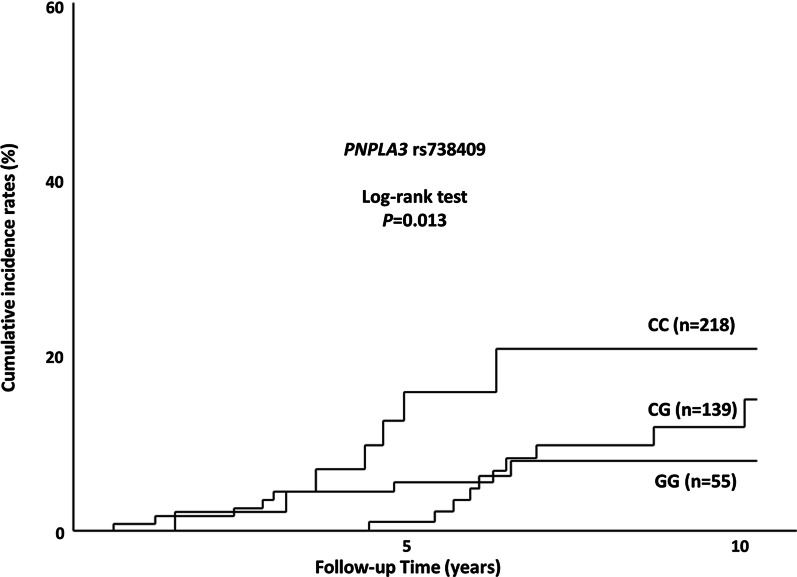
Four-hundred twelve patients, who were confirmed to have no previous or current CVDs NAFLD diagnosis, underwent analysis to determine the cumulative incidence rates of CVDs according to PNPLA3 genotype. Cumulative incidence rates were significantly different among the three genotypes of PNPLA3 [CC, CG, and GG (n = 218, 139, and 55, respectively); P = 0.013 based on log-rank test] as shown in Fig. 2. In particular, the rates of the CC type were significantly higher than those of CG type (P = 0.049; log-rank test) and GG type (P = 0.002; log-rank test). The rates of the CG type were not different from those of the GG type (P = 0.400; log-rank test).
Fig. 2.
Cumulative incidence rates of cardiovascular diseases (CVDs) according to PNPLA3 genotype. The rates were significantly different among the three genotypes (CC, CG, and GG type; P = 0.013; log-rank test). In particular, the rates of CC type were significantly higher than those of CG and GG types (P = 0.049; log-rank test type and P = 0.002; log-rank test, respectively). The rates of CG type were not different from those of GG type (P = 0.400; log-rank test)
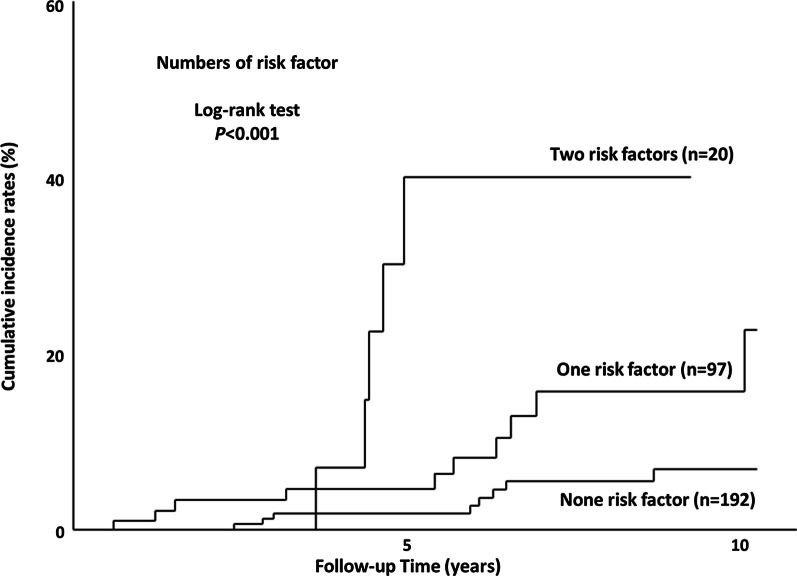
Three-hundred nine patients, who were evaluated based on the three CVD risk factors were analyzed to determine the cumulative incidence rates according to a combination of risk factors. The cumulative incidence rates were significantly different among the three subgroups based on the number of risk factors [no, one, two, or three risk factors (corresponding to n = 192, 97, 20, or 0); P < 0.001] based on the log-rank test, Fig. 3. In particular, the incidence rates for patients with two risk factors were significantly higher than in those with one risk factor (P = 0.018; log-rank test) and no risk factors (P < 0.001; log-rank test). Furthermore, the incidence rates for patients with one risk factor were also significantly higher than those for no risk factors (P = 0.002; log-rank test).
Fig. 3.
Cumulative survival rates according to the number of risk factors for CVDs. Cumulative survival rates according to the number of risk factors for CVDs, including PNPLA3 genotype CC, presence of chronic kidney disease (CKD), and Fibrosis-4 (FIB-4) index ≥ 2.67. The cumulative incidence rates were significantly different among the three subgroups, based on the number of risk factors (P < 0.001; Log-rank test). Subgroup with 3 risk factors was nobody in the present study
Incidence and noninvasive predictors of malignancies (except for liver cancer) in NAFLD
The characteristics of the 426 patients confirmed to have no previous or current malignancies (except for liver cancer) at NAFLD diagnosis were evaluated for the rate of development of malignancies (except for liver cancer). During the follow-up period, 32 patients (7.5%) developed malignancies (except for liver cancer). The cumulative incidence rates were 4.7% and 8.3% at the end of 5 and 10 years, respectively. The yearly incidence rates of malignancies (except for liver cancer) over the investigated 10-year period were 0.83% (Fig. 1b). Of the 32 patients, eight (25.0%) patients were diagnosed with colon cancer, five (15.6%) with lung cancer, four (12.5%) with pancreatic cancer, three (9.4%) with gastric cancer, three (9.4%) with prostate cancer, and nine (28.1%) with other cancers (Table 4). Especially, five of 19 males (26.3%) were diagnosed with lung cancer, and seven of the 13 females (53.8%) were diagnosed with colon cancer.
Table 4.
Incidence of malignancies, except for liver cancer
| Total (n = 32) | Male (n = 19) | Female (n = 13) | |
|---|---|---|---|
| Colon cancer | 8 | 1 | 7 |
| Lung cancer | 5 | 5 | 0 |
| Pancreas cancer | 4 | 3 | 1 |
| Gastric cancer | 3 | 2 | 1 |
| Prostate cancer | 3 | 3 | 0 |
| Breast cancer | 2 | 0 | 2 |
| Uterine cancer | 2 | 0 | 2 |
| Malignant lymphoma | 2 | 2 | 0 |
| Esophageal cancer | 1 | 1 | 0 |
| Renal cancer | 1 | 1 | 0 |
| Bladder cancer | 1 | 1 | 0 |
Univariate analysis identified six noninvasive parameters that correlated significantly with the incidence of malignancies except for liver cancer: (1) waist circumference, (2) previous or current liver-related events, (3) type 2 diabetes mellitus, (4) ALDH2, (5) PNPLA3, and (6) FIB-4 index. These factors were entered into multivariate analysis, which identified one noninvasive factor that significantly and independently influenced the incidence of malignancies (except for liver cancer), namely, the FIB-4 index (≥ 2.67; HR 25.2, 95% CI = 2.81–226; P = 0.004) as shown in Table 5.
Table 5.
Non-invasive predictors associated with the incidence of malignancies, except for liver cancer
| Factor | Category | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | (95% CI) | P value* | Hazard ratio | (95% CI) | P value* | ||
| FIB-4 index | < 2.67 | 1 | 1 | ||||
| ≥ 2.67 | 2.51 | (1.10–5.74) | 0.029 | 25.2 | (2.81–226) | 0.004 | |
| Waist circumference** | Small | 1 | |||||
| Large** | 5.78 | (1.09–30.3) | 0.039 | ||||
| ALDH2 rs671 | GG | 1 | |||||
| AA, AG | 5.46 | (1.82–16.4) | 0.002 | ||||
| PNPLA3 rs738409 | CC, CG | 1 | |||||
| GG | 3.64 | (1.41–9.44) | 0.008 | ||||
| Previous or current liver-related events | Absence | 1 | |||||
| Presence | 3.42 | (1.14–10.3) | 0.028 | ||||
| Type 2 diabetes mellitus | Absence | 1 | |||||
| Presence | 2.49 | (1.19–5.21) | 0.015 | ||||
*Significance was determined using the Cox proportional hazard model. Variables that were statistically significant on univariate analysis were entered into multivariate analysis to identify significant independent factors
**Large waist circumference was defined as ≥ 85 cm in men and ≥ 90 cm in women. The characteristics of the 426 patients confirmed to have no previous or current malignancies except for liver cancer at NAFLD diagnosis were evaluated for the rate of development of malignancies except for liver cancer. During the follow-up period, 32 patients developed malignancies except for liver cancer. Liver-related events included liver cancer, hepatic encephalopathy, esophago-gastric varices with bleeding, ascites, and jaundice
Incidence and noninvasive predictors of liver-related events in NAFLD
The characteristics of the 432 patients who were confirmed to have no previous or current liver-related events at NAFLD diagnosis were evaluated for the rate of development of liver-related events. During the follow-up period, 13 patients (3.0%) developed liver-related events. The cumulative incidence rates were 3.0% and 3.0% at the end of 5 and 10 years, respectively. The yearly incidence rate of liver-related events over the 10-year study period was 0.30% (Fig. 1c). Of the 13 patients, eight (61.5%) developed liver cancer, five (38.5%) esophago-gastric varices, three (23.1%) hepatic encephalopathy, one (7.7%) developed ascites, and one (7.7%) developed jaundice. Of the 13 patients, four patients had several of the above-listed conditions.
Univariate analysis identified two noninvasive parameters that correlated significantly with the incidence of liver-related events: (1) gamma-glutamyl transpeptidase and (2) FIB-4 index. These factors were entered into multivariate analysis, which identified two noninvasive factors that significantly and independently influenced the incidence of liver-related events: (1) FIB-4 index (≥ 2.67; HR 23.2, 95% CI = 5.66–95.1, P < 0.001) and (2) gamma-glutamyl transpeptidase (≥ 219 U/L; HR 5.53, 95% CI = 1.70–18.0; P = 0.004) as shown in Table 6.
Table 6.
Non-invasive predictors associated with the incidence of liver-related events
| Factor | Category | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | (95% CI) | P value* | Hazard ratio | (95% CI) | P value* | ||
| FIB-4 index | < 2.67 | 1 | 1 | ||||
| ≥ 2.67 | 17.5 | (4.49–67.8) | < 0.001 | 23.2 | (5.66–95.1) | < 0.001 | |
| Gamma-glutamyl transpeptidase | < 219 U/L | 1 | 1 | ||||
| ≥ 219 U/L | 3.45 | (1.11–10.7) | 0.032 | 5.53 | (1.70–18.0) | 0.004 | |
*Significance was determined using the Cox proportional hazard model. Variables that were statistically significant on univariate analysis were entered into multivariate analysis to identify significant independent factors. The characteristics of the 432 patients who were confirmed to have no previous or current liver-related events at NAFLD diagnosis were evaluated for the rate of development of liver-related events. During the follow-up period, 13 patients developed liver-related events. Liver-related events included liver cancer, hepatic encephalopathy, esophago-gastric varices, ascites, and jaundice
In the present study, the cumulative incidence rates of liver cancer were 1.6% and 2.3% at the end of 5 and 10 years, respectively. The yearly incidence rate of liver-related events over the 10-year study period was 0.23%. Univariate analysis did not identify noninvasive parameters that correlated significantly with the incidence of liver cancer.
Discussion
Previous reports have demonstrated that the stage of fibrosis in patients with NAFLD is one of the most important predictors for the incidence of the three complications described in this study (CVDs, malignancies [except for liver cancer], and liver-related events [10]. Our results confirmed the above results in which the multivariate analysis identified a FIB-4 index ≥ 2.67 as an advanced stage of fibrosis in addition to a significant and independent predictor of three complications. However, the present results concerning CVDs and malignancies (except for liver cancer) should be carefully interpreted due to the small number of events during the study period. FIB-4 index could be identified as the most powerful predictor of the three factors associated with fibrosis stage (platelet count), inflammation (AST, ALT), and age. The previous study was based on NAFLD patients who were diagnosed by US at a heath checkup in a tertiary hospital in Korea and demonstrated that a high FIB-4 index showed a strong association with the development of malignancies (except for liver cancer) [17]. However, a recent report based on adults in Sweden with NAFLD confirmed by biopsy indicated that a significant excess mortality risk was found across all stages of fibrosis associated with NAFLD, and this increased risk was primarily due to deaths from malignancies (except for liver cancer) [18]. Hence, the usefulness of the FIB-4 index for the prediction of the incidence of CVDs and malignancies (except for liver cancer) should be also evaluated in terms of the impact of not only the stage of fibrosis but also with respect to the age of the patient [19].
It is still unclear whether the impact of genetic factors, including the PNPLA3 genotype, might affect CVDs in patients with NAFLD. The influence of the PNPLA3 genotype on retinol metabolism might occur at the level of hepatic stellate cells, or hepatocytes lipid droplets could potentially play a role in NAFLD progression. The remodeling of specific lipids and retinol plays a pivotal role in NAFLD development and contributes to fat accumulation, inflammation, and fibrogenesis [20]. Previous Japanese report showed that it has been reported that levels of fasting plasma glucose and triglyceride were higher in patients with PNPLA3 CC type [21]. Interestingly, in the present cohort, patients with CC type significantly indicated the higher rates of type 2 diabetes mellitus and hyperuricemia than those with non-CC type, respectively (type 2 diabetes mellitus; CC 47.4% versus non-CC 33.1%, P = 0.048; Chi-squared test) (hyperuricemia; CC 24.6% versus non-CC 11.0%, P = 0.010; Chi-squared test). The present findings support that there might be the tendency for patients with CC type to have more complications related to CVDs risk. However, mendelian randomization analysis recently indicated that the PNPLA3 genetic variant might not be causally associated with the risk of CVDs. Among other genetic variants related to NAFLD, TM6SF2 appears to be protective, whereas MBOAT7 could favor venous thromboembolism [22, 23]. Furthermore, the previous Japanese report indicated that the PNPLA3 GG type might affect development of liver-related events, including liver cancer [24]. The present study showed that the PNPLA3 CC type might be one risk factor for CVD in patients with NAFLD. The differences in these results might be due to racial differences, study design, small number of events, collider bias, and differences in the impact of PNPLA3 genotype on two complications (phenotype of CVDs or liver-related events). Further studies that matched the patients’ backgrounds, including racial background or ethnicity, should be performed to investigate the impact of genetic factors on the incidence of complications in patients with NAFLD.
Our results highlight the importance of the combination of the three risk factors (PNPLA3 genotype, CKD, and FIB-4 index) for early prediction of CVDs in patients with NAFLD. Recent reports show that a high frequency of NAFLD could be observed among the patients with CKD and a possible association of NAFLD with the increase in CVDs among those patients [25]. The present study identified CKD as one risk factor for the incidence of CVDs in patients with NAFLD and agreed to this recent report [25]. The mechanism of the increase in the CVDs is still unknown in NAFLD patients with CKD. Further studies based on the large number of patients should be performed to investigate the impact of CKD on CVDs in patients with NAFLD.
Differences in malignancies (except for liver cancer) according to gender should be investigated. A previous report from Korea showed that male patients with NAFLD had a higher association of developing colon cancer and female patients developed breast cancer as malignancies other than liver cancer [17]. Overall, the present study identified the increase in colon cancer and particularly showed higher rates of lung cancer in males and colon cancer in females. The present study presents certain limitations. This discrepancy between the previous report and the present study could be due to differences in racial factors and the small number of events during the study period. Further studies with a larger number of NAFLD patients and longer follow-up periods should be performed to investigate malignancies (except for liver cancer) according to gender.
In conclusion, the present results highlight the importance of genetic factors and FIB-4 index on the incidence of complication in Japanese patients with histo-pathologically confirmed NAFLD, especially with respect to the incidence of CVDs. Early diagnosis based on the presence of one or more risk factor in addition to early treatment might improve the prognosis in patients with NAFLD. Further prospective and multi-center study based on the large number of patients should be performed to investigate the reliability of the present findings.
Conclusions
Our results highlight the importance of the PNPLA3 genotype and FIB-4 index ≥ 2.67 on the incidence of complications in Japanese patients with NAFLD, especially the incidence of CVDs.
Acknowledgements
The authors thank Drs. Keiichi Kinowaki and Takeshi Fujii (Department of Pathology, Toranomon Hospital) and also Drs. Fukuo Kondo and Toshio Fukusato (Department of Pathology, Teikyo University School of Medicine) for assistance in histopathological diagnosis.
Abbreviations
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- AUROC
Area under the receiver-operating characteristic curve
- CI
Confidence interval
- CKD
Chronic kidney disease
- CVDs
Cardiovascular diseases
- FIB-4 index
Fibrosis-4 index
- HR
Hazard ratio
- NAFLD
Non-alcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
Non-alcoholic steatohepatitis
- PPV
Positive predictive value
- NPV
Negative predictive value
Authors' contributions
NA, YK, YA, SS, SF, HS, TH, MK (Masahiro Kobayashi), MK (Mariko Kobayashi), YS, FS, KI, and HK contributed to this work. NA, YK, and YA analyzed the data. NA wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported in part by Grant-in-Aid from Japan Agency for Medical Research and Development (JP21fk0210058, JP21fk0210065, JP21fk0210073, JP21fk0210090).
Availability of data and materials
The datasets generated and/or analyzed in the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Human Ethics Review Committee at Toranomon Hospital and each patient provided a signed informed consent at the time of liver histological diagnosis. The study was conducted in compliance with the International Conference on Harmonization guidelines for Good Clinical Practice (E6) and the 2013 Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
(1) Hiromitsu Kumada has received honoraria from Gilead Sciences, AbbVie Inc., Eisai Co., Ltd, and Dainippon Sumitomo Pharma. (2) Yusuke Kawamura has received honoraria from Eisai Co., Ltd. All other authors declare no conflict of interest.
Disclaimers
This paper has not been published or presented elsewhere in part or in entirety, and is not under consideration by another journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Norio Akuta, Email: akutanorio@gmail.com.
Yusuke Kawamura, Email: k-yusuke@toranomon.gr.jp.
Yasuji Arase, Email: es9y-ars@asahi-net.or.jp.
Satoshi Saitoh, Email: sa3110@f2.dion.ne.jp.
Shunichiro Fujiyama, Email: shunichiro-fujiyama@toranomon.gr.jp.
Hitomi Sezaki, Email: hitomis@mx1.harmonix.ne.jp.
Tetsuya Hosaka, Email: hosa-p@toranomon.gr.jp.
Masahiro Kobayashi, Email: mshkobayashi@toranomon.gr.jp.
Mariko Kobayashi, Email: vj7m-kbys@asahi-net.or.jp.
Yoshiyuki Suzuki, Email: suzunari@interlink.or.jp.
Fumitaka Suzuki, Email: fumitakas@toranomon.gr.jp.
Kenji Ikeda, ikedakenji@tora.email.ne.jp.
Hiromitsu Kumada, Email: kumahiro@toranomon.gr.jp.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Williams R. Global changes in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 3.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 4.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–485. doi: 10.3748/wjg.v20.i2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akuta N, Kawamura Y, Arase Y, Saitoh S, Fujiyama S, Sezaki H, et al. Hepatocellular carcinoma is the most common liver-related complication in patients with histopathologically-confirmed NAFLD in Japan. BMC Gastroenterol. 2018;18:165. doi: 10.1186/s12876-018-0900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: Pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: Interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 10.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 12.Sinn DH, Cho SJ, Gu S, Seong D, Kang D, Kim H, et al. Persistent nonalcoholic fatty liver disease increases risk for carotid atherosclerosis. Gastroenterology. 2016;151:481–488. doi: 10.1053/j.gastro.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 14.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 15.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 16.Nagai K, Asahi K, Iseki K, Yamagata K. Estimating the prevalence of definitive chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2021 doi: 10.1007/s10157-021-02049-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2018;68:140–146. doi: 10.1016/j.jhep.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2020 doi: 10.1136/gutjnl-2020-322786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishiba H, Sumida Y, Tanaka S, Yoneda M, Hyogo H, Ono M, et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi-center study. J Gastroenterol. 2018;53:1216–1224. doi: 10.1007/s00535-018-1474-y. [DOI] [PubMed] [Google Scholar]
- 20.Pirazzi C, Valenti L, Motta BM, Pingitore P, Hedfalk K, Mancina RM, et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum Mol Genet. 2014;23:4077–4085. doi: 10.1093/hmg/ddu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotta K, Yoneda M, Hyogo H, Ochi H, Mizusawa S, Ueno T, et al. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC Med Genet. 2010;11:172. doi: 10.1186/1471-2350-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauridsen BK, Stender S, Kristensen TS, Kofoed KF, Køber L, Nordestgaard BG, et al. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J. 2018;39:385–393. doi: 10.1093/eurheartj/ehx662. [DOI] [PubMed] [Google Scholar]
- 23.Dongiovanni P, Paolini E, Corsini A, Sirtori CR, Ruscica M. Nonalcoholic fatty liver disease or metabolic dysfunction-associated fatty liver disease diagnoses and cardiovascular diseases: from epidemiology to drug approaches. Eur J Clin Invest. 2021;51:e13519. doi: 10.1111/eci.13519. [DOI] [PubMed] [Google Scholar]
- 24.Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol Res. 2017;47:1083–1092. doi: 10.1111/hepr.12840. [DOI] [PubMed] [Google Scholar]
- 25.Behairy MA, Sherief AF, Hussein HA. Prevalence of non-alcoholic fatty liver disease among patients with non-diabetic chronic kidney disease detected by transient elastography. Int Urol Nephrol. 2021 doi: 10.1007/s11255-021-02815-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in the present study are available from the corresponding author on reasonable request.