Abstract
The IL-17 family is an evolutionarily old cytokine family consisting of six members (IL-17A-F). IL-17 family cytokines signal through heterodimeric receptors that include the shared IL-17RA subunit which is widely expressed throughout the body on both hematopoietic and non-hematopoietic cells. The founding family member, IL-17A, is usually referred to as IL-17 and has received most attention for proinflammatory roles in autoimmune diseases like psoriasis. However, IL-17 is associated with a wide array of diseases with perhaps surprisingly variable pathologies. This review will focus on recent advances in the known and emerging roles of IL-17 during health and in disease pathogenesis. To decipher the functions of IL-17 in diverse disease processes it is useful to first consider the physiological functions that IL-17 contributes to health. We will then discuss how these beneficial functions can be diverted towards pathogenic amplification of deleterious pathways driving chronic disease.
Keywords: IL-17, autoimmunity, microbiota, wound healing, fibrosis, cancer
Introduction: IL-17 protects barrier surfaces
In healthy humans and mice, IL-17 expression is limited to barrier surface tissues: intestine, gingiva, conjunctiva, vaginal mucosa, skin. At these surfaces, IL-17 is produced at low amounts in response to the beneficial resident microbiota, and induces production of antimicrobial peptides by the epithelium to maintain a healthy bacterial and fungal population. IL-17 also stimulates epithelial cells to produce GCSF and chemokines that recruit neutrophils, pro-inflammatory cytokines such as IL-6, and IL-17 supports antibody production (Figure 1). By inducing sub-clinical amounts of these acute-phase responses in local tissue, homeostatic IL-17 not only helps to maintain healthy populations of microbiota, IL-17 signaling raises the epithelial antimicrobial threshold to protect against infection(1, 2). Although much emphasis has been placed on bacterial microbiota roles in barrier surfaces, it is likely that fungal residents of the ‘mycobiome’ also contribute to IL-17 homeostatic roles as demonstrated recently in mice(2–4).
Figure 1: IL-17 in health and disease overview.
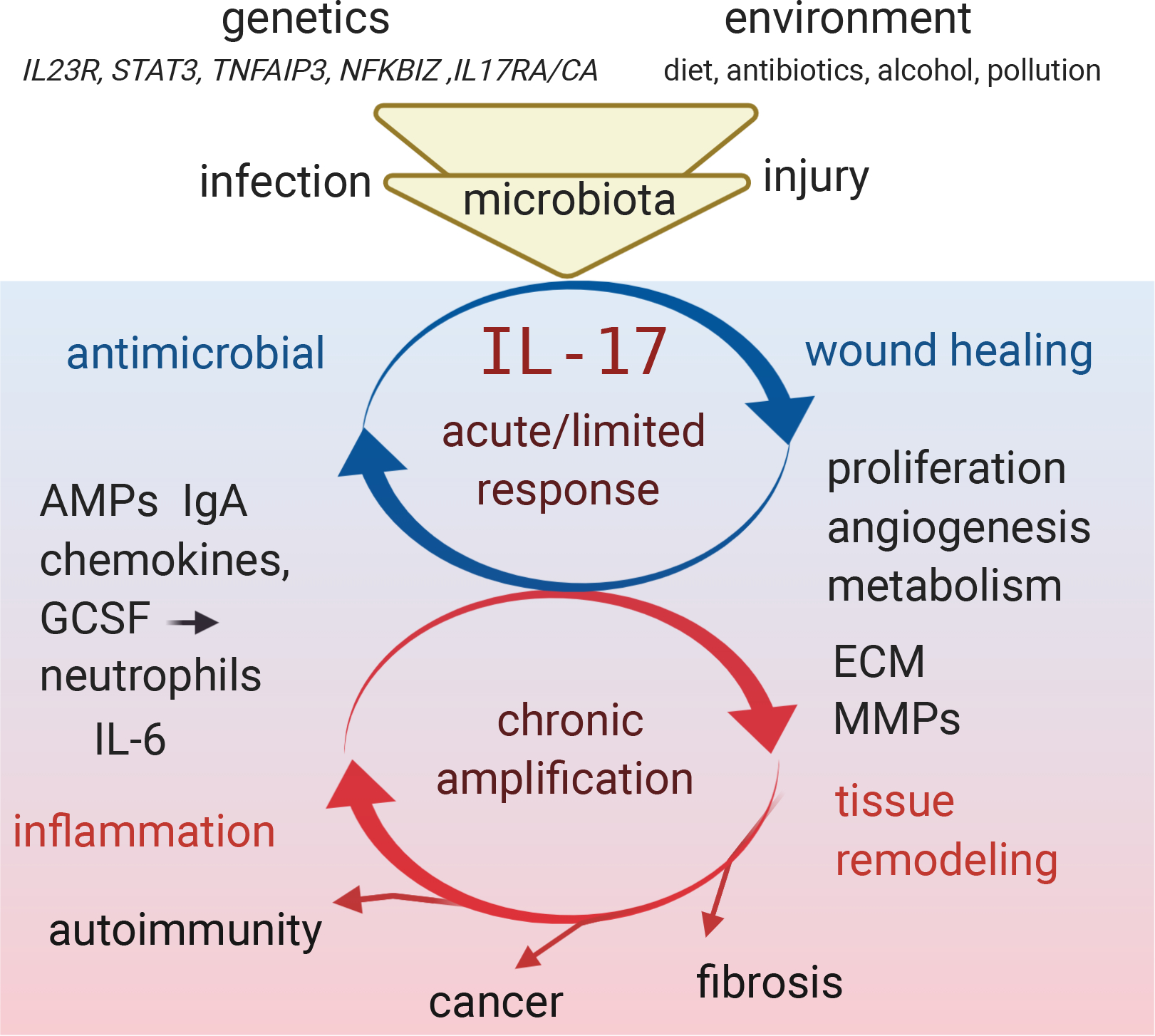
IL-17 production and signaling is multifactorially regulated by interplay between genetics, environment and resulting microbiota populations at barrier surfaces, leading to homeostatic maintenance in healthy individuals. Injury or infection increase the IL17 effector functions that drive antimicrobial effector response and promote repair of the tissue. If the IL-17 response is inappropriately amplified due to altered input from genetic and environment factors or due to chronic stimulation that occurs during autoimmunity, persistent infection or cancer, then the antimicrobial and repair functions convert towards pathologic inflammation and tissue remodeling that promote fibrosis, tumorigenesis or autoimmune disease. Figure adapted from image created with Biorender.com.
If pathogens breach epithelial barriers, then tissue damage along with increased immune activation increases the magnitude of the IL-17 response to control and clear the invasion, with accompanying signs of inflammation. Mice or humans deficient in IL-17 signaling take longer to clear infections such as Candida albicans and Staphyloccocus aureus, and develop infections that spread across a wider surface area as well as penetrating underlying tissues(5, 6). Interestingly, the Kaplan lab recently demonstrated that local IL-17 responses are activated in the non-infected tissues adjacent to Candida infection, in a process termed ‘anticipatory immunity” that acts to contain the infection and protect surrounding tissues(7).
The delicate balance between IL-17 and microbiota is most elegantly demonstrated in mice lacking IL-17 receptor specifically on intestinal epithelium: they develop intestinal dysbiosis due to outgrowth of normally IL-17-regulated bacterial strains. In turn, dysbiosis drives enhanced Th17 activation and IL-17 production in an attempt to restore balance. The consequence for the host of this enhanced mucosal IL-17 response is increased autoimmune disease severity in a model of multiple sclerosis. Indeed, it is now widely thought that dysregulation of healthy microbiota populations contributes to autoimmune disease susceptibility in humans, in part by disrupting the balance of type-17 responses in the gut that then influences systemic Th17 activation. The use of photoconvertible cell tracking in mice has shown that in some instances, Th17 cells that originate in the gut are found in inflamed peripheral tissues including kidney and joints, further supporting a role for microbiota-regulated Th17 cells in autoimmune disease(8, 9). Autoimmunity could thus be considered an unintended collateral damage outcome resulting from mucosal surface immunity.
IL-17 and lymphoid organs
A feature of chronically inflamed tissues is the generation of tertiary lymphoid organs (TLO), which are semi-organized structures resembling lymph nodes containing B and T cells. The role of TLO in autoimmune disease and cancer remains unclear, but it is thought that they help to sustain local activation of adaptive immunity that could contribute to the ongoing disease process. Both TLO and secondary lymphoid organs (lymph node and spleen) are maintained and architecturally zoned by specialized fibroblast-like stromal cells broadly termed fibroblastic reticular cells (FRC) and follicular dendritic cells (FDC). FRC produce CCL19 and IL-7 to sustain T cell zones, and FDC produce CXCL13 to recruit B cells and T follicular helper cells to form germinal centers. Chronic lung inflammation due to infection or repeated lps stimulation drives formation of TLO called inducible bronchial associated lymphoid tissue (iBALT) in an IL-17-dependent manner(10, 11). IL-17 induces chemokines CXCL13 and CCL19 to recruit lymphocytes to iBALT, implicating FRC involvement in establishing these structures(10, 11). IL-17 is also required for formation of TLO in meninges in a mouse model of multiple sclerosis by driving the expansion and differentiation of meningeal myofibroblasts into FRC-like cells(12, 13) (Figure 2).
Figure 2: IL-17 activates lymphoid structure stromal cells.
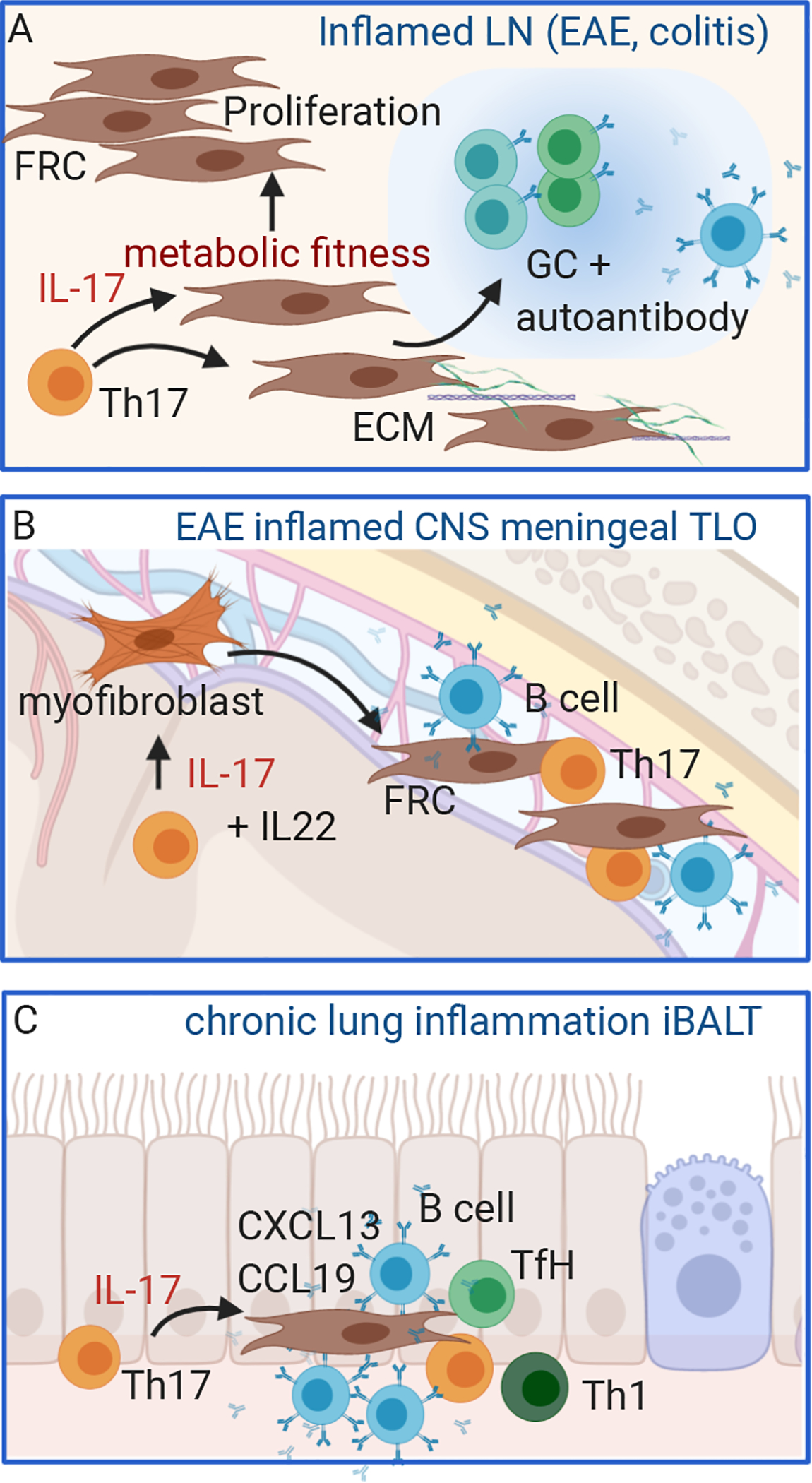
A. During Th17-driving immune responses, fibroblastic reticular cells proliferate in inflamed draining LN, and IL-17 is required for increased metabolic fitness that promotes FRC survival and ECM production and optimizes B cell germinal center activation. B: During experimental autoimmune encephalomyelitis (EAE), IL-17 promotes development of tertiary lymphoid organ (TLO) structures by activating meningeal myofibroblasts differentiation to FRC-like stromal cells. C: IL-17 promotes inflammatory bronchial alveolar lymphoid tissue, a form of TLO, in chronically inflamed lung tissue by inducing chemokines that recruit T and B cells to support anti-bacterial Th1 responses and antibody production. Figure adapted from image created with Biorender.com.
Lymphoid tissue inducer (LTi) cells are similar to ILC3 and require RORγt for their ability to establish secondary lymphoid organs during fetal development. However, LTi produce lymphotoxin for this function, and mice deficient in IL-17 do not have obvious defects in their secondary lymphoid structures, with normal size LN and spleen. However, during adaptive immune responses the size of the LN increases, with an accompanying increase in numbers of supporting FRC cells. It was recently established that this expansion of the existing FRC population does require IL-17 signaling, at least during type-17 driving inflammatory responses. When IL-17 receptor was specifically deleted in FRC cells, they failed to expand following immunization or colitis despite hypercellularity of the inflamed LN occurring as normal. While IL-17 is known to drive proliferation of epithelial cells, this study indicated that IL-17 supports proliferation and survival of activated stromal cells by increasing their metabolic fitness, a role that was not previously known for IL-17 but has interesting connotations in terms of regulating IL-17 driven inflammatory outcomes in other organs. On a related note, IL-17 has been shown to regulate metabolic thermogenesis in adipose tissues(14).
Similarly to peripheral inflamed tissues, IL-17 recruits neutrophils to the LN during Th17 responses(15), which can provide an additional source of IL-1β for Th17 differentiation(16). However, it appears that FRC are not the predominant cell type that recruits neutrophils suggesting that other stromal cells also respond to IL-17 and influence the LN response(15). The consequence of failed FRC expansion in absence of IL-17 was instead reduced germinal center B cells and impaired autoantibody production(15). IL-17 cells have previously been shown to support antibody production in autoimmunity and infection and proposed to act directly on B cells(17, 18),(19, 20). It has also been suggested that Th17 cells convert to T follicular helper cells in Peyer’s patches to support IgA production against commensals and following oral immunization(19). This new data provides an alternative explanation by demonstrating that IL-17 can promote antibody responses via signaling to LN stromal cells to act as an intermediary between Th17 and B cells recognizing autoantigen. Whether the same principal applies to infections that require antibody and to Th1 or Th2 dominated immune responses has yet to be determined. However, as most autoimmune diseases are associated with both IL-17 and autoantibody (etiher as diagnostic or pathogenic markers), this link between IL-17 responses and enhanced autoantibody responses is intriguing.
Pathogenic versus homeostatic functions of IL-17: consider the source
IL-17 is predominantly produced by immune cells of the adaptive and innate lymphocyte lineages, including CD4+ Th17 cells, CD8+ Tc17 cells, γδT17 cells, MAIT cells, innate lymphoid cells ILC3, collectively the cells producing IL-17 are called ‘type-17’ hereafter. Commensal-driven type 17 immune responses tend to regulate microbiota without causing classical signs of inflammation, and instead promote healing of skin wounds and enhanced defense against invading pathogens(1). This is in contrast to now well-known inflammatory roles of IL-17-producing cells in autoimmune disease pathogenesis and the proposed roles in cancer and fibrosis discussed below. How are these pleiotropic functions of IL-17 achieved to cause different outcomes? Here we describe three main mechanisms: synergy at the responder cell level, feed-forward loops, and finally regulation of co-expressed cytokines at the producing cell level. The common theme is that IL-17 is rarely a lone driver, but rather acts to modulate and amplify signals in a local and context-dependent fashion.
One important aspect of IL-17 signaling is that it heavily relies on synergy with other cytokines for output. In fact, IL-17 by itself is a rather weak activator of signaling proteins and downstream gene expression. Instead, IL-17 synergizes with many cytokines from obvious pro-inflammatory cytokines such as TNF and IFNγ to seemingly anti-inflammatory TGFβ, and can also promote LPS signaling through TLR4. In many instances, the mechanisms through which this synergy is achieved have not been established. IL-17 signaling has been recently reviewed in detailed so we refer the reader to ref (21) and only briefly discuss mechanisms of synergy here. IL-17 and TNF synergy has been most intensively studied and includes induction and/or activation of RNA binding proteins that act to stabilize and promote translation of target mRNA transcripts, and induction of transcriptional regulators Iκbζ and C/EBP that further enhance cytokine receptor signaling outputs(21). For example, Iκbζ co-activates NF-κb for gene transcription of IL-6. Iκbζ expression is induced by IL-17 but not TNF(22), while TNF is a strong activator of NF-κb compared to TNF, hence the result of both is synergistic increase in Iκbζ-regulated targets(22–24). IL-17 also enhances expression of cytokines that can then act on the responder cells in a feed-forward loop. An example is the induction of LIF in synovial fibroblasts that then acts to enhance and sustain IL-6 expression(25).
Another mechanism that has emerged as a key modulator of IL-17 effects is at the level of the cells that produce IL-17. Skin-resident Tc17 cells induced by the commensal S. epidermidis produce IL-17 along with immunoregulatory and tissue repair factors, including IL-10, TGFβ, FGF, amphiregulin and VEGF(1). In addition, they are poised for co-production of type-II cytokines depending on the tissue cytokine milieu, with IL-18 identified as a switch towards type-II(26). In this context, it is interesting that γδT17 and Th17 cells express IL-18R, but whether IL-18 alters the pro-inflammatory versus reparative bias of these cells is currently unknown. However, the balance of cytokines that activate Th17 cells is thought to be one factor driving a more pro-inflammatory (IL-23 and IL-1β driven) versus non-pathogenic Th17 phenotype (TGFβ driven)(27, 28). Human Th17 cells can be induced in vitro against C albicans or Staphylococcus aureus, both opportunistic pathogens known to elicit protective Th17 responses. IL-23, IL-6 and IL-1β were both required, but concentration of IL-1β was identified as a switch that could inhibit IL-10 production while promoting IFNγ(29). A recent studied compared gene expression signatures of intestinal Th17 cells induced in response to infection with the commensal SFB or the pathogen Citrobacter rodentium, both attaching-effacing bacteria but with different outcomes in terms of clearance and inflammation(30). Commensal-induced Th17 cells co-expressed IL-10 and IL-22 along with IL-17, while pathogen-induced Th17 cells showed an increased propensity for plasticity towards Th1 phenotype, increased pathogenic Th17 signature and metabolic activity suggesting greater activation and proliferation(30). An independent study has verified that commensal-driven gut Th17 cells express IL-17, IL-22 and IL-10 and further identified that they are uniquely dependent on DC expression of the C-type lectin receptor Mincle, which induces IL-6 and IL-23 expression(31).
An interesting component of type-17 cell activation at barrier surfaces is the quite limited reliance on dendritic cell activation through classical pathways that drive DC activation and costimulation expression for T cell activation (Figure 3). IL-6 is produced by non-hematopoietic cells in response to mechanical stress and to cytokines including IL-17 itself(32). Inflammasome activation by damaged cell death releases IL-1β. Epithelial cells can detect the pathogenic determinant Candidalysin and produce IL-1β in response to oral Candida infection(33). In the skin, cutaneous sensory neurons detect Candida albicans hyphal invasion and promote dendritic cell production of IL-23 through release of the neuropeptide CGRP(7). Attaching-effacing bacteria in the gut induce epithelial serum amyloid A production that then drives IL-23 and IL-1β production in DC(34). For ILCs and γδT cells, major producers of IL-17 which do not express classical T cell receptors, cytokines are the critical drivers of their proliferation and effector functions in tissues(35–37). Tc17 cells responding to skin commensals are activated through non-classical MHC Ib as well as cytokines(1). We recently described that STAT3 activation, downstream of IL-6 and IL-23 signaling, licenses effector Th17 cells to respond to antigen by maintaining mitochondrial membrane potential (Poholek et al, JEM 2020 In Press). This again suggests that the cytokine milieu is a strong regulator of IL-17 production even in antigen-specific Th17 cells. In addition, human Th17 cells do not require and in fact are inhibited by CD28 costimulation, unlike Th1 cells(38). Instead, IL-23 and IL-1β provide activation signals including metabolic reprogramming normally associated with CD28, albeit at a lower magnitude with correspondingly reduced proliferation(38). Hence, we propose that the cytokine conditions present in healthy barrier tissues promote the preferential induction of small populations of metabolically inert Th17 cells that in turn regulate barrier surface immunity without inducing overt inflammation. However, type-17 cells are poised to rapidly expand and increase their pro-inflammatory functions in case of tissue injury or pathogen invasion by sensing changes in cytokine composition.
Figure 3: Atypical signals induce IL-17 promoting cytokines IL-23, IL-1β and IL-6 at barrier surfaces.
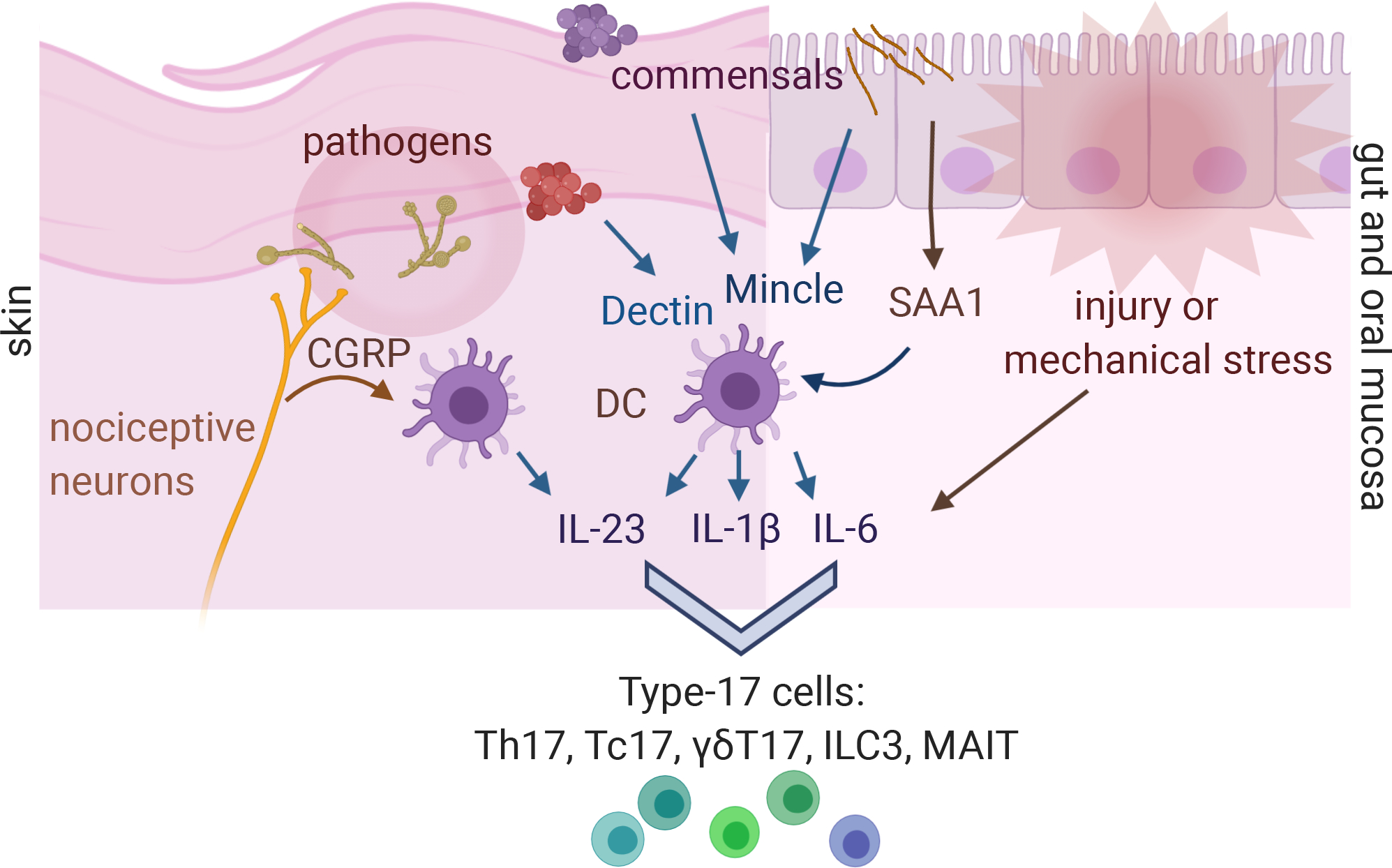
Cutaneous neurons detect Candida albicans hyphal invasion and release CGRP to promote dendritic cell production of IL-23. Both commensals and pathogens that activate type-17 responses preferentially activate dendritic cells through C-type lectin receptors including Mincle. Attaching-effacing commensal bacteria drive production of the acute phase protein serum amyloid A by gut epithelia to activate DC. IL-6 is produced by non-hematopoietic cells in response to mechanical stress such as chewing hard food, and to cytokines including IL-17 itself in a positive feedback loop, and damaged and dying cells release IL-1β. Figure adapted from image created with Biorender.com.
IL-17 goes viral?
IL-17 is most critical for control of extracellular bacteria and fungi, as evidenced by the high susceptibility to these pathogens in humans with genetic mutations affecting the IL-17 pathway(6). Indeed, the well-established roles of IL-17 in promoting production of AMPs and recruitment of neutrophils are well-suited to controlling these types of infections. However, important contributions of IL-17 signaling have been found during infections with various viruses and intracellular bacteria. In mouse models, IL-17 has been reported to promote cytotoxic T cell function against West Nile virus, and to promote recruitment of CD8+ cytotoxic T cells to the liver during acute hepatitis (39),(40). During lung infection with Mycobacterium tuberculosis, early IL-17 supports the Th1 response by induction of chemokines that enhance recruitment to the site of infection(41). IL-17 has also been reported to promote the antibody response during H5N1 influenza infection by recruiting B cells to the lungs(42),(43). Similarly, CD4+ tissue-resident memory Th1 cells are recruited and maintained in the vaginal mucosa after HSV-2 infection in an IL-17-dependent manner, and IL-17−/− mice are highly susceptible to reinfection(44). Hence induction of chemokines to aid in positioning of the anti-viral immune response to the site of infection can be a beneficial function of IL-17 that is likely induced by viral tissue damage.
On the other hand, recruitment of immune cells and induction of pro-inflammatory cytokines, particularly neutrophils and IL-6, can have detrimental effects in an already-injured tissue(45). In a recent study with pediatric patients, the authors found that IL-17 production is significantly increased in the bronchoalveolar lavages (BAL) of children with community-acquired pneumonia (CAP)(46). Profiling of immune cells identifies MAIT cells to be the major producers of IL-17 in BAL. Along with IL-22, IL-23 and IL-6, levels of IL-17 correlated to the CAP severity (46). IL-17 levels were found to increase in patients with severe pandemic Influenza A H5N1 associated disease, and neutralizing IL-17 in a mouse model of H1N1 reduced lung injury (47). Similarly, infants with severe Respiratory Syncytial virus (RSV) infection had increased IL-17 and IL-6 in their BAL, and mouse models show that IL-17-mediated CXCl1 and MMP expression in the airways leads to increased neutrophil accumulation and amplified lung tissue destruction(48),(49). Overall then, increased IL-17 appears to have negative consequences in viral lung disease, contributing to increased pathology in damaged lungs.
The most extreme version of inflammatory lung damage results in acute respiratory distress syndrome (ARDS), where the lungs fill with debris, immune cells and mucus impeding their ability to perform oxygen exchange. The current COVID-19 coronavirus pandemic has dramatically illustrated the life or death consequences of an overactive cytokine response, as around 10–20% of confirmed cases require hospitalization and oxygen support in the second phase of the disease if the virus triggers ARDS. Another clinical feature of Covid-19 induced lung damage has been the extensive fibrotic changes that further compromise respiration and may have long-term consequences for surviving patients. Although we are still in the preliminary stages of understanding the pathology associated with Covid-19 induced ARDS, IL-17 and it’s downstream intermediary IL-6 have been proposed as drivers of immunopathology and at least one of the ongoing clinical trials in China is testing the potential role of ixekizumab (a neutralizing IL-17A antibody used for psoriasis) against SARS-CoV-2(50–52).
When tissue repair goes wrong: cancer, fibrosis, autoimmunity
Following injury, IL-17 plays dual roles in protecting the host, both protecting against microbes that invade the breached barrier and promoting healing. Chronic injury for example in persistent infection or autoimmune attack can lead to prolonged attempts at repair that become pathologic (Figure 4). There is now a multitude of evidence pointing towards a pro-tumorigenic role for IL-17 in human cancer (Table 1a), although a few studies point towards protective effects (Table 1b). Similarly, IL-17 is clearly associated with pathologic processes in autoimmune diseases and fibrotic disease. Here we will discuss the role of IL-17 in beneficial reparative processes and how those become pathogenic during chronic stimulation and tissue injury.
Figure 4: Pleotropic IL-17 regulates fibrosis, cancer development and wound healing:
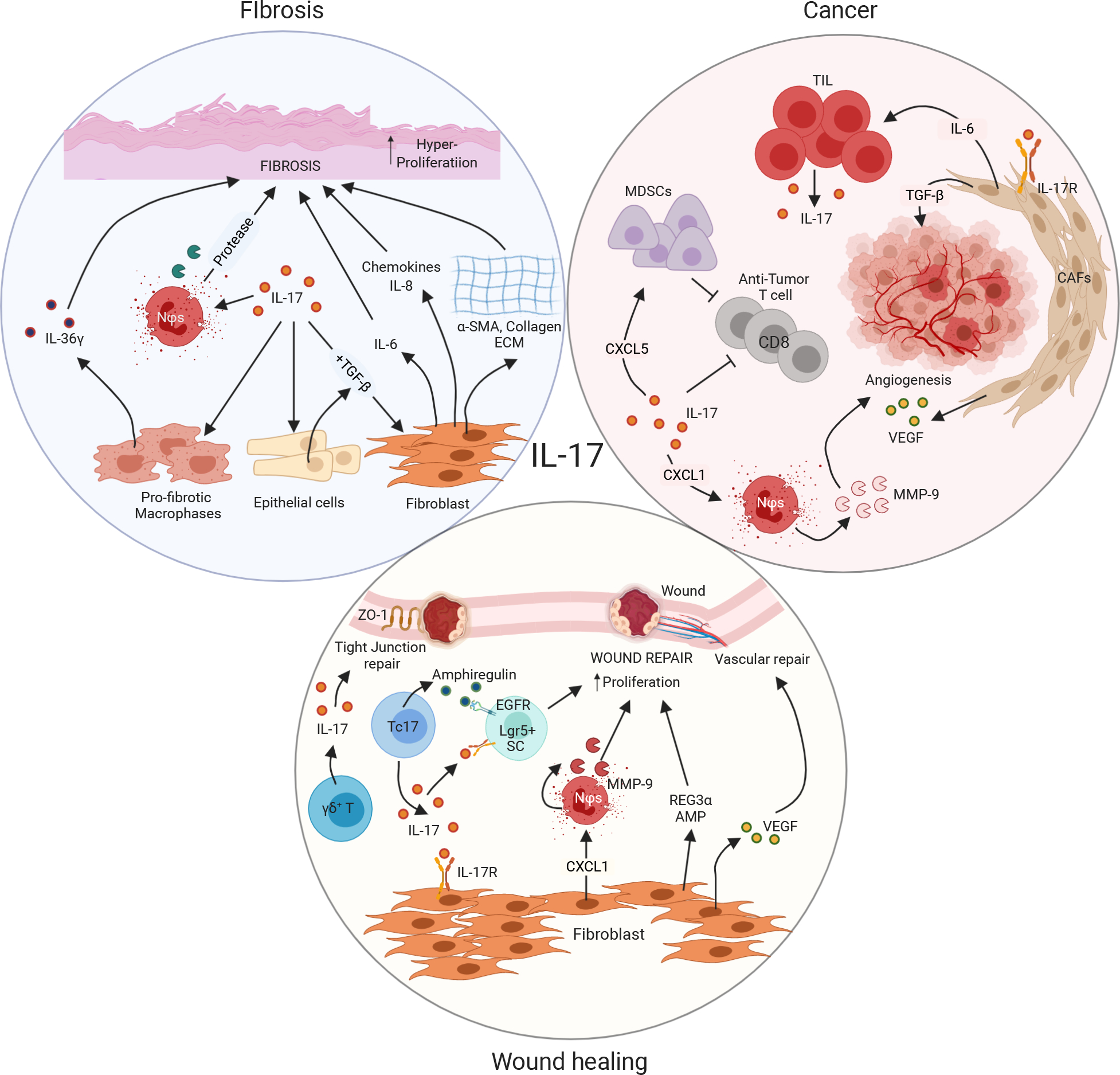
(A) IL-17 promotes fibrosis by acting on fibroblasts, epithelial cells and pro-fibrotic macrophages. IL-17 signals on epithelial cells to secrete profibrotic TGF-β. TGF-β along with IL-17 act on fibroblasts to promote IL-6 production which has pro-fibrotic functions. Other than IL-6, Fibroblasts in presence of IL-17 signaling also secrete chemokines such as IL-8 and CXCL1. CXCL1 then recruits neutrophils, which synthesizes matrix metalloproteinases (MMP) critical for fibrosis development. Besides fibroblasts in presence of IL-17 also produce α-SMA, collagen and ECM proteins necessary for fibrosis. Similarly, IL-17 acting on macrophages produce IL-36g which is important for the generation of fibrosis. (B) Besides, directly promoting tumor formation, IL-17 mainly acts on cancers associated fibroblasts (CAFs) to manifest its pro-tumor functions. IL-17 signaling in CAFs generates VEGF, IL-6 and chemokines all of which has critical protumor roles. VEGF is important for angiogenesis, one of the hallmarks of cancer. IL-6 can directly act of tumor infiltrating lymphocytes to produce more IL-17. CXCL1 on the other hand recruits’ neutrophils to synthesize MMP9 important for angiogenesis. Moreover, IL-17 can block anti-tumor CD8 T cells, critical for fighting cancers, either directly or though CXCL5 driven myeloid derived suppressor cells (MDSCs.) (C) IL-17 function is indispensable for wound healing. IL-17 derived from either conventional CD4+ T cells, γδ+ T cells or CD8+ T cells (Tc17) plays critical role in repairing wounds. IL-17 acting on fibroblasts generate VEGF, CXCL1, REG3α all critical for wound healing. VEGF plays an important role in vascular repair following wound formation. CXCL1 again is important for the recruitment of neutrophils secreting MMP-9, critical for wound repair. Lgr5+ stem cells are also important for wound repair. Synergistic signaling of IL-17R and EGFR on these stem cells are critical for wound repair following injury. Beside IL-17 secretion, Tc17 cells also produce amphiregulin, an important protein for wound repair. Moreover, several studies reported that IL-17 secreted from γδ+ T cells is critical for repairing tight junction proteins such as ZO-1 following DSS mediated gut injuries. Figure adapted from image created with Biorender.com.
Table 1a:
Pro-tumorigenic roles of IL-17
| Species | Cancer Type | Major Findings/Mechanisms |
|---|---|---|
| Mouse | Colorectal cancer | Barrier disruption by microbial products trigger tumor-elicited inflammation, which in turn drives tumor growth (106) |
| Human | Advanced colorectal cancer | Metastatic disease was associated with elevated Th17-associated cytokines such as IL-23, IL-17F in both colonic tissue and circulation (107) |
| Mouse | Colorectal cancer | IL-17RA signals directly within transformed colonic epithelial cells (enterocytes) to promote early tumor development via an ERK, p38 MAPK and NF-κB signaling pathway(108) |
| Human | Advanced-stage colorectal cancer | Th17 cells Inhibit CD8+ T Cell Migration by downregulation of CXCR3 expression via IL-17A/STAT3 axis. (109) |
| Human | Colorectal cancer | Patients with lower IL-17 levels have increased survival of 5 years. (110) |
| Mouse | Sporadic colorectal cancer | Tumor-prone mice colonized with onco-toxin producing bacteria showed increased IL-17 in the colon and DNA damage in colonic epithelium with faster tumor onset and greater mortality.(111) |
| Mouse | Colon cancer | Damage to intestinal epithelium activates IL-17A signaling in PLET1 cells leading to aberrant wound healing favoring tumor growth. (54) |
| Mouse | Colon cancer | IL-17 targets colonic epithelial cells (CECs) to promote ETBF mediated carcinogenesis via NF-kb signaling triggering CXC chemokine to drive pro-tumoral neutrophil infiltration to distal colon. (62) |
| Mouse | Multiple myeloma | Gavaging tumor-prone mice with P. heparinolytica promotes differentiation of Th17 cells in gut and migration to bone marrow favoring multiple myeloma growth. (112) |
| Mouse | Skin cancer | IL-23 required for spontaneous skin tumors(113). Damage to skin activates IL-17A signaling in Lrig1+ stem cells leading to aberrant wound healing favoring tumor growth. (53) |
| Mouse | Liver cancer | IL-17A induced CXCL5 production by tumor cells enhance the infiltration of myeloid-derived suppressor cells thereby reducing anti-tumor immunity.(56) |
| Mouse | Lung cancer | IL-17A weakens the antitumor immunity by inhibiting apoptosis of MDSCs.(57) |
| Human | Gastric cancer | IL-17A from CD8+T cells regulates the influx of MDSCs to the tumor site via a CXCL12-CXCR4 axis to mitigate anti-tumor CD8+ T cell functions(60) |
| Human | Gastric cancer | Both IL-17A (rs2275913) and IL-17F (rs763780) polymorphisms significantly increase gastric cancer risk. (114, 115) |
| Mouse | Breast cancer | IL-17A from γδ+T cells induces the infiltration of neutrophils to suppress the CD8+ T cells function and promote metastasis.(59) |
| Mouse | Lung cancer | Commensal bacteria drive IL-17 production from γδ+ T cells to promote neutrophil infiltration and tumor cell proliferation. (61) |
| Mouse | Non-Small-Cell Lung Cancer | lL-17 drives angiogenesis by stimulating VEGF production of cancer cells via STAT3/GIV signaling. (64) |
| Human | Gall bladder cancer | IL-17 producing γδ+T cells drive VEGF production to promote blood-vessel formation (66) |
| Human | Gastric cancer | IL-17 producing neutrophils drive MMP-9 production to promote angiogenesis and tumor growth. (116) |
| Mouse | Liver Cancer, Pancreatic Cancer | IL-17 promotes chemokine signaling driven angiogenesis (68, 69) |
Table 1b:
Anti-tumor roles of IL-17
| Species | Cancer Type | Mechanism/s |
|---|---|---|
| Human | Cervical adenocarcinoma | Increased number of IL-17+ cells in patients were significantly correlated with the absence of vaso-invasion, less infiltration depths and smaller tumor growths. (117) |
| Mouse | fibrosarcoma | IL-17-overexpresssion drives upregulation of MHC I and II, thereby making fibrosarcoma cells increasingly susceptible to anti-tumor T cells. (118) |
| Human | Esophageal cancer | IL-17 drives chemokine production from the tumors, leading to the infiltration of cytotoxic neutrophils, CD8+CTLs and dendritic cells resulting in better tumor control and patient survival. (119, 120) |
| Mouse | Lung Cancer | IL-17 controls tumor growth and metastasis by enhancing the cytotoxic potential of anti-tumor CD8+ T cells (121), and by increasing IFN-γ production from anti-tumor T cells and NK cells. (122) |
| Mouse | Breast Cancer | IL-17 inhibits the accumulation of MDSCs in tumor microenvironment by suppressing their proliferation and triggering apoptosis. (123) |
| Human | Colorectal cancer | Individuals with higher IL-17 expression exhibited better disease control and survival which is linked to increased infiltration of cytotoxic CD15+ neutrophils (124). |
Following skin wounding, mice that are deficient in IL-17 have delayed wound closure. In the gut, IL-17 promotes epithelial repair following injury by promoting increased proliferation of epithelial stem cells to replace the damaged cells and enhancing the restoration of an effective barrier with expression of tight junction proteins that prevent microbial translocation from the gut. IL-17 neutralizing biologics are highly effective in treating psoriasis, supporting the pro-inflammatory and pro-proliferative roles of IL-17 in the skin. However, the same drugs were disappointingly ineffective in Crohn’s disease and exacerbated disease in some patients, suggesting that on balance the beneficial roles of IL-17 in microbial homeostasis and repair of the gut outweigh the contributions to pathologic inflammation.
Pathologic proliferation of synovial fibroblasts also contributes to rheumatoid arthritis, where it is thought that IL-17 may contribute perhaps in the earlier phases of disease since IL-17 targeting biologic therapy was only effective in a subset of patients with established RA. Another potential pathology that can result from excess proliferation is the depletion of precursor stem cells that ultimately contributes to failed repair of inflamed tissue. In a model of multiple sclerosis, hyperproliferation of oligodendrocyte precursors responding to IL-17 has been proposed to increase their death, thus contributing to oligodendrocyte decline and increased demyelination in the central nervous system.
In spontaneous tumorigenesis models that combine tissue damage with a carcinogenic stimulus, IL-17 promotes increased proliferation of epithelial stem cells in response to tissue injury. Recently, a novel IL-17A-activated EGFR signaling pathway was discovered that drives the expansion and migration of Lrig1+ stem cells during skin injury, leading to skin tumorigenesis and suggesting repeated injuries can promote dysregulated IL-17-dependent wound repair leading to neoplastic growth (53). Similarly, another study from the same group, demonstrates that inhibition of IL-17 in a mouse gut-injury model of colitis resulted in restricted tumor growth (54).
Tissue injury rapidly recruits neutrophils for microbial control and debris clearance(55), and as already discussed IL-17 is a major recruiter of these cells during sustained inflammation. In cancer, myeloid-derived suppressor cells (MDSCs) and neutrophils are two important myeloid cells often found in the tumor microenvironment. IL-17 can recruit suppressive MDSCs and neutrophils that inhibit cytotoxic T cells and produce matrix metalloproteases (MMPs) to enhance metastasis of cancer cells (56–58).(59).(60). (61, 62). While IL-17 is primarily considered to act through non-hematopoeitic cells, it is worth noting a recent single cell analysis of foreign body induced fibrosis identified a pro-fibrotic macrophage subtype that expresses both IL-17 receptor subunits and responds to IL-17 by producing IL-36γ, an IL-1 family member also highly expressed by psoriatic keratinocytes. This suggests that tissue-resident macrophages could be subverted along with stromal cells towards an IL-17-responsive pro-fibrotic phenotype under chronic stimulation conditions.
Tissue growth and repair is an energy-intensive process. A recent immune-profiling study of more than 1000 breast cancer patients in the Cancer Genome Atlas project demonstrated that the cohort with highly glycolytic breast cancers were linked with lower infiltration of tumor killing cells, higher expression of checkpoint inhibitors such as PDL1 and poor prognosis. Interestingly, the top upregulated pathway in this group of patients is the IL-17 signaling pathway, further linking IL-17 with pro-tumorigenic functions (63). We recently demonstrated that IL-17 signaling has profound effects on lymph node stromal cell metabolism, boosting glucose uptake, glycolysis and oxidative phosphorylation(15). IL-17 receptor deficient fibroblastic reticular cells had very low spare respiratory capacity, displayed signs of nutrient stress and underwent increased apoptosis in vivo(15). We speculate that metabolic changes driven by IL-17 signaling through Iκbζ and NF-κb could also enhance the proliferation and survival of CAFS, or indeed tumor cells, though this has yet to be tested.
In addition to replacement of damaged cells, one of the critical aspects of wound healing is vascular repair to provide nutrients to the recovering organ, and this often requires angiogenesis (formation of new blood vessels). IL-17 drives the production of vascular endothelial growth factor (VEGF) from epithelial and fibroblastic cells to stimulate angiogenesis, as observed in the highly vascularized red areas underlying psoriasis lesions. Fast-growing tumors require rapid vascularization in order to avoid necrosis, and one of the major pro-tumorigenic roles of IL-17 likely depends on these pro-angiogenic properties(64) (65),(66), (67, 68) (69).
Stromal cells, or fibroblast-like cells, produce and organize extracellular matrix components (ECM) to provide structural support of organs. In addition, stromal cells produce growth factors to promote the function of adjacent cells that are tissue-specific, and it is increasingly appreciated that they exist as heterogeneous and specialized functional subsets within a tissue and between organs. During wound healing, local fibroblasts provide a scaffold for migrating epithelium to help close the wound, and produce ECM with a balance of proteases to produce an organized scar that is as close to the original tissue as possible. Extensive or inappropriate production of ECM and scar formation, particularly over an extended period of time as occurs with chronic injury due to autoimmunity, infection or cancer ultimately results in dysfunction. During autoimmune attack of the CNS, astrocytes contribute to glial scarring in MS plaques. IL-17 certainly activates astrocytes to promote chemokine and inflammatory cytokine production in the mouse model of MS(70), but roles in aberrant astrocyte scar formation have not been investigated.
Systemic sclerosis (SSc) is an autoimmune connective tissue disease in which excess fibroblast proliferation and activation causes fibrosis, most commonly in the skin leading to decreased pliability and movement around joints that can be disabling. Even more severe morbidity and mortality occurs in SSc patients experiencing fibrosis of internal organs especially lungs, who ultimately require transplant for survival. Fibrotic tissues have signatures of inflammatory cytokines including IL-6 and IL-17, but with high expression of TGFβ considered the major driver of ECM production(71). Mice deficient in IL-17 are resistant to bleomycin-induced lung fibrosis(72). IL-6 is a major target of IL-17 signaling in almost every cell type tested to date, and IL17 has also been reported to enhance production of TGFβ in human lung alveolar epithelial cells(73). It is interesting to note that Th17 cells themselves express TGFβ, which has been verified to act in an autocrine manner in mice(74). In both healthy and SSc dermal fibroblasts, IL-17 synergized with TGFβ to increase IL-6 production by approximately 100 fold compared to either cytokine alone(75). The authors in this study make the important point that fibroblasts do not express IL-6R and so rely on IL-6 trans-signaling through soluble IL-6R produced by other cells in the tissue in order to display the pro-fibrotic effects of IL-6: this is something that needs to be considered for the many in vitro studies of human fibroblast cells in which IL-17 function is assessed. Nevertheless, this study also revealed a potentially interesting dichotomy in which IL-17 synergized with TGFβ for IL-6 production but inhibited TGFb-induced ECM production (in absence of IL-6 signaling), further emphasizing the complexity of cytokine interactions in fibrosis(75).
Patients with chronic viral hepatitis are at risk for developing fibrotic liver disease (cirrhosis) as well as liver cancer. Increased intrahepatic IL-17A and IL-22 at biopsy is considered a signature of advanced liver fibrosis with worse prognosis (76), (77). Hepatic stellate cells are the major driver of liver fibrosis, and IL-17 has been shown to drive collagen formation by stellate cells, in part by increasing the expression of receptor for TGFβ (78). Similarly, we found that IL-17 enhanced expression of genes encoding collagen and fibronectin in lymph node stromal cells that were pre-activated in vivo by immunization, as well as promoting their proliferation(15). During chronic inflammation of LN, for example in HIV patients or third world residents experiencing frequent infections, fibrosis of the LN itself leads to reduced T cell survival and reduced response to vaccination(79, 80),(81). We speculate that repeated infections or exposure to gut microbes as occurs in ‘leaky gut’ of HIV patients could promote LN fibrosis through increased inflammation and locally induced IL-17 signaling. However, it is the local loss of gut-resident IL-17 producing T cells that is thought to lead to increased ‘leakiness’ due to reduced tight junction proteins in HIV and the non-human primate model SIV (82),(83).(84). As a side note, there is another interesting connection between HIV and IL-17: Human Th17 cells express receptors important for HIV entry and have been found to preferentially produce higher viral capsid proteins due to reduced expression of RNAse A, an important enzyme which limits HIV replication (85). Hence, a fraction of Th17 cells act as a reservoir to allow HIV persistence despite antiretroviral therapy (86).
Tumor stromal architecture not only guides initial tumor growth, but controls all stages of cancer progression by dynamically interacting with tumor cells and the immune system (87). Cancer associated fibroblasts (CAF) are increasingly appreciated for their role in limiting access and function of cytotoxic T cells into tumors, along with architectural support of invading cancer cells. It is still unclear exactly how CAFs promote immune evasion by tumors. One mechanism could be production and organization of ECM including collagen to ‘wall off’ the tumor. TGFβ is a major driver of fibrosis as well as inhibitor of cytotoxic T cells. TGFβ-driven CAFs are a key indicator of non-responsiveness to anti-PD-L1 therapy in cancer patients, and it was noted that these tumors also more frequently had T cells that were trapped in the surrounding collagen-rich fibroblast zones(88, 89). CAF-derived IL-6 can promote IL-17 production by tumor-infiltrating T cells(90). As IL-17 drives and enhances IL-6 and TGFβ production, it is highly probable that IL-17 can also act on CAFs as a feed-forward loop to modulate their proliferation and function during cancer progression, thereby controlling the disease outcome.
Future horizons
IL-17 has now been associated with immunopathologies beyond classic inflammatory autoimmune disease, and mouse models support a functional role, but in many cases the definitive test in clinical trials has not been done. From the experience targeting IL-17 in autoimmune disease, it appears that two components may be critical to evaluate the likelihood of success of anti-IL-17 therapy: 1) understanding whether IL-17 is an initiator, driver or amplifier of the disease, and 2) determining whether IL-17 is contributing any important benefit that may outweigh the pathological contribution as is now thought for Crohn’s disease (91). In many cases, it seems that targeting IL-17 as an adjunct therapy could improve the success of stand-alone therapies. An example would be in fibrosis where IL-17 appears to enhance the pro-fibrotic effects of TGFβ. Current evidence suggests that adjunct blockade of IL17 could improve immunotherapy and reduce chemoresistance in cancer. Studies of anti-PD-1 therapy response identified an increased IL-17 gene signature in colorectal cancer patient non-responders and increased Th17 cell frequency in melanoma patient non-responders(92),(93). The potential to improve the autoimmune disease that can occur as a side effect to check point inhibitors is another attractive benefit of neutralizing IL-17 in these patients. It has also been suggested that IL-17 may promote development of cisplatin resistance in colorectal cancer (94). Given the mixed results of IL-17 neutralizing therapies in autoimmune diseases, determination of appropriate biomarkers to identify cancers in which IL-17 is a driver of disease progression is critical.
An exciting new frontier in IL-17 biology is in the growing field of neuro-immune interactions. Several lines of evidence already demonstrate that IL-17 is involved in neural-immune circuits that can affect inflammatory disease. Skin neurons promote local IL-17 production to increase psoriatic or pathogen-induced inflammation(95, 96). IL-17 and IL-17 inucing gut microbiota contribute to degree of lesion severity following ischemic stroke (97). Conversely, by regulating gut microbiota, IL-17 can alter systemic microbial products that are increasingly thought to affect mental health, and Th17 cells were increased and promoted depression-like symptoms in mouse models. In this context, it is interesting to note that depression is a relatively common co-morbidity in autoimmune patients, often attributed to effects of living with chronic disease but perhaps exacerbated by the underlying disease processes? Alcoholic humans have increased levels of IL-17 thought to be driven by liver injury, and in mice IL-17 was found to promote alcohol-seeking behavior suggesting an important feedback loop in addiction (101). IL-17 is increased in lesions from pediatric patients with intractable epilepsy and causes neuron hyperexcitablility in mouse models of epilepsy, multiple sclerosis and pain (98) (99, 100). In a mouse model of autism induced by causing inflammation in the pregnant dam, IL-17 is required for autistic trait development in the offspring(103). However, boosting IL-17 in autistic mice provided temporary restoration of non-autistic social behaviors(104). This study was initiated because of the clinical observation that autistic children experiencing infection with fever sometimes show transient increases in social behaviors, and the authors suggest that increased IL-17 during fetal development causes a heightened threshold for later IL-17 signaling that promotes typical social behaviors after birth(104). Although it is surprising to think that IL-17 could act in the brain to regulate behavior, there is precedent for cytokines acting this way: IFNγ increases in response to social interactions in mice and conversely regulates social behavior (105). In the current age of social distancing, it is seems timely to consider that our neuro-immune circuits may be evolutionarily far ahead of us in linking pathogen-induced immune responses with change in social behavior.
Acknowledgements:
Funding provided by NIHAI137029 (MJM) and American Association of Immunology training fellowship (SM).
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, Sen SK, Shaik J, Smelkinson M, Tamoutounour S, Collins N, Bouladoux N, Dzutsev A, Rosshart SP, Arbuckle JH, Wang CR, Kristie TM, Rehermann B, Trinchieri G, Brenchley JM, O’Shea JJ, Belkaid Y. 2018. Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell 172: 784–96 e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang TT, Shao TY, Ang WXG, Kinder JM, Turner LH, Pham G, Whitt J, Alenghat T, Way SS. 2017. Commensal Fungi Recapitulate the Protective Benefits of Intestinal Bacteria. Cell Host Microbe 22: 809–16 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, Arditi M, Underhill DM, Iliev ID. 2016. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 19: 865–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limon JJ, Skalski JH, Underhill DM. 2017. Commensal Fungi in Health and Disease. Cell Host Microbe 22: 156–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 120: 1762–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Casanova JL, Puel A. 2018. Mucocutaneous IL-17 immunity in mice and humans: host defense vs. excessive inflammation. Mucosal Immunol 11: 581–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen JA, Edwards TN, Liu AW, Hirai T, Jones MR, Wu J, Li Y, Zhang S, Ho J, Davis BM, Albers KM, Kaplan DH. 2019. Cutaneous TRPV1(+) Neurons Trigger Protective Innate Type 17 Anticipatory Immunity. Cell 178: 919–32 e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krebs CF, Paust HJ, Krohn S, Koyro T, Brix SR, Riedel JH, Bartsch P, Wiech T, Meyer-Schwesinger C, Huang J, Fischer N, Busch P, Mittrucker HW, Steinhoff U, Stockinger B, Perez LG, Wenzel UO, Janneck M, Steinmetz OM, Gagliani N, Stahl RAK, Huber S, Turner JE, Panzer U. 2016. Autoimmune Renal Disease Is Exacerbated by S1P-Receptor-1-Dependent Intestinal Th17 Cell Migration to the Kidney. Immunity 45: 1078–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morton AM, Sefik E, Upadhyay R, Weissleder R, Benoist C, Mathis D. 2014. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc Natl Acad Sci U S A 111: 6696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eddens T, Elsegeiny W, Garcia-Hernadez ML, Castillo P, Trevejo-Nunez G, Serody K, Campfield BT, Khader SA, Chen K, Rangel-Moreno J, Kolls JK. 2017. Pneumocystis-Driven Inducible Bronchus-Associated Lymphoid Tissue Formation Requires Th2 and Th17 Immunity. Cell Rep 18: 3078–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. 2011. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 12: 639–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pikor NB, Astarita JL, Summers-Deluca L, Galicia G, Qu J, Ward LA, Armstrong S, Dominguez CX, Malhotra D, Heiden B, Kay R, Castanov V, Touil H, Boon L, O’Connor P, Bar-Or A, Prat A, Ramaglia V, Ludwin S, Turley SJ, Gommerman JL. 2015. Integration of Th17- and Lymphotoxin-Derived Signals Initiates Meningeal-Resident Stromal Cell Remodeling to Propagate Neuroinflammation. Immunity 43: 1160–73 [DOI] [PubMed] [Google Scholar]
- 13.Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, Wucherpfennig K, Turley S, Carroll MC, Sobel RA, Bettelli E, Kuchroo VK. 2011. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 35: 986–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Koay HF, Nguyen HN, Mina AI, Paras T, Tavakkoli A, von Andrian U, Uldrich AP, Godfrey DI, Banks AS, Shay T, Brenner MB, Lynch L. 2018. gammadelta T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol 19: 464–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majumder S, Amatya N, Revu S, Jawale CV, Wu D, Rittenhouse N, Menk A, Kupul S, Du F, Raphael I, Bhattacharjee A, Siebenlist U, Hand TW, Delgoffe GM, Poholek AC, Gaffen SL, Biswas PS, McGeachy MJ. 2019. IL-17 metabolically reprograms activated fibroblastic reticular cells for proliferation and survival. Nat Immunol 20: 534–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGinley AM, Sutton CE, Edwards SC, Leane CM, DeCourcey J, Teijeiro A, Hamilton JA, Boon L, Djouder N, Mills KHG. 2020. Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1betaProducing Myeloid Cells that Promote Pathogenic T Cells. Immunity 52: 342–56 e6 [DOI] [PubMed] [Google Scholar]
- 17.Xie S, Li J, Wang JH, Wu Q, Yang P, Hsu HC, Smythies LE, Mountz JD. 2010. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol 184: 2289–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. 2010. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A 107: 14292–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. 2013. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol 14: 372–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solans L, Debrie AS, Borkner L, Aguilo N, Thiriard A, Coutte L, Uranga S, Trottein F, Martin C, Mills KHG, Locht C. 2018. IL-17-dependent SIgA-mediated protection against nasal Bordetella pertussis infection by live attenuated BPZE1 vaccine. Mucosal Immunol 11: 1753–62 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. 2019. IL-17 receptor-based signaling and implications for disease. Nat Immunol 20: 1594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. 2005. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol 77: 388–99 [DOI] [PubMed] [Google Scholar]
- 23.Slowikowski K, Nguyen HN, Noss EH, Simmons DP, Mizoguchi F, Watts GFM, Gurish MF, Brenner MB, Raychaudhuri S. 2020. CUX1 and IkappaBzeta (NFKBIZ) mediate the synergistic inflammatory response to TNF and IL-17A in stromal fibroblasts. Proc Natl Acad Sci U S A 117: 5532–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonder SU, Saret S, Tang W, Sturdevant DE, Porcella SF, Siebenlist U. 2011. IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J Biol Chem 286: 12881–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen HN, Noss EH, Mizoguchi F, Huppertz C, Wei KS, Watts GFM, Brenner MB. 2017. Autocrine Loop Involving IL-6 Family Member LIF, LIF Receptor, and STAT4 Drives Sustained Fibroblast Production of Inflammatory Mediators. Immunity 46: 220–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison OJ, Linehan JL, Shih HY, Bouladoux N, Han SJ, Smelkinson M, Sen SK, Byrd AL, Enamorado M, Yao C, Tamoutounour S, Van Laethem F, Hurabielle C, Collins N, Paun A, Salcedo R, O’Shea JJ, Belkaid Y. 2019. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8: 1390–7 [DOI] [PubMed] [Google Scholar]
- 28.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. 2010. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467: 967–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. 2012. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 484: 514–8 [DOI] [PubMed] [Google Scholar]
- 30.Omenetti S, Bussi C, Metidji A, Iseppon A, Lee S, Tolaini M, Li Y, Kelly G, Chakravarty P, Shoaie S, Gutierrez MG, Stockinger B. 2019. The Intestine Harbors Functionally Distinct Homeostatic Tissue-Resident and Inflammatory Th17 Cells. Immunity 51: 77–89 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Lopez M, Iborra S, Conde-Garrosa R, Mastrangelo A, Danne C, Mann ER, Reid DM, GaboriauRouthiau V, Chaparro M, Lorenzo MP, Minnerup L, Saz-Leal P, Slack E, Kemp B, Gisbert JP, Dzionek A, Robinson MJ, Ruperez FJ, Cerf-Bensussan N, Brown GD, Bernardo D, LeibundGut-Landmann S, Sancho D. 2019. Microbiota Sensing by Mincle-Syk Axis in Dendritic Cells Regulates Interleukin-17 and −22 Production and Promotes Intestinal Barrier Integrity. Immunity 50: 446–61 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, Calderon G, Hong BY, Break TJ, Bowdish DME, Lionakis MS, Jones SA, Trinchieri G, Diaz PI, Belkaid Y, Konkel JE, Moutsopoulos NM. 2017. On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity 46: 133–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho J, Yang X, Nikou SA, Kichik N, Donkin A, Ponde NO, Richardson JP, Gratacap RL, Archambault LS, Zwirner CP, Murciano C, Henley-Smith R, Thavaraj S, Tynan CJ, Gaffen SL, Hube B, Wheeler RT, Moyes DL, Naglik JR. 2019. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat Commun 10: 2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee JY, Ziel JW, Miraldi ER, Domingos AI, Bonneau R, Littman DR. 2015. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 163: 381–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. 2009. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31: 321–30 [DOI] [PubMed] [Google Scholar]
- 36.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31: 331–41 [DOI] [PubMed] [Google Scholar]
- 37.Verma AH, Zafar H, Ponde NO, Hepworth OW, Sihra D, Aggor FEY, Ainscough JS, Ho J, Richardson JP, Coleman BM, Hube B, Stacey M, McGeachy MJ, Naglik JR, Gaffen SL, Moyes DL. 2018. IL-36 and IL-1/IL-17 Drive Immunity to Oral Candidiasis via Parallel Mechanisms. J Immunol 201: 627–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Revu S, Wu J, Henkel M, Rittenhouse N, Menk A, Delgoffe GM, Poholek AC, McGeachy MJ. 2018. IL-23 and IL-1beta Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Rep 22: 2642–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya D, Wang P, Paul AM, Dai J, Gate D, Lowery JE, Stokic DS, Leis AA, Flavell RA, Town T, Fikrig E, Bai F. 2017. Interleukin-17A Promotes CD8+ T Cell Cytotoxicity To Facilitate West Nile Virus Clearance. J Virol 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou L, Jie Z, Desai M, Liang Y, Soong L, Wang T, Sun J. 2013. Early IL-17 production by intrahepatic T cells is important for adaptive immune responses in viral hepatitis. J Immunol 190: 621–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8: 369–77 [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Chan CC, Yang M, Deng J, Poon VK, Leung VH, Ko KH, Zhou J, Yuen KY, Zheng BJ, Lu L. 2011. A critical role of IL-17 in modulating the B-cell response during H5N1 influenza virus infection. Cell Mol Immunol 8: 462–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Ma K, Chen M, Ko KH, Zheng BJ, Lu L. 2016. IL-17A Promotes Pulmonary B-1a Cell Differentiation via Induction of Blimp-1 Expression during Influenza Virus Infection. PLoS Pathog 12: e1005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagri P, Anipindi VC, Nguyen PV, Vitali D, Stampfli MR, Kaushic C. 2017. Novel Role for Interleukin-17 in Enhancing Type 1 Helper T Cell Immunity in the Female Genital Tract following Mucosal Herpes Simplex Virus 2 Vaccination. J Virol 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muir R, Osbourn M, Dubois AV, Doran E, Small DM, Monahan A, O’Kane CM, McAllister K, Fitzgerald DC, Kissenpfennig A, McAuley DF, Ingram RJ. 2016. Innate Lymphoid Cells Are the Predominant Source of IL17A during the Early Pathogenesis of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 193: 407–16 [DOI] [PubMed] [Google Scholar]
- 46.Lu B, Liu M, Wang J, Fan H, Yang D, Zhang L, Gu X, Nie J, Chen Z, Corbett AJ, Zhan MJ, Zhang S, Bryant VL, Lew AM, McCluskey J, Luo HB, Cui J, Zhang Y, Zhan Y, Lu G. 2020. IL-17 production by tissue-resident MAIT cells is locally induced in children with pneumonia. Mucosal Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C, Yang P, Sun Y, Li T, Wang C, Wang Z, Zou Z, Yan Y, Wang W, Wang C, Chen Z, Xing L, Tang C, Ju X, Guo F, Deng J, Zhao Y, Yang P, Tang J, Wang H, Zhao Z, Yin Z, Cao B, Wang X, Jiang C. 2012. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res 22: 528–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mebratu YA, Tesfaigzi Y. 2018. IL-17 Plays a Role in Respiratory Syncytial Virus-induced Lung Inflammation and Emphysema in Elastase and LPS-injured Mice. Am J Respir Cell Mol Biol 58: 717–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. 2009. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol 183: 5301–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Megna M, Napolitano M, Fabbrocini G. 2020. May IL-17 have a role in COVID-19 infection? Med Hypotheses 140: 109749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pacha O, Sallman MA, Evans SE. 2020. COVID-19: a case for inhibiting IL-17? Nat Rev Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lythgoe MP, Middleton P. 2020. Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol Sci 41: 363–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Cai G, Liu C, Zhao J, Gu C, Wu L, Hamilton TA, Zhang CJ, Ko J, Zhu L, Qin J, Vidimos A, Koyfman S, Gastman BR, Jensen KB, Li X. 2019. IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1(+) stem cells. J Exp Med 216: 195–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zepp JA, Zhao J, Liu C, Bulek K, Wu L, Chen X, Hao Y, Wang Z, Wang X, Ouyang W, Kalady MF, Carman J, Yang WP, Zhu J, Blackburn C, Huang YH, Hamilton TA, Su B, Li X. 2017. IL-17A-Induced PLET1 Expression Contributes to Tissue Repair and Colon Tumorigenesis. J Immunol 199: 3849–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. 2013. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498: 371–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, Shi L, Wu D, Dong C, Liu H. 2014. IL-17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res 74: 1969–82 [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Zhang Y, Yin K, Xu P, Tian J, Ma J, Tian X, Wang Y, Tang X, Xu H, Wang S. 2017. IL-17A weakens the antitumor immuity by inhibiting apoptosis of MDSCs in Lewis lung carcinoma bearing mice. Oncotarget 8: 4814–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veglia F, Perego M, Gabrilovich D. 2018. Myeloid-derived suppressor cells coming of age. Nat Immunol 19: 108–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels L, Jonkers J, de Visser KE. 2015. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522: 345–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, Pang KC, Liu XF, Liu T, Zhang JY, Zeng H, Liu KY, Guo G, Tong WD, Shi Y, Tang B, Li N, Yu S, Luo P, Zhang WJ, Lu DS, Yu PW, Zou QM. 2012. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 143: 951–62 e8 [DOI] [PubMed] [Google Scholar]
- 61.Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S, Whary MT, Meyerson M, Germain R, Blainey PC, Fox JG, Jacks T. 2019. Commensal Microbiota Promote Lung Cancer Development via gammadelta T Cells. Cell 176: 998–1013 e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chung L, Thiele Orberg E, Geis AL, Chan JL, Fu K, DeStefano Shields CE, Dejea CM, Fathi P, Chen J, Finard BB, Tam AJ, McAllister F, Fan H, Wu X, Ganguly S, Lebid A, Metz P, Van Meerbeke SW, Huso DL, Wick EC, Pardoll DM, Wan F, Wu S, Sears CL, Housseau F. 2018. Bacteroides fragilis Toxin Coordinates a Procarcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 23: 203–14 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li W, Xu M, Li Y, Huang Z, Zhou J, Zhao Q, Le K, Dong F, Wan C, Yi P. 2020. Comprehensive analysis of the association between tumor glycolysis and immune/inflammation function in breast cancer. J Transl Med 18: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan B, Shen J, Cao J, Zhou Y, Shang L, Jin S, Cao S, Che D, Liu F, Yu Y. 2015. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep 5: 16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang B, Kang H, Fung A, Zhao H, Wang T, Ma D. 2014. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediators Inflamm 2014: 623759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. 2016. IL17 producing gammadeltaT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer 139: 869–81 [DOI] [PubMed] [Google Scholar]
- 67.Kato T, Furumoto H, Ogura T, Onishi Y, Irahara M, Yamano S, Kamada M, Aono T. 2001. Expression of IL17 mRNA in ovarian cancer. Biochem Biophys Res Commun 282: 735–8 [DOI] [PubMed] [Google Scholar]
- 68.Liu L, Sun H, Wu S, Tan H, Sun Y, Liu X, Si S, Xu L, Huang J, Zhou W, Yang Z, Wang Z. 2019. IL17A promotes CXCR2dependent angiogenesis in a mouse model of liver cancer. Mol Med Rep 20: 1065–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu HH, Hwang-Verslues WW, Lee WH, Huang CK, Wei PC, Chen CL, Shew JY, Lee EY, Jeng YM, Tien YW, Ma C, Lee WH. 2015. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J Exp Med 212: 333–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, Stohlman S, Tuohy VK, Li X. 2010. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 32: 414–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paquissi FC. 2017. Immunity and Fibrogenesis: The Role of Th17/IL-17 Axis in HBV and HCV-induced Chronic Hepatitis and Progression to Cirrhosis. Front Immunol 8: 1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. 2010. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 207: 535–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang T, Liu Y, Zou JF, Cheng ZS. 2017. Interleukin-17 induces human alveolar epithelial to mesenchymal cell transition via the TGF-beta1 mediated Smad2/3 and ERK1/2 activation. PLoS One 12: e0183972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. 2011. Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity 34: 396–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dufour AM, Alvarez M, Russo B, Chizzolini C. 2018. Interleukin-6 and Type-I Collagen Production by Systemic Sclerosis Fibroblasts Are Differentially Regulated by Interleukin-17A in the Presence of Transforming Growth Factor-Beta 1. Front Immunol 9: 1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fabre T, Molina MF, Soucy G, Goulet JP, Willems B, Villeneuve JP, Bilodeau M, Shoukry NH. 2018. Type 3 cytokines IL-17A and IL-22 drive TGF-beta-dependent liver fibrosis. Sci Immunol 3 [DOI] [PubMed] [Google Scholar]
- 77.Wang Q, Zhou J, Zhang B, Tian Z, Tang J, Zheng Y, Huang Z, Tian Y, Jia Z, Tang Y, van Velkinburgh JC, Mao Q, Bian X, Ping Y, Ni B, Wu Y. 2013. Hepatitis B virus induces IL-23 production in antigen presenting cells and causes liver damage via the IL-23/IL-17 axis. PLoS Pathog 9: e1003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, Sun B. 2013. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol 191: 1835–44 [DOI] [PubMed] [Google Scholar]
- 79.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, Carlis JV, Haase AT. 2011. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest 121: 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Estes JD, Reilly C, Trubey CM, Fletcher CV, Cory TJ, Piatak M Jr., Russ S, Anderson J, Reimann TG, Star R, Smith A, Tracy RP, Berglund A, Schmidt T, Coalter V, Chertova E, Smedley J, Haase AT, Lifson JD, Schacker TW. 2015. Antifibrotic therapy in simian immunodeficiency virus infection preserves CD4+ T-cell populations and improves immune reconstitution with antiretroviral therapy. J Infect Dis 211: 744–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kityo C, Makamdop KN, Rothenberger M, Chipman JG, Hoskuldsson T, Beilman GJ, Grzywacz B, Mugyenyi P, Ssali F, Akondy RS, Anderson J, Schmidt TE, Reimann T, Callisto SP, Schoephoerster J, Schuster J, Muloma P, Ssengendo P, Moysi E, Petrovas C, Lanciotti R, Zhang L, Arevalo MT, Rodriguez B, Ross TM, Trautmann L, Sekaly RP, Lederman MM, Koup RA, Ahmed R, Reilly C, Douek DC, Schacker TW. 2018. Lymphoid tissue fibrosis is associated with impaired vaccine responses. J Clin Invest [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, Tabb B, Canary LA, Dang Q, Hirsch VM, Alter G, Belkaid Y, Lifson JD, Silvestri G, Milner JD, Paiardini M, Haddad EK, Brenchley JM. 2012. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol 5: 646–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, Cua DJ. 2015. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity 43: 727–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan ES, Micci L, Fromentin R, Paganini S, McGary CS, Easley K, Chomont N, Paiardini M. 2016. Loss of Function of Intestinal IL-17 and IL-22 Producing Cells Contributes to Inflammation and Viral Persistence in SIV-Infected Rhesus Macaques. PLoS Pathog 12: e1005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christensen-Quick A, Lafferty M, Sun L, Marchionni L, DeVico A, Garzino-Demo A. 2016. Human Th17 Cells Lack HIV-Inhibitory RNases and Are Highly Permissive to Productive HIV Infection. J Virol 90: 7833–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Planas D, Routy JP, Ancuta P. 2019. New Th17-specific therapeutic strategies for HIV remission. Curr Opin HIV AIDS 14: 85–92 [DOI] [PubMed] [Google Scholar]
- 87.Hinshaw DC, Shevde LA. 2019. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res 79: 4557–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Senbabaoglu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Hoglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T. 2018. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554: 544–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dominguez CX, Muller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, Breart B, Foreman O, Bainbridge TW, Castiglioni A, Senbabaoglu Y, Modrusan Z, Liang Y, Junttila MR, Klijn C, Bourgon R, Turley SJ. 2020. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15(+) Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov 10: 232–53 [DOI] [PubMed] [Google Scholar]
- 90.Barnas JL, Simpson-Abelson MR, Brooks SP, Kelleher RJ Jr., Bankert RB. 2010. Reciprocal functional modulation of the activation of T lymphocytes and fibroblasts derived from human solid tumors. J Immunol 185: 2681–92 [DOI] [PubMed] [Google Scholar]
- 91.Ghilardi N, Pappu R, Arron JR, Chan AC. 2020. 30 Years of Biotherapeutics Development-What Have We Learned? Annu Rev Immunol 38: 249–87 [DOI] [PubMed] [Google Scholar]
- 92.Llosa NJ, Luber B, Tam AJ, Smith KN, Siegel N, Awan AH, Fan H, Oke T, Zhang J, Domingue J, Engle EL, Roberts CA, Bartlett BR, Aulakh LK, Thompson ED, Taube JM, Durham JN, Sears CL, Le DT, Diaz LA, Pardoll DM, Wang H, Anders RA, Housseau F. 2019. Intratumoral Adaptive Immunosuppression and Type 17 Immunity in Mismatch Repair Proficient Colorectal Tumors. Clin Cancer Res 25: 5250–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, AustinBreneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. 2018. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359: 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sui G, Qiu Y, Yu H, Kong Q, Zhen B. 2019. Interleukin-17 promotes the development of cisplatin resistance in colorectal cancer. Oncol Lett 17: 944–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. 2015. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 43: 515–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. 2014. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510: 157–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. 2016. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med 22: 516–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo H, Liu HZ, Zhang WW, Matsuda M, Lv N, Chen G, Xu ZZ, Zhang YQ. 2019. Interleukin-17 Regulates Neuron-Glial Communications, Synaptic Transmission, and Neuropathic Pain after Chemotherapy. Cell Rep 29: 2384–97 e5 [DOI] [PubMed] [Google Scholar]
- 99.Xu D, Robinson AP, Ishii T, Duncan DS, Alden TD, Goings GE, Ifergan I, Podojil JR, Penaloza-MacMaster P, Kearney JA, Swanson GT, Miller SD, Koh S. 2018. Peripherally derived T regulatory and gammadelta T cells have opposing roles in the pathogenesis of intractable pediatric epilepsy. J Exp Med 215: 1169–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, Laube G, Luche H, Lehnardt S, Fehling HJ, Griesbeck O, Zipp F. 2010. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 33: 424–36 [DOI] [PubMed] [Google Scholar]
- 101.Xu J, Ma HY, Liu X, Rosenthal S, Baglieri J, McCubbin R, Sun M, Koyama Y, Geoffroy CG, Saijo K, Shang L, Nishio T, Maricic I, Kreifeldt M, Kusumanchi P, Roberts A, Zheng B, Kumar V, Zengler K, Pizzo DP, Hosseini M, Contet C, Glass CK, Liangpunsakul S, Tsukamoto H, Gao B, Karin M, Brenner DA, Koob GF, Kisseleva T. 2020. Blockade of IL-17 signaling reverses alcohol-induced liver injury and excessive alcohol drinking in mice. JCI Insight 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beurel E, Harrington LE, Jope RS. 2013. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry 73: 622–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gumusoglu SB, Hing BWQ, Chilukuri ASS, Dewitt JJ, Scroggins SM, Stevens HE. 2020. Chronic maternal interleukin-17 and autism-related cortical gene expression, neurobiology, and behavior. Neuropsychopharmacology 45: 1008–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reed MD, Yim YS, Wimmer RD, Kim H, Ryu C, Welch GM, Andina M, King HO, Waisman A, Halassa MM, Huh JR, Choi GB. 2020. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 577: 249–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC, Gadani SP, Turner SD, Weng Z, Peerzade SN, Chen H, Lee KS, Scott MM, Beenhakker MP, Litvak V, Kipnis J. 2016. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535: 425–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. 2012. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491: 254–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharp SP, Avram D, Stain SC, Lee EC. 2017. Local and systemic Th17 immune response associated with advanced stage colon cancer. J Surg Res 208: 180–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu LW, Taniguchi K, Feng Y, Fearon E, Grivennikov SI, Karin M. 2014. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity 41: 1052–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang D, Yu W, Lian J, Wu Q, Liu S, Yang L, Li F, Huang L, Chen X, Zhang Z, Li A, Liu J, Sun Z, Wang J, Yuan W, Zhang Y. 2020. Th17 cells inhibit CD8(+) T cell migration by systematically downregulating CXCR3 expression via IL-17A/STAT3 in advanced-stage colorectal cancer patients. J Hematol Oncol 13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. 2011. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 71: 1263–71 [DOI] [PubMed] [Google Scholar]
- 111.Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, Sears CL. 2018. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359: 592–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Calcinotto A, Brevi A, Chesi M, Ferrarese R, Garcia Perez L, Grioni M, Kumar S, Garbitt VM, Sharik ME, Henderson KJ, Tonon G, Tomura M, Miwa Y, Esplugues E, Flavell RA, Huber S, Canducci F, Rajkumar VS, Bergsagel PL, Bellone M. 2018. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat Commun 9: 4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. 2006. IL-23 promotes tumour incidence and growth. Nature 442: 461–5 [DOI] [PubMed] [Google Scholar]
- 114.Dai ZM, Zhang TS, Lin S, Zhang WG, Liu J, Cao XM, Li HB, Wang M, Liu XH, Liu K, Li SL, Dai ZJ. 2016. Role of IL-17A rs2275913 and IL-17F rs763780 polymorphisms in risk of cancer development: an updated meta-analysis. Sci Rep 6: 20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu X, Zeng Z, Chen B, Yu J, Xue L, Hao Y, Chen M, Sung JJ, Hu P. 2010. Association between polymorphisms in interleukin-17A and interleukin-17F genes and risks of gastric cancer. Int J Cancer 127: 86–92 [DOI] [PubMed] [Google Scholar]
- 116.Li TJ, Jiang YM, Hu YF, Huang L, Yu J, Zhao LY, Deng HJ, Mou TY, Liu H, Yang Y, Zhang Q, Li GX. 2017. Interleukin-17-Producing Neutrophils Link Inflammatory Stimuli to Disease Progression by Promoting Angiogenesis in Gastric Cancer. Clin Cancer Res 23: 1575–85 [DOI] [PubMed] [Google Scholar]
- 117.Punt S, van Vliet ME, Spaans VM, de Kroon CD, Fleuren GJ, Gorter A, Jordanova ES. 2015. FoxP3(+) and IL-17(+) cells are correlated with improved prognosis in cervical adenocarcinoma. Cancer Immunol Immunother 64: 745–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, Dong M, Yamasawa K, Tamura K. 2001. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology 61: 79–89 [DOI] [PubMed] [Google Scholar]
- 119.Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao JJ, Weng DS, Pan QZ, Wang DD, Jiang SS, Chang AE, Li Q, Xia JC. 2013. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J Immunother 36: 451–8 [DOI] [PubMed] [Google Scholar]
- 120.Chen CL, Wang Y, Huang CY, Zhou ZQ, Zhao JJ, Zhang XF, Pan QZ, Wu JX, Weng DS, Tang Y, Zhu Q, Yuan LP, Xia JC. 2017. IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology 7: e1373234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. 2009. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31: 787–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. 2009. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 114: 357–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma M, Huang W, Kong D. 2018. IL-17 inhibits the accumulation of myeloid-derived suppressor cells in breast cancer via activating STAT3. Int Immunopharmacol 59: 148–56 [DOI] [PubMed] [Google Scholar]
- 124.Lin Y, Xu J, Su H, Zhong W, Yuan Y, Yu Z, Fang Y, Zhou H, Li C, Huang K. 2015. Interleukin-17 is a favorable prognostic marker for colorectal cancer. Clin Transl Oncol 17: 50–6 [DOI] [PubMed] [Google Scholar]


