Abstract
Objective:
To test the null hypothesis that there is no difference between latex and nonlatex orthodontic elastics with respect to tissue compatibility and surface structure.
Materials and Methods:
Latex and nonlatex elastics were implanted in the subcutaneous connective tissue of 45 Wistar rats. In the control groups, no material was implanted (sham). After 24 hours, 3, 7, 14, and 21 days, the animals were euthanized; tissue samples were processed and analyzed by descriptive and semi-quantitative microscopic analysis and quantification of plasma extravasation. The surface structure of elastics was evaluated by scanning electron microscopy (SEM). The results were analyzed by analysis of variance (ANOVA), Tukey test and Kruskal-Wallis test at 5% significance level.
Results:
Peri-implant plasma extravasation was significantly higher (P < .05) in the animals that received latex elastics compared with those with nonlatex elastics and those that were control animals. The microscopic analysis revealed a more intense inflammatory infiltrate in the initial periods without statistically significant difference (P > .05) between the experimental and control groups. The SEM analysis revealed that the latex elastics presented microspheres and porosities, while the nonlatex elastics exhibited crystals on their surface and absence of porosities.
Conclusion:
The null hypothesis was rejected since the latex elastics were more irritating to the connective tissue than the nonlatex elastics in the initial periods and presented a more porous surface.
Keywords: Latex, Nonlatex, Orthodontic elastics, Inflammatory response, SEM
INTRODUCTION
Elastics have long become an essential part of the orthodontic clinic, and their importance and widespread use have attracted interest in orthodontic research.1 The composition of orthodontic elastics divides them into two categories. Natural latex rubber is the most traditional material for elastics and it was the first to be produced on an industrial basis. Its most important source is Hevea brasiliensis, a rubber wood tree native to rainforests in the Amazon region of South America. Latex (or natural) elastics are composed of cis-1,4 poly-isoprene chains with preservatives, usually ammonia, added to achieve the unique properties like elasticity, flexibility, and resilience.2 The production of latex on an industrial scale involves the mixture of natural rubber with stabilizers such as zinc oxide and other chemicals, which are heated to specific temperatures to promote a homogeneous final product. There is no standardization in the composition of latex elastics, resulting in products with different properties.3
Nonlatex elastics are composed of synthetic rubber, which is any type of artificial polymer that reproduces, to a higher or lower degree, the physical properties of natural rubber, namely silicone,4 polyurethane plastic,5 and styrene-butadiene rubber (SBR).
The mixtures of components found in latex elastics make these materials more prone to elicit allergies and even cause immediate hypersensitivity reactions in the patients.6 Latex-mediated allergic reactions include the development of stomatitis, erythematous oral lesions, respiratory distress, systemic reactions, and anaphylaxis in more severe cases.7,8 In view of this, the demand for alternative latex-free products has increased substantially.9
Developing dental materials with tissue compatibility remains a challenge to researchers and manufacturers.10 Any material in contact with oral tissues for several hours per day, months, or years may unchain an unfavorable biological response. However, material compatibility may be affected by its physical characteristics and its capacity to resist the oral conditions. This means that the biological compatibility depends on the material’s properties and capacity to resist degradation.11
The results of cell culture studies show that latex elastics induce more cell lysis.6 Nevertheless, there are so far no studies evaluating comparatively latex and nonlatex elastics with respect to compatibility with vital tissues.
The aim of this experiment was to test the null hypothesis that there is no difference in the tissue compatibility and the surface structure of latex and nonlatex (SBR) elastics, analyzed by quantification of plasma extravasation, microscopic analysis and scanning electron microscopy (SEM).
MATERIALS AND METHODS
This study was conducted according to the International Organization for Standardization (ISO) standard 10993-6 (2007)12 following the recommendations for in vivo toxicity tests and number of samples. The study was approved by the Ethics Committee for Animal Experimentation of the School of Dentistry of Ribeirão Preto, São Paulo, Brazil under protocol 2014.1.349.58.0. Care about the welfare of experimental animals followed the norms and ethical principles adopted by the Ethics Committee.
Forty-five male Wistar rats (Rattus norvegicus albinus), approximately 120 days old and weighing 500 g, were used for two experiments: quantification of plasma extravasation and optical microscopy analysis. The animals were housed under climate-controlled conditions (12 hours light/12 hours dark; 22 ± 3°C) with free access to ground solid ration and water.
Evaluation of Plasma Extravasation
Operative procedures
Six animals were assigned to three groups, two experimental groups (n = 2), implanted with latex elastics (natural rubber; Dentaurum, Newtown, Pa; 3/16-inch diameter, medium force) or nonlatex elastics (SBR; Dentaurum; 3/16-inch diameter, medium force), and one control group (sham surgery; n = 2), subjected to a fictitious (sham) surgery, in which no material was implanted.
All animals were anesthetized by an intramuscular injection of a combination of 10% ketamine hydrochloride (Agener União Química Farmacêutica Nacional S/A, Embu-Guaçu, SP, Brazil; 0.20 mL/kg body weight) and xylazine hydrochloride (Dopaser; Laboratorios Calier SA, Barcelona, Spain; 0.8 mL/kg body weight). The dorsal skin of the animals was shaved and cleaned with 0.12% chlorhexidine digluconate (Bioquant, Nashville, Tenn). Four 6-mm wide incisions were made with a number 15 scalpel blade, two at each side of the spine with a 10-mm space between the incisions.
Elastics previously sterilized with ethylene oxide were inserted in the subcutaneous connective tissue. In each animal from the experimental groups, four elastics of the same type (latex or nonlatex) were implanted. The animals in the sham (control) group did not have elastics implanted. All incisions were sutured with silk thread (Vicril Rapid 4.0; Ethicon, Johnson & Johnson, Brussels, Belgium).
Sample collection and quantification of plasma extravasation
Plasma extravasation was evaluated 24 hours after implantation of the elastics. Two hours before sample collection, Evans blue dye (60 mg/kg body weight) was injected into the lateral tail vein of each animal. The animals were euthanized in a carbon dioxide (CO2) chamber with 60% saturation, and tissue fragments containing the elastics (approximate 2 cm2 area) were excised. The collected tissues were stored in an aqueous formamide solution (1/1) for 24 hours at 37°C. The optical density of the supernatant containing the extravasated Evans blue dye was determined by spectrophotometry (630 nm, Model U-2001; Hitachi, Tokyo, Japan), and dye concentration was determined by linear regression from a curve of known dye concentrations. The results were expressed in optical density of Evans blue dye per gram of tissue.
Optical microscopy: descriptive and semi-quantitative analyses
Thirty-nine animals were assigned to three groups, two experimental groups (n = 13 per group), implanted with latex or nonlatex elastics and one control group (sham surgery; n = 13).
The animals were euthanized 3, 7, 14 days (n = 3 animals per period per group) and 21 days (n = 4 animals per group) after implantation, and tissue fragments containing the elastics were excised. The samples were fixed in 10% buffered formalin for 72 hours and subjected to the routine histotechnical processing. Serial 6-μm thick sections were stained with hematoxylin and eosin (HE) for analysis with an optical microscope (Axio Imager M1; Carl Zeiss MicroImaging GmbH, Gottingen, Germany). The histologic sections were evaluated according to the microscopic parameters and scores shown in Table 1.
Table 1.
Microscopic Parameters and Scores Used in the Microscopic Analysis of Tissue Compatibility at 3, 7, 14 and 21 Days
SEM analysis
Twelve elastics of each type (latex and nonlatex) were removed from their original packages, sectioned in the middle with a scalpel blade or maintained intact, and examined by SEM. Processing and analysis of specimens were carried out as described by Magno et al.13
Statistical Analysis
The results were analyzed by analysis of variance (ANOVA), Tukey posttest, and Kruskal-Wallis test. Graph Pad Prism 5.0a (Graph Pad Software Inc, San Diego, Calif) software was used for all analyses and a significance level of 5% was adopted.
RESULTS
Plasma Extravasation
In the 24-hour period, all groups exhibited plasma extravasation around the implant site. In the tissues implanted with latex elastics, Evans blue dye extravasation per gram of tissue (0.059 ± 0.019) was significantly greater (P < .05) than in the animals that received nonlatex elastics (0.031 ± 0.017) and in the sham group (0.020 ± 0.008). On the other hand, the nonlatex elastics produced plasma extravasation similar (P > .05) to that of the sham group. The mean values (± standard error of the mean) for plasma extravasation in each group are shown in Figure 1.
Figure 1.
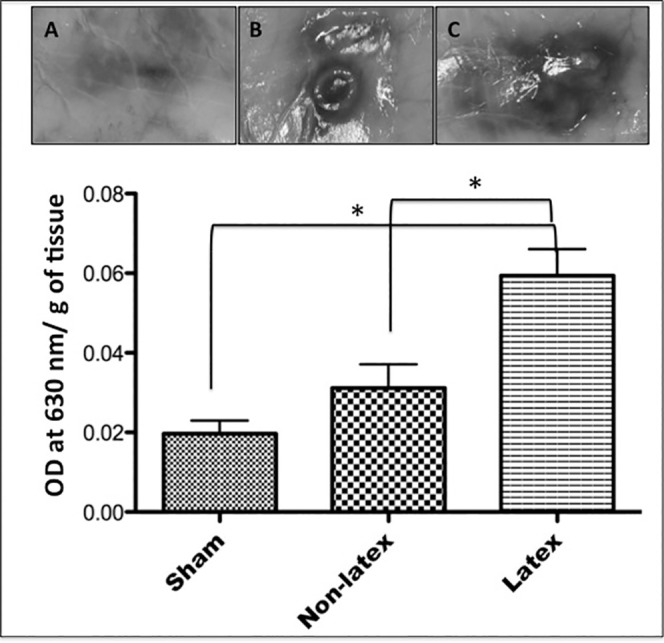
Mean values (± standard deviation) of the amount of Evans blue dye (OD indicates optical density) overflown per gram of tissue, measured by spectrophotometry 24 hours after implantation in the subcutaneous tissue. (A) Sham. (B) Nonlatex. (C) Latex. NS indicates nonsignificant statistical difference. * P > .05.
Descriptive Microscopic Analysis
Experimental groups (latex and nonlatex elastics) (Figures 2 and 3)
Figure 2.
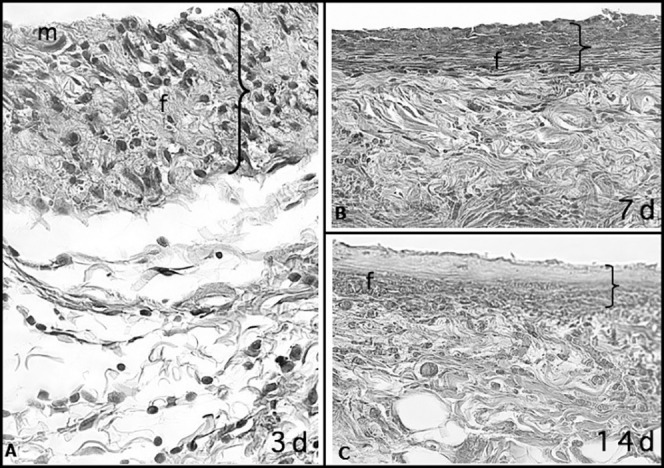
Capsular tissue around a nonlatex elastic with numerous macrophages (m) and few neutrophils at 3 days. At 7 and 14 days, fiber formation (f) was more intense than at 3 days and with the formation of fibrous capsule. The greater thickness of the capsule at 3 days is probably due to the greater accumulation of cells and edema, which were significantly reduced at 7 and 14 days (HE; 40× magnification).
Figure 3.

Capsular tissue around a latex elastic with numerous macrophages (m) and some neutrophils at 3 days. Fiber formation (f) was greater at 7, 14, and 21 days than at 3 days, and the fibers were organized in the form of a fibrous capsule. The greater thickness at 3 days refers to higher accumulation of cells and edema, which were significantly reduced at 7, 14, and 21 days (HE; 40× magnification).
As these groups presented similar features, the results are presented together as follows.
At 3 days, macrophages and neutrophils were observed on the surface of both types of elastics, forming a reactionary interface. Macrophages were juxtaposed and adhered to elastic surface, while neutrophils were randomly and diffusely scattered. There was intense edema, mild collagen fiber formation characterized by occasional and delicate collagen fibers, and numerous interwoven newly formed vessels.
At 7 days, macrophages adhered to the elastic surfaces were roughly organized in juxtaposition and very few of them were multi-nucleated giant cells and occasional neutrophils. Lesser edema was observed and fiber formation was characterized by more, denser and longer collagen fibers. The number of newly formed vessels ranged from moderate to intense.
At 14 days, the macrophages on the surface of elastics were juxtaposed more densely, and several of them were multi-nucleated giant cells. In the fibrous capsule formed around the material, fiber formation was moderate or intense, presenting dense collagen fibers permeated by fibroblasts. Edema was absent or very discrete and the neutrophils were practically absent. Few vessels remained on the external portion of the organized fibrous capsule.
At 21 days, macrophages and multi-nucleated giant cells were observed at the tissue/elastic interface. These cells were well condensed, forming one or two juxtaposed cell layers. Edema and neutrophils were not observed. The intense fiber formation was characterized by areas of hyalinization or dense collagen fiber bundles organized as fiber capsules. The blood vessels were only slightly more numerous than normal.
Control group (sham surgery)
On the third day, the sham-operated sites were filled by exudate or inflammatory edema with fibrin permeated by polymorphonuclear neutrophils and newly formed vessels. The presence of few delicate collagen bundles characterized mild fiber formation. At 7 days, the surgical spaces were filled by granulation tissue in an advanced phase of transformation into fibrous collagen tissue. Vessels and fibroblasts permeated well-organized collagen bundles, characterizing a moderate to intense fiber formation. At 14 days, normal collagen tissue filled the surgical sham spaces, with a slightly larger number of blood vessels. At 21 days, the sites presented normal fibrous collagen tissue, with no difference from the surrounding tissues (Figure 4).
Figure 4.
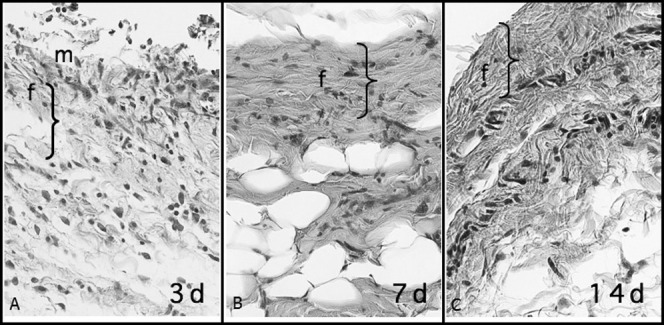
Fibrous connective tissue under reorganization in the sham-operated area with macrophages (m) and some neutrophils at 3 days. At 7 and 14 days, fiber formation (f) was more intense and newly formed connective tissue was randomly organized, similar to the preoperative condition but more mature than at 3 days. The greater thickness at 3 days refers to higher accumulation of cells and edema, which were significantly reduced at 7 and 14 days (HE; 40× magnification).
Semiquantitative Microscopic Evaluation
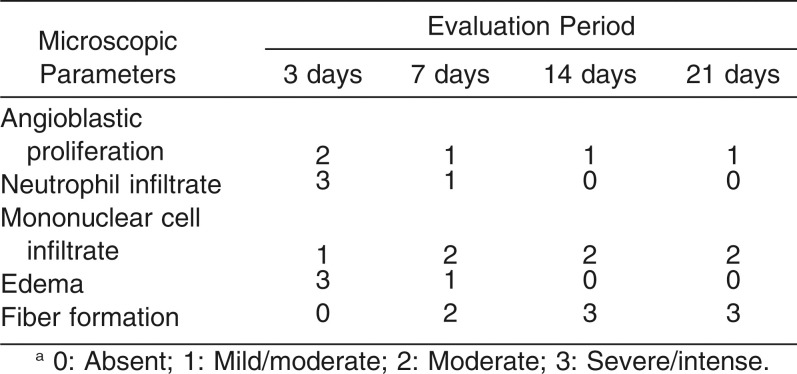
Table 2 presents the scores attributed to the histologic parameters (angioblastic proliferation, neutrophils, mononuclear cells, edema, and fiber formation) evaluated at 3, 7, 14, and 21 days, in the latex and nonlatex groups.
Table 2.
The Median Scores Attributed to Each Microscopic Parameter Evaluated for the Latex and Non-latex Groups at 3, 7, 14 and 21 Daysa
For both types of elastics, there was a decrease in angioblastic proliferation, number of neutrophils and intensity of edema over the evaluation periods. On the other hand, the number of mononuclear cells and fiber formation increased from day 3 to day 21 (Table 2).
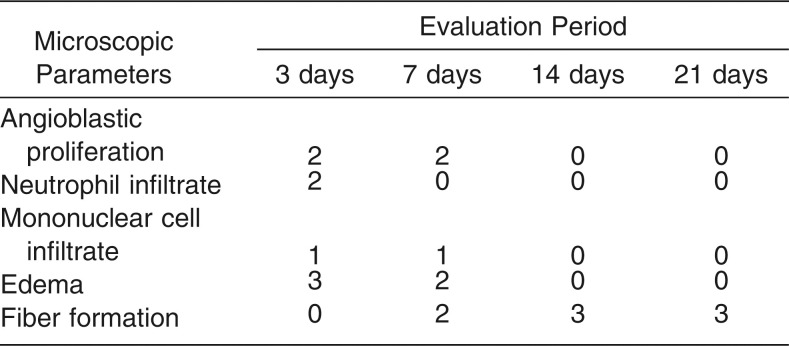
Table 3 presents the median scores attributed to the histologic parameters in the control (sham) group at each evaluation period. Fiber formation increased, while all other histologic parameters decreased over time.
Table 3.
The Median Scores Attributed to Each Microscopic Parameter in the Sham Group at 3, 7, 14 and 21 Days
No statistically significant differences (P > .05) were found among the groups (latex elastics, nonlatex and sham) for all evaluated parameters and periods.
SEM Analysis of the Elastics
Latex
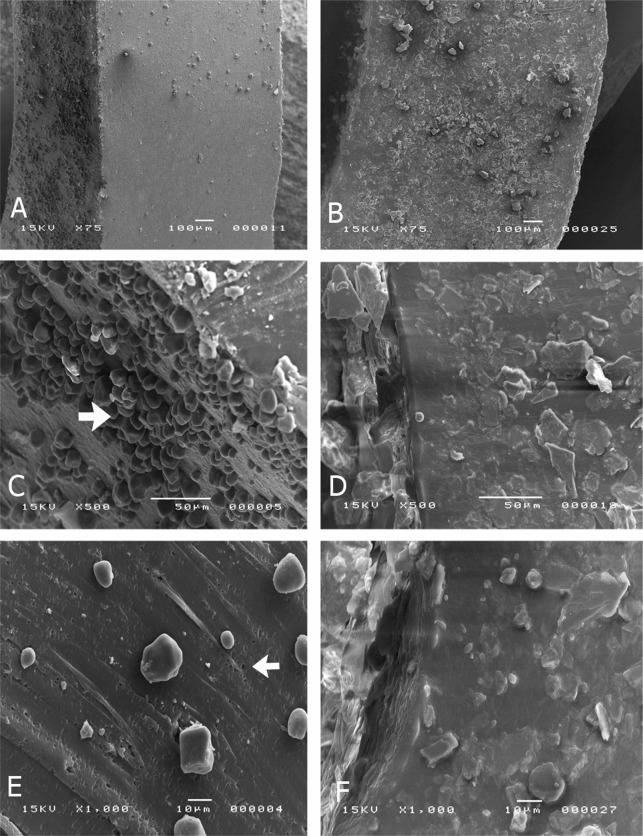
The examination of the intact elastics revealed microspheres on the surface, probably formed at the moment of the industrial cutting of the elastic rings during the manufacturing process (Figure 5A, 75× magnification). The inner surface of the elastics presented a large number of rubber microspheres (arrow) (Figure 5C, 500× magnification). The analysis of the cross-sections revealed porosities (arrow) as well as fewer and sparsely distributed microspheres compared with the outer and inner surface of the elastics (Figure 5E, 1000× magnification).
Figure 5.
SEM micrographs of latex elastics (A, C, E) and nonlatex elastics (B, D, F) at 75× to 1000× magnifications. The intact latex elastics revealed the presence of microspheres on the surface (arrow C). When sectioned, these elastics presented microspheres and porosities on their inner surface (arrow E). The intact nonlatex elastics presented crystalloid structures on the surface (B, D). When sectioned, these elastics did not present porosities (F).
Nonlatex
The examination of the intact elastics revealed the existence of crystalloid structures (Figure 5B, 75× magnification), probably produced during the manufacturing process. Both inner and outer surfaces presented as a homogeneous mass, without porosities (Figure 5D, 500× magnification). The analysis of the cross-sections showed an appearance similar to that of intact elastic, with presence of crystalloid formations and absence of porosities (Figure 5F, 1000× magnification).
DISCUSSION
The inflammatory tissue reaction elicited by the elastics was evaluated histopathologically at 3, 7, 14, and 21 days. These evaluation periods were chosen to evaluate the kinetics of tissue response as a function of time and also to assess after how much time the elastic should be replaced by the patient. dos Santos et al.,14 evaluated the cytotoxicity of latex and nonlatex elastics in cell culture at periods of 1, 2, 3, and 7 days, and found that nonlatex elastics induced less cell lysis compared with latex elastics. In the present study, evaluation for longer periods did not show significant differences between both types of elastics, but a more intense plasma exudation was observed in the initial periods in the group that received latex elastics.
In the present study, plasma extravasation was evaluated 24 hours after subcutaneous implantation of the elastics and exudation into the surrounding tissues was observed with more intensity in the animals implanted with latex elastics. Apart from the reaction elicited by the material itself in the subcutaneous connective tissue, latex elastics caused greater initial aggression with higher plasma extravasation. No statistically significant differences were found between the nonelastic latex and control (sham) groups. A previous study suggested that latex elastics could be associated with cell death.6
Similar inflammatory results were obtained with both methodologies. The edemogenic test (Evans blue dyes) revealed a better initial (24 hours) tissue reaction to the nonlatex elastics (SBR). The increase in vascular permeability and consequent increase of edema probably diluted the aggressive agent to cause its eventual neutralization. This could justify the similar results observed between the two types of elastics after implantation in the subcutaneous connective tissue for 3, 7, 14, and 21 days.
In clinical practice, 3/16-inch orthodontic elastics are normally used as intermaxillary elastics, and their change is recommended several times a day, every day, or every 3 days at most. The microscopic analysis showed greater scores of the histopathologic parameters in the 3-day evaluation period and a decrease of these scores from the days 7 to 21, with no significant difference between the experimental (latex and nonlatex) and control groups. Such findings suggest that the inflammatory reaction occurs mainly at the start of the treatment and with remissions along the treatment. In this way, according to the results from this experimental animal model, longer intervals for elastic change could be recommended to avoid recurrence of tissue irritations. These findings agree with dos Santos et al.,14 who tested different brands of elastics in cell cultures and reported tissue compatibility after the third day of the experiment.
In the present study, SEM examination of intact and sectioned elastics at different magnifications revealed the presence of rubber microspheres and porosities. These surface irregularities formed during manufacturing and orthodontic handling can become important retention sites to harbor bacteria, fungi and viruses, and their byproducts.13 Since tissue compatibility depends not only on the material composition, but also on its physical structure and capacity to resist the oral conditions, such imperfections could contribute to increase its incompatibility with the oral tissues and cause untoward reactions.
Although the SEM analysis revealed crystal-like structures on the surface of nonlatex elastics, overall they presented a homogeneous, regular and pore-free structure, which is less favorable to the adherence of microorganisms and consequently less aggressive to the surrounding tissues.
In view of the above, the nonlatex elastic was the material with better behavior. It is important to mention that the present results were obtained in an in vivo experiment in which elastics were inserted in the subcutaneous connective tissue of animals. In clinical practice, orthodontic elastics are not inserted in the oral tissues and do not remain for long periods, but rather maintain brief and continuous contacts with the oral soft tissues. It should be emphasized that tissue response may be influenced not only by the material used for fabrication of the elastic (natural rubber or SBR), but also by differences in chemical composition and product quality. Based on the results of this study, a stronger inflammatory response was verified in the initial periods after the contact with the tissue, with gradual decrease along time. Clinical studies are recommended to assess whether changes of elastics after shorter periods would elicit continuous inflammatory reactions as well as to reinforce the importance of seeking materials that undergo less (or slower) deterioration over time so that changes of elastics at longer intervals can be indicated without compromising the orthodontic mechanics and movements.
CONCLUSIONS
According to the results obtained and the used methodologies, the null hypothesis was rejected from the biologic standpoint.
The latex elastics were more irritating to the connective tissue than the nonlatex elastics in the initial evaluation periods and presented a less homogenous physical structure and a more porous surface than the nonlatex elastics.
REFERENCES
- 1.Pithon MM, Santos RL, Martins FO, Romanos MTV, Araújo MT. Cytotoxicity of orthodontic elastic chain bands after sterilization by different methods. Orthod Waves. 2010;8:1–5. [Google Scholar]
- 2.Perrella FW, Gaspari AA. Natural rubber latex protein reduction with an emphasis on enzyme treatment. Methods. 2002;4:77–86. doi: 10.1016/S1046-2023(02)00055-5. [DOI] [PubMed] [Google Scholar]
- 3.Bishara SE, Andreasen GF. A comparison of time related forces between plastic elastics and latex elastics. Angle Orthod. 1970;40:319–328. doi: 10.1043/0003-3219(1970)040<0319:ACOTRF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Hwang CJ, Cha JY. Mechanical and biological comparison of latex and silicone rubber bands. Am J Orthod Dentofacial Orthop. 2003;124:379–386. doi: 10.1016/s0889-5406(03)00564-x. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson JS, Kusy RP. Force application and decay characteristic of untreated and treated polyurethane elastomeric chains. Angle Orthod. 1994;64:455–467. doi: 10.1043/0003-3219(1994)064<0455:FAADCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Hanson M, Lobner D. In vitro neuronal cytotoxicity of latex and non-latex orthodontic elastics. Am J Orthod Dentofacial Orthop. 2004;126:65–70. doi: 10.1016/j.ajodo.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Everett FG, Hice TL. Contact stomatitis resulting from the use of orthodontic rubber elastics: report of case. J Am Dent Assoc. 1974;88:1030–1031. doi: 10.14219/jada.archive.1974.0221. [DOI] [PubMed] [Google Scholar]
- 8.Tomazic VJ, Withrow TJ, Fisher BR, Dillard SF. Latex-associated allergies and anaphylactic reactions. Clin Immunol Immunopathol. 1992;64:89–97. doi: 10.1016/0090-1229(92)90185-q. [DOI] [PubMed] [Google Scholar]
- 9.Russell KA, Milne AD, Khanna RA, Lee JM. In vitro assessment of the mechanical properties of latex and non-latex orthodontic elastics. Am J Orthod Dentofacial Orthop. 2001;120:36–44. doi: 10.1067/mod.2001.114642. [DOI] [PubMed] [Google Scholar]
- 10.Jorge JH, Giampaolo ET, Pavarina AC. Cytotoxicity of dental materials. A literature review. Rev Odontol UNESP. 2004;33:65–68. [Google Scholar]
- 11.Jonke E, Franz A, Freudenthaler J, König F, Bantleon HP, Schedle A. Cytotoxicity and shear bond strength of four orthodontic adhesive systems. Eur J Orthod. 2008;30:495–502. doi: 10.1093/ejo/cjn042. [DOI] [PubMed] [Google Scholar]
- 12.International Standard for Organization Biological evaluation of medical devices—tests for local effects after implantation. 2007:1–21. ISO 10993-6. [Google Scholar]
- 13.Magno AFF, Itikawa CE, Ito IY, Matsumoto MAN, Faria G, Nelson-Filho P. In-vivo evaluation of the contamination of Super Slick elastomeric rings by Streptococcus mutans in orthodontic patients. Am J Orthod Dentofacial Orthop. 2008;133:104–109. doi: 10.1016/j.ajodo.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 14.dos Santos RL, Pithon MM, Martins FO, Romanos MT, de Oliveira Ruellas AC. Evaluation of the cytotoxicity of latex and non-latex orthodontic separating elastics. Orthod Craniofacial Res. 2010;13:28–33. doi: 10.1111/j.1601-6343.2009.01469.x. [DOI] [PubMed] [Google Scholar]