Abstract
Objective:
To evaluate adhesive performance in terms of debonding forces of precoated metal and ceramic brackets 4 years after expiration.
Materials and Methods:
Buccal and lingual surfaces of embedded extracted maxillary premolars were etched with 34% Tooth Conditioner Gel (Dentsply Caulk, Milford, Del), rinsed, and dried. Transbond MIP (3M Unitek, Monrovia, Calif) was applied prior to placing adhesive precoated brackets (APC II Victory stainless steel and APC Plus Clarity ceramic brackets, 3M Unitek). The preexpiration brackets had 29–35 months before, and the postexpiration brackets were 45–52 months past, their expiration dates. Sample size was 17–21 per group. Debonding forces were determined by subjecting the bonded brackets to a shear force in a universal testing machine. Debonding forces were compared using two-way ANOVA. Debonded surfaces were examined under a stereomicroscope to determine failure modes, which were compared using the chi-square test.
Results:
No statistically significant difference was found in debonding forces (P = .8581) or failure modes (P = .4538) between expired and unexpired brackets. Metal brackets required statistically significantly higher debonding forces than did ceramic brackets (P = .0001). For both expired and unexpired brackets, failure modes were mostly cohesive in the adhesive layer for ceramic brackets, and mixed between adhesive and cohesive failure in the adhesive layer for metal brackets.
Conclusions:
Adhesive precoated brackets did not have any reduction in enamel-adhesion properties up to 4 years after their expiration date. Extended shelf life testing for precoated dental brackets may be worth considering.
Keywords: Expiration date; Adhesive; Precoated bracket; Bonding, Failure mode; Shelf life
INTRODUCTION
Precoating brackets with adhesive has been advocated as a cost-effective and efficient method of supplying brackets to clinicians. The strength of the bonds achieved with precoated brackets is comparable to that obtained by application of adhesive to uncoated brackets.1 Consequently, many practitioners use precoated brackets without concern for bond strength.
Adhesives for orthodontic use are typically packaged with expiration or beyond-use date and practitioners maintain and use inventory according to that date. According to the US Food and Drug Administration Code of Federal Regulations, Title 21, the expiration date means the date by which the device must or should be used.2 Inventory control measures within a practice can sometimes fail, resulting in the use of an outdated bracket pack. A survey from the US Army Institute of Dental Research in 1991 found that almost half of dental restorative materials used at US Army dental care facilities were beyond the manufacturer’s expiration date.3
Most products are assigned a 2- to 3-year expiration date from the time of manufacture. This does not necessarily mean that the material “goes bad” after the expiration date, but rather that the manufacturer assures the user that the material is still safe to use and that it will perform as required at the assigned expiration date.4,5 For many drug products, it was found that shelf life could be extended to an average of 66 months past their expiration date if properly stored.6 The US Department of Defense investigated expiration dates for drugs and found that shelf life of most could be extended 5 or more years without ill effects.7 It has been claimed that stability testing for extended shelf life by the Department of Defense cost $4 million but saved more than $260 million in inventory costs. The Council on Scientific Affairs of the American Medical Association therefore urged all parties involved—industry, consumers, and legislators—to consider longer stability testing for extended expiration dates.7
For the few published studies in dentistry investigating expiration date effects on mechanical properties of resin-based materials, the results have been mixed.8–10 In orthodontics, the dilemma of whether to use expired brackets is associated with both the economics of disposing of a potentially useful bracket and the risk of altered bond strength leading to either bracket bond failures and extended treatment times or compromised patients. At the authors’ institution, the Department of Orthodontics had approximately 1500 “expired” brackets in its inventory. These brackets had been continuously stored in their original packaging in an unrefrigerated, room-temperature environment following the manufacturer’s recommendation of storage between 2°C and 27°C.11,12 The disposition of those brackets and the lack of information about the effect of overrunning the expiration date on bracket adhesives prompted this comparative investigation.
The objective of this study was to answer the question about the significance of the expiration date relative to adhesive performance by comparing pre- and postexpiration date debonding forces used with adhesive precoated metal and ceramic brackets. The null hypothesis of this study was that there is no difference in adhesion between expired and unexpired precoated brackets.
MATERIALS AND METHODS
The effect of expiration dates on adhesion was tested by comparing the in vitro debonding forces of expired and unexpired adhesive precoated brackets. Extracted maxillary premolars (IRB approval 13-02375-XM) were embedded in acrylic resin (Orthodontic Resin, Dentsply Caulk, Milford, DE). Buccal and lingual surfaces were etched for 30 seconds with 34% phosphoric acid (Tooth Conditioner Gel, Dentsply Caulk), rinsed with water, and dried. Immediately prior to bracket placement, Transbond MIP (3M Unitek, Monrovia, CA) was applied to the prepared surface. Unexpired and expired adhesive precoated maxillary premolar brackets (APC II Victory stainless steel and APC Plus Clarity ceramic brackets) were placed and light-cured for 30 seconds using a halogen curing light. Because of the limited availability of extracted maxillary premolars, expired (45–52 months postexpiration) and unexpired (29–35 months preexpiration) brackets of the same type (stainless steel vs ceramic) were randomly placed on buccal and lingual surfaces. The minimum sample size was calculated to be 15 in order to have a 95% confidence level in detecting a mean difference of 0.5 standard deviation between groups. The actual sample sizes in this study were 17–21, depending on availability of the matched brackets. Expiration information and sample sizes are shown in Table 1.
Table 1.
Mean ± Standard Deviation of Debonding Forces in Newtons, Expiration Status, and Sample Sizes (N)
The bonded specimens were stored in water at 37°C for 24 hours. Bond strengths were tested using a universal testing machine (Instron 5567, Norwood, MA). The bracket base was loaded in shear mode using a knife-edge stainless steel bar until debonding occurred under displacement control at a crosshead rate of 0.5 mm/min. Debonding forces were recorded with a 10 kN load cell and analyzed using a two-way ANOVA and pairwise comparisons.
The fracture surfaces of the brackets and the teeth were viewed under a stereomicroscope to determine failure mode as diagrammatically shown in Figure 1. The modes of failure were categorized as adhesive failure (type A-1, between bracket and adhesive; type A-2, between adhesive and enamel), cohesive failure (type C-1, within the adhesive layer; type C-2, within enamel), or mixed-mode failures (a combination of adhesive and cohesive failures). The failure modes of expired and unexpired metal and ceramic brackets were compared using a chi-square test.
Figure 1.

Diagram showing failure mode identification. Adhesive failures: Type A-1, bracket/adhesive, type A-2, adhesive/enamel. Cohesive failures: type C-1, within the adhesive layer, type C-2, within enamel. Mixed mode failures: a combination of adhesive and cohesive failures.
RESULTS
Debonding forces are shown in Table 1. The debonding forces followed a normal distribution (Anderson-Darling normality test; 59% – 93% confidence), and thus the two-way ANOVA was used for statistical comparison. Metal brackets required higher debonding forces than ceramic brackets, regardless of expiration status (P = .0001). No significant difference was found between pre- and postexpiration brackets with either the pooled data (P = .6011) or within the bracket type (P = .6947 for metal and P = .7290 for ceramic brackets, respectively). Two-way ANOVA also indicated that the interaction between bracket type and expiration status did not significantly affect the debonding forces (P = .9665).
Occurrences of various failure modes are shown in Table 2. Failure mode was significantly affected by bracket type (chi-square test, P = .0001). The most frequent mode of failure for ceramic brackets was a cohesive failure in the adhesive layer with 12 of the 17 expired brackets and 9 of the 21 unexpired brackets. Figures 2A and 3A are examples of ceramic brackets with cohesive failure in the adhesive layer. For metal brackets, mixed mode was the predominant failure mode for both expired and unexpired brackets. Figures 2B and 3B show debonded metal brackets that had cohesive failure in the adhesive layer (C-1) and adhesive failure between bracket and adhesive (A-1), and between adhesive and enamel (A-2). There was no evidence that expiration status affected the failure modes (chi-square test, metal P = .7015; ceramic P = .3990; pooled metal and ceramic P = .4538). Debonding resulting in cohesive failure of the enamel occurred in a total of five brackets. This failure mode was identified in all groups without any specific association (Table 2).
Table 2.
Failure Modesa
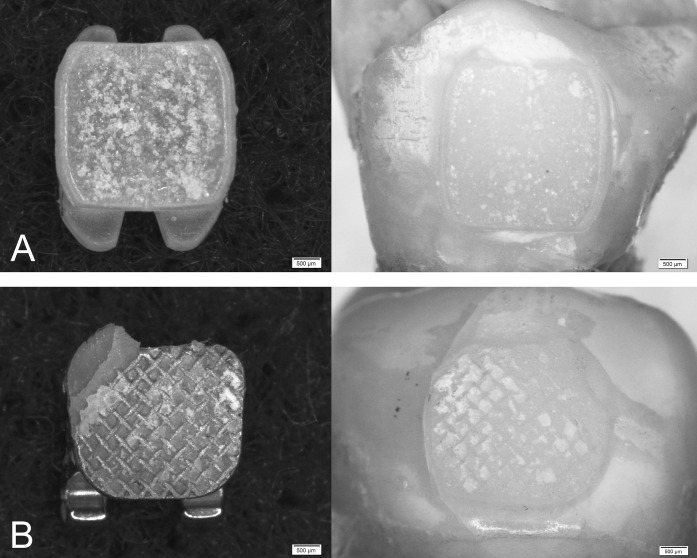
Figure 2.
(A) Debonded ceramic bracket showing cohesive failure in the adhesive layer and the corresponding debonded enamel surface. (B) Debonded metal bracket with mixed failure mode (A-1, C-1, A-2) and the corresponding debonded enamel surface.
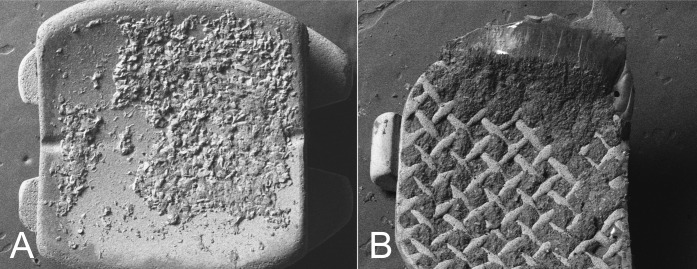
Figure 3.
Scanning electron micrographs of debonded ceramic bracket (A) and metal bracket (B).
DISCUSSION
The bond strength of orthodontic adhesives should be sufficient to secure attachments for the duration of a typical course of orthodontic treatment. A 4.9-MPa in vitro bracket bond strength is considered adequate for clinical application.13 In vitro bond strengths for ceramic and metal brackets often vary between studies due to differences in bracket designs and testing conditions.14–16 It is equally important, however, that brackets can be safely and easily removed. Currently available adhesive precoated brackets meet these requirements.
In this study, we investigated whether the use of precoated brackets after their expiration dates affects the adhesive properties by measuring the force required for debonding. Bond strength can be defined as a material property (reported in terms of stress) or as a structural property (reported as a force). We chose to report the forces because it can be argued that a force has a more direct clinical relevance than stress. Forces reflect the effort of the practitioner or the external challenge and avoid simplifications that are applied when stress is assumed to equal force divided by surface area. That simplification does not take into account effects such as bracket surface curvature or load application. However, for comparative purposes, the forces found in this study can be divided by the bracket bonding areas (9.3 mm2 for metal and 11.2 mm2 for ceramic brackets) to estimate the maximum bond stresses as 25 MPa for metal brackets and 14 MPa for ceramic. Regardless of strength definition, the results of this study showed that the adhesion of the precoated metal and ceramic brackets was not significantly affected by expiration status. Moreover, it was found that failure modes were not significantly different between the expired and unexpired samples. Failure modes were affected by bracket types, with ceramic brackets failing more often in the adhesive layer and metal brackets more often in mixed failure modes. Of interest is that there were five occurrences of cohesive failure within the tooth (enamel fracture), which is an undesired and injurious side effect of the bonding/debonding process. These occurrences, however, were too small to speculate further as to their cause. It is possible that different debonding patterns would be experienced in a clinical environment. Based on general observations, it can be concluded that neither adhesive strength nor failure mode showed evidence of changes in adhesive performance 45–52 months after bracket expiration. Therefore, the null hypothesis, that is, no effect of the expiration date, was accepted.
Despite important health and economical implications, effects of expiration dates on the adhesive properties of precoated dental brackets have not been reported previously. Few studies have tested the effect of expiration dates on properties of dental composites and bonding agents, and the results of those studies are mixed. One study showed no change in mechanical properties of light-cured composites over a 7-year period regardless of storage conditions.8 Others reported reduced microhardness for some expired composites.9,10 This was attributed to degradation of the photoinitiator, leading to a lower degree of polymerization and thus reduced microhardness.9 Volatile acetone-based dental adhesives were shown to develop reduced bond strengths due to evaporation of the solvent.17 The shelf life of precoated adhesive, which is more comparable to dental composites than to dental adhesives, is likely primarily determined by the influence of storage on ingredients that affect the polymerization process. Packaging is thus an important aspect. Composites packaged in syringes maintained hardness, depth of cure, and handling characteristics that were equal to the unexpired composites.18 The adhesive precoated brackets used in this study were packed individually in a foil package, with minimal light exposure, which may have helped maintain the original adhesive properties even 45–52 months after their expiration date.
Many dental products have an expiration date of 2 or 3 years from the time of manufacture. This time period may have been inspired more by standard shelf life used for pharmaceutical products than scientific reasons specific to dental materials. As discussed before, dental materials’ shelf life is likely to vary depending on type of material and packaging, and composites are quite stable whereas bonding agents with volatile substances are not. Even for pharmaceutical products, the actual shelf life has often been shown to be greater than their labeled expiration dates.6,7 Manufacturers have argued that to extend expiration dates by testing drug stability for an extended expiration period will add cost to an already expensive industry. However, others claim that the cost savings of extended shelf life outweigh the extra testing costs.7 Therefore, it would be useful for industry and regulatory bodies to revisit the definition and determination of expiration dates and consider making it more specific to material type, composition, and storage conditions.
In the United States, the Food and Drug Administration (FDA) regulates packaging and labeling of medical devices and in the European Union (EU), similar regulations and guidelines are included in “New Approach” legislation. The FDA definition of expiration date with respect to package labeling is: “Expiration date means the date by which the label of a device states the device must or should be used.”2 The EU mandatory CE mark indicates compliance with a similar regulatory process. As in the United States, the EU requirement for labeling is “an indication of the date by which the device should be used, in safety.”19 Whether the device is safe to use after the labeled date is ultimately the responsibility of the end user. In the event of bracket bond failure, injury to the patient could take many forms from soft tissue injury to aspiration of the individual bracket. The decision to use an adhesive precoated bracket beyond the expiration date should consider risk to the patient and the medicolegal implications associated with such use and injury. Responsibility for the injury would pass from the manufacturer to the user if intentional use of a product beyond the labeled date could be proven in the legal system. While the results of an in vitro study such as this might be used to defend a disregard for package labeling, this study did not consider other properties of the adhesive material such as release of chemical components, susceptibility to discoloration, or bond strength performance beyond 24 hours. Manufacturer’s recommendations should thus be followed in using any material, and good inventory control should prevent holding any product beyond the labeled expiration date regardless of storage conditions. Nevertheless, this study shows that the expiration date does not necessarily represent the expiration of a material but rather a limit to which a manufacturer is committed to test its product.
CONCLUSIONS
Within the limitations in this study:
Adhesive properties and failure behavior of precoated metal and ceramic brackets were unaffected by exceeding their expiration dates for 45–52 months.
It may therefore be worthwhile to consider conducting longer stability testing on dental products for extending expiration dates.
ACKNOWLEDGMENT
We would like to acknowledge 3M Unitek Orthodontic Products Division for the donation of the unexpired brackets and Transbond MIP used in this study and Brian Morrow for helping with the SEM.
REFERENCES
- 1.Bishara SE, Olsen M, Von Wald L. Comparisons of shear bond strength of precoated and uncoated brackets. Am J Orthod Dentofacial Orthop. 1997;112:617–621. doi: 10.1016/s0889-5406(97)70226-9. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration. Code of Federal Regulations Title 21, Volume 8 Definitions 21 CFR801.3. Revised April 1, 2014. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=801.3 Accessed March 4, 2015.
- 3.Hondrum SO. The U.S. Army Institute of Dental Research dental materials shelf-life survey: lot request results. Mil Med. 1991;156:491–493. [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. Shelf life of medical devices. April 1991. http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidance%20documents/ucm081366 . Accessed March 4, 2015. [Google Scholar]
- 5.Pharmacist’s Letter. 2010 Technician Training Tutorial: Drug Expiration Dates, http://pharmacytechniciansletter.therapeuticresearch.com/pl/DetailDocuments/260130.pdf. Accessed March 4, 2015.
- 6.Lyon RC, Taylor JS, Porter DA, Prasanna HR, Hussain AS. Stability profiles of drug products extended beyond labeled expiration dates. J Pharm Sci. 2006;95:1549–1560. doi: 10.1002/jps.20636. [DOI] [PubMed] [Google Scholar]
- 7.American Medical Association. Pharmaceutical Expiration Dates. Report 1 of the Council on Scientific Affairs. July 25, 2001. http://www.ama-assn.org/meetings/public/annual01/csa_reports.pdf . Accessed March 4, 2015. [Google Scholar]
- 8.Hondrum SO, Fernandez R., Jr The storage stability of dental composite resins: seven-year results. Gen Dent. 1997;45:382–389. [PubMed] [Google Scholar]
- 9.Tirapelli C, Panzeri F de C, Panzeri H, Pardini LC, Zaniquelli O. Radiopacity and microhardness changes and effect of x-ray operating voltage in resin-based materials before and after the expiration date. Mat Res. 2004;7:409–412. [Google Scholar]
- 10.Garcia L da F, Roselino L de M, Pires-de-Souza F de C, Consani S. Evaluation of the conversion degree, microhardness, and surface roughness of composite resins used after their expiration date. Gen Dent. 2010;58:e262–e267. [PubMed] [Google Scholar]
- 11.3M Unitek Publication, Instructions for Use, APC II, 011-583-6, 2009. http://multimedia.3m.com/mws/media/156052O/apc-ii-adhesive-coated-appliance-system-instructions.pdf?fn=011-583-6_APCII_ML_1205_ML_Web.pdf . Accessed March 4, 2015.
- 12.3M Unitek Publication, Instructions for Use, APC Plus, 011-592-7, 2005. http://multimedia.3m.com/mws/media/441402O/apc-plus-adhesive-coated-appliance-system-ifu.pdf?&fn=011-592-7_APCPlus_ML_1205_HR.pdf . Accessed March 4, 2015.
- 13.Reynolds IR. A review of direct orthodontic bonding. Br J Orthod. 1975;2:171–178. [Google Scholar]
- 14.Bishara SE, Oonsombat C, Soliman MMA, Warren JJ, Laffoon JF, Ajlouni R. Comparison of bonding time and shear bond strength between a conventional and a new integrated bonding system. Angle Orthod. 2005;75:237–242. doi: 10.1043/0003-3219(2005)075<0233:COBTAS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes TMF, Janson G, Somensi J, et al. Effects of modifying the bonding protocol on the shear bond strength of metallic and ceramic orthodontic brackets. Gen Dent. 2012;60:51–55. [PubMed] [Google Scholar]
- 16.Reddy YG, Sharma R, Singh A, Agrawal V, Agrawal V, Chaturvedi S. The shear bond strengths of metal and ceramic brackets: an in-vitro comparative study. J Clin Diagn Res. 2013;7:1495–1497. doi: 10.7860/JCDR/2013/5435.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perdigão J, Swift EJ, Jr, Lopes GC. Effects of repeated use on bond strengths of one-bottle adhesives. Quintessence Int. 1999;30:819–823. [PubMed] [Google Scholar]
- 18.Penugonda B, Penugonda B. Evaluation of expired composite materials’ efficacy. J Dent Res. 2006;85 (Spec Iss B): 2127. https://iadr.confex.com/iadr/2006Brisb/techprogram/abstract_78361.htm . Accessed March 4, 2015. [Google Scholar]
- 19.Concerning Medical Devices, Council Directive 93/42/EEC, The Council of The European Communities, 14 June 1993. Accessed March 4, 2015 http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1993L0042:20071011:en:PDF . [Google Scholar]