Abstract
Objective:
To investigate combined effect of photobiomodulation with a matrix metalloproteinase (MMP) inhibitor on the relapse rate in relation to MMP expression in rats.
Materials and Methods:
Fifty-two rats were divided into four groups according to the treatment modality: control group, irradiation group, doxycycline group, and irradiation with doxycycline group. During a relapse period of 5 days after orthodontic movement, maxillary central incisors were treated by low-level laser therapy (LLLT) as a photobiomodulation and/or doxycycline as a synthetic MMP inhibitor. Relapse rate was evaluated in association with MMP expression at the gene and protein levels.
Results:
Relapse rates were increased by LLLT (1.57-fold) and decreased by doxycycline (0.83-fold) compared with the control, showing positive correlation with the levels of expression for all MMPs in the periodontal ligament (PDL). LLLT concomitant with doxycycline administration resulted in no significant differences of relapse rate and MMP expression from the control.
Conclusions:
The combined effect of photobiomodulation with an MMP inhibitor around the relapsing teeth proved to be antagonistic to PDL remodeling activity during relapse. This study suggests a basis for developing a novel biologic procedure targeting the MMP-dependent PDL remodeling to control the relapse rate.
Keywords: Orthodontic relapse, Photobiomodulation, Doxycycline
INTRODUCTION
Immediate relapse after orthodontic tooth movement (OTM) occurs through the remodeling of the transformed periodontal ligament (PDL) and alveolar bone around the moved teeth.1 During active OTM, accumulated strains on the PDL fiber proliferate and generate an opposite force to that of the previous tooth movement, initiating relapse movement in the absence of secure retention after appliance removal; conversely, the PDL fibers move in opposite configurations from compressed to stretched or from stretched to compressed conditions.2 PDL remodeling can progress as a result of degeneration, degradation, and regeneration of extracellular matrix (ECM) components such as type I and III collagens.
Matrix metalloproteinases (MMPs) are proteolytic enzymes that degrade the ECM of connective tissues1,3; they are produced by periodontal fibroblasts and counteracted by tissue inhibitors of metalloproteinases in the PDL.4 The expression of MMPs was investigated to support the biological mechanism of periodontal remodeling during OTM.5,6 Collagenase subfamilies (MMP-1, MMP-8, and MMP-13) were reported to increase cleavage of type I and III collagens of the PDL, and gelatinase subfamilies (MMP-2 and MMP-9) increased removal of degenerated collagen-like gelatin or hyalinized tissues in the compressed PDL during OTM. As relapse can be considered an active tooth movement, both transcriptional and translational changes in the regulation of MMPs can serve as biologic markers of periodontium remodeling during relapse.
As a pharmacologic approach, tetracycline or chemically modified tetracyclines have been applied as synthetic MMP inhibitors to inhibit periodontal remodeling during OTM as well as pathologic periodontal degradation.7 Doxycycline, a more potent MMP inhibitor than other tetracyclines, was reported to decrease the rate of OTM by decreasing bone-resorbing activity and root resorption.8–11 Administration of doxycycline might help verify the role of MMPs in PDL remodeling in the initial relapse state, thus providing the basis for further development of biologic retention protocols targeting the control of MMPs and collagens in the PDL.
As a photobiomodulation approach, low-level laser therapy (LLLT) was reported to affect the rate of relapse movement by stimulating MMP gene expression in the periodontium in our previous studies.12,13 LLLT on moved teeth without retainers increased the relapse rate while maintaining a balance between collagen degradation and synthesis in the activated remodeling state. In contrast, LLLT on teeth fixed with retainers facilitated collagen synthesis exceeding collagen degradation, which could be indicated by the relative expression levels of MMPs and collagen in the PDL. Related studies regarding the effect of LLLT on MMP expression in the connective tissues were performed. In aortic smooth muscle cells, LLLT upregulated MMP-1 and MMP-2 expression concurrently with collagen synthesis stimulation,14 whereas it increased MMP-1, MMP-8, and MMP-13 gene expression with reduced collagen expression in the PDL during OTM.15 Focusing on the postorthodontic relapse state, it was questionable whether the biostimulation induced by LLLT would act on MMP expression in the PDL synergistically or antagonistically with a pretreated MMP inhibitor.
The aim of this study was to investigate the combined effects of photobiomodulation with doxycycline on the expression levels of MMPs in rat periodontium during the initial relapse after OTM. Differential expression levels of five subtypes of MMPs will be elucidated at the protein and gene levels in relation to the relapse rate.
MATERIALS AND METHODS
The animal experimental protocol used in this study was approved by Kyung Hee Medical Center Institutional Animal Care and Use Committee (Approval No. KHMC IACUC 11-029).
A total of 52 male, 8-week-old Sprague-Dawley rats, weighing 200 ± 20 g, were enrolled. Animals were randomly divided into four groups as follows: a control group without any treatment after OTM (group C, n = 12); an irradiation group (group L, n = 12); a doxycycline group (group D, n = 12); and an irradiation + doxycycline group (group LD, n = 12). Animals in each group were euthanized at days 1, 3, and 5 after relapse. Another four rats were used to set up a baseline reference MMP messenger RNA (mRNA) expression level.
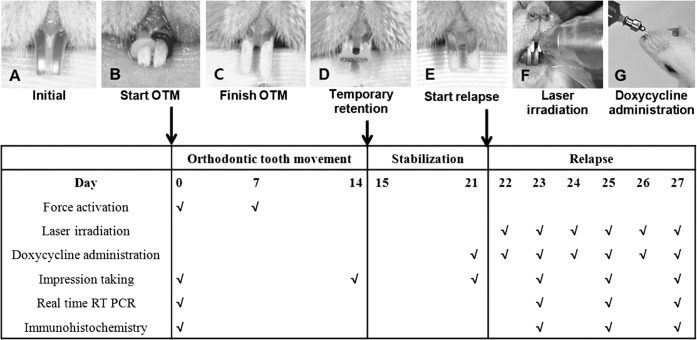
Under general anesthesia with Zoletil (0.25 mg/kg; Virac Lab, Carros, France), the distal movement of maxillary central incisors was performed by inserting elastomeric rings (Unistick colored ligature, American Orthodontics, Sheboygan, Wis) in all groups (Figure 1B). After the completion of OTM for 2 weeks, the distally moved central incisors were temporarily fixed by a resin-wire splint for stabilization for a week after elastic removal (Figure 1C, D). Then, the resin-wire splint was removed to induce relapse movement (Figure 1E). In groups D and LD, doxycycline (CollaGenex Pharmaceuticals Inc, Newton, Pa), dissolved in phosphate buffered saline (PBS) to the minimum effective dose of 5 mg/kg per day,16 was orally administered every day from day 1 before elastic removal to the day of euthanasia; (PBS) was administered in the other groups (Figure 1G). In groups L and LD, LLLT was performed once a day from the day of elastic removal to the day of euthanasia. A gallium-aluminum-arsenide diode laser (Laser Hand, MM Optics Ltd, São Carlos, Brazil) with a wavelength of 780 nm was used in the biostimulation mode. Continuous waves were delivered on the gingiva over the root areas of each central incisor in a contact mode with the total energy dose of 20J/cm2 per day (Figure 1F).
Figure 1.
A rat model for this study on relapse after orthodontic tooth movement (OTM) and experimental schedule. (A) Before intervention. (B) Initiating OTM by inserting elastomeric rings. (C) Separating incisors when orthodontic force stopped. (D) Teeth immobilized by wire-resin splint retainer. (E) Initiating relapse movement after removal of retainer. (F) Laser irradiation. (G) Doxycycline administration.
The distance of OTM was measured between the most mesial points of the central incisors at the gingival margin level on stone models. Relapse distance was defined as the difference between the distance of OTM and the remaining space at each time. All measurements were repeated twice by an examiner to determine the error of method 2 weeks apart. Relapse rate (%) was calculated as the ratio of relapse distance to the OTM at three observation time points: 1, 3, and 5 days.
Expression of MMP-1, MMP-8, MMP-13, MMP-2, and MMP-9 was determined using the TaqMan Gene Expression Assays kit (Applied Biosystems Inc, Carlsbad, Calif). Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed using Chromo4 RT-PCR analysis (Bio-Rad Laboratories, Hemel Hempstead,UK). Bio-Rad Laboratories, Hemel Hempstead, UK, was used for the relative quantification of gene expression with GAPDH as a housekeeping gene. These data were converted into relative values as the value of the cage control group without any intervention was converted into 1.00 as a reference. The relative mRNA expression of five MMPs in the respective groups was compared at 1, 3, and 5 days after relapse.
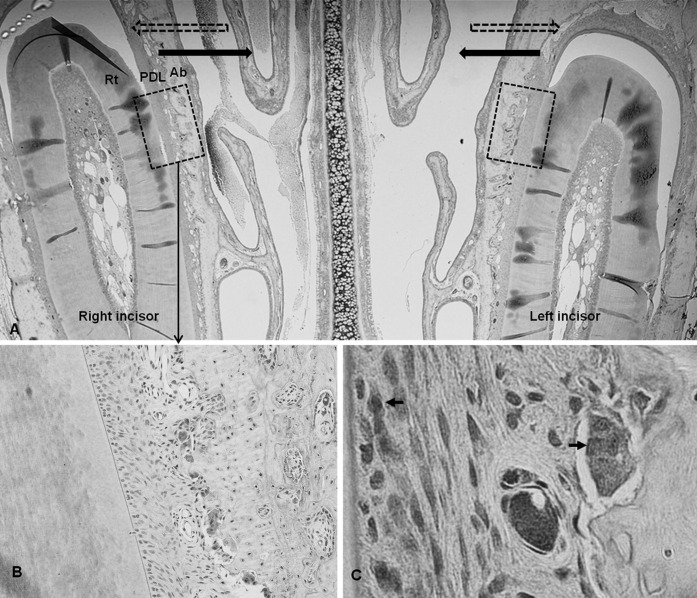
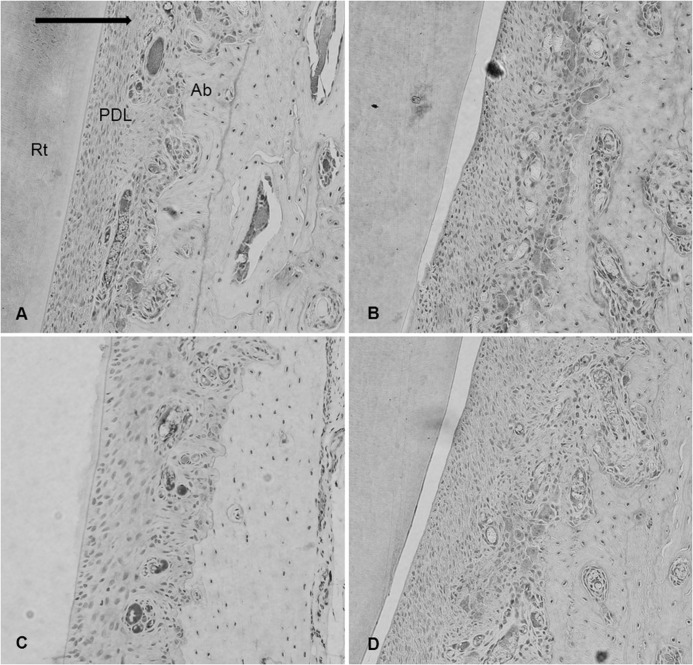
The immunohistochemical analysis was performed with primary antibodies for five MMPs using a biotin-free polymeric horseradish peroxidase-linker antibody conjugate system. On the microphotographs taken under light microscopy, the number of immunoreactive cells within the range of interest on the mesial side of each central incisor was counted three times by a pathologist (Figure 2).
Figure 2.
Microphotograph of immunohistochemical staining in the experimental groups. (A) Representative microphotograph of the whole observation area, including teeth and periodontal tissues used in the experiment, ×20. Dashed arrow indicates direction of previous orthodontic tooth movement; solid arrow, direction of relapse movement. Former tension sides were changed into compression sides. Dashed box indicates the range of interest. (B) Immunoreactivity to MMPs was seen in the multinucleated cells along the resorbed bone surface, ×200.(C) Immunoreactive cells, ×1000. Rt indicates incisor root; PDL, periodontal ligament; Ab, alveolar bone.
Repeated-measures analysis of variance (ANOVA) was performed to compare the relapse rate over time between the groups. One-way ANOVA was used to compare the mRNA expression level of each MMP and the mean number of immunoreactive cells to each MMP between the groups, followed by Duncan post-doc analysis. Spearman correlation analysis was performed between the relapse rate and the mRNA expression level of each MMP. A P value of < .05 was considered statistically significant.
RESULTS
Relapse Rate
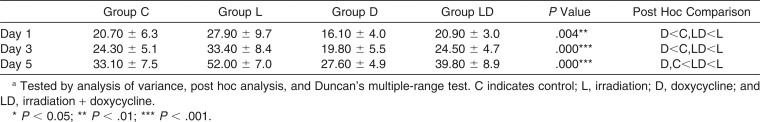
There was no significant intergroup difference in the distance of previous OTM (P > .05). Relapse rates increased in a time-dependent manner throughout the experimental period in all groups (Figure 3). The increment ratio over time in group LD was significantly lower than that in group L and higher than that in group D (P < .05). As for the relapse rate at each time point, group LD showed no significant difference from group C at all times. On the other hand, group L showed a significant relapse rate increase of 1.35-fold at day 1, 1.37-fold at day 3, and 1.57-fold at day 5, while group D showed a decrease of 0.78-fold at day 1, 0.82-fold at day 3, and 0.83-fold at day 5, respectively, in comparison with group C (Table 1).
Figure 3.
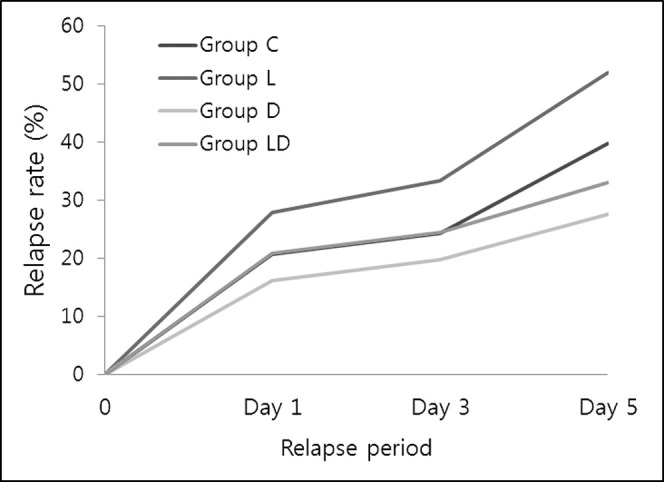
Intergroup comparison of the increment ratio of relapse rate over time. Tested by repeated-measures ANOVA and post hoc analysis, Duncan's multiple range test. * P < .05; ** P < .01; *** P < .001.
Table 1.
Intergroup Comparison of Relapse Rate (%) at Each Observation Time Pointa
Relative mRNA Expression of Five MMPs
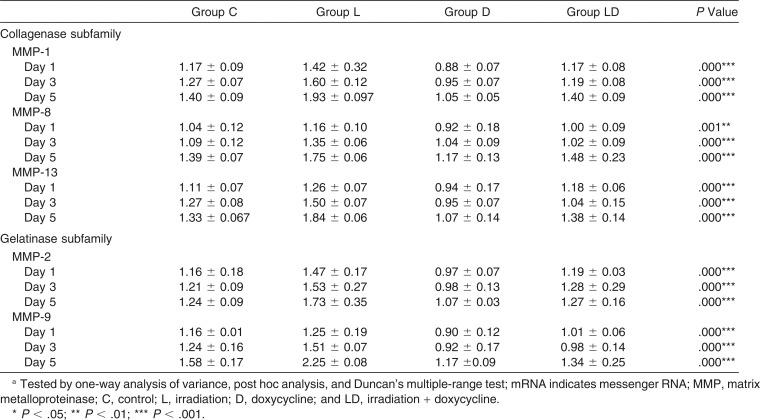
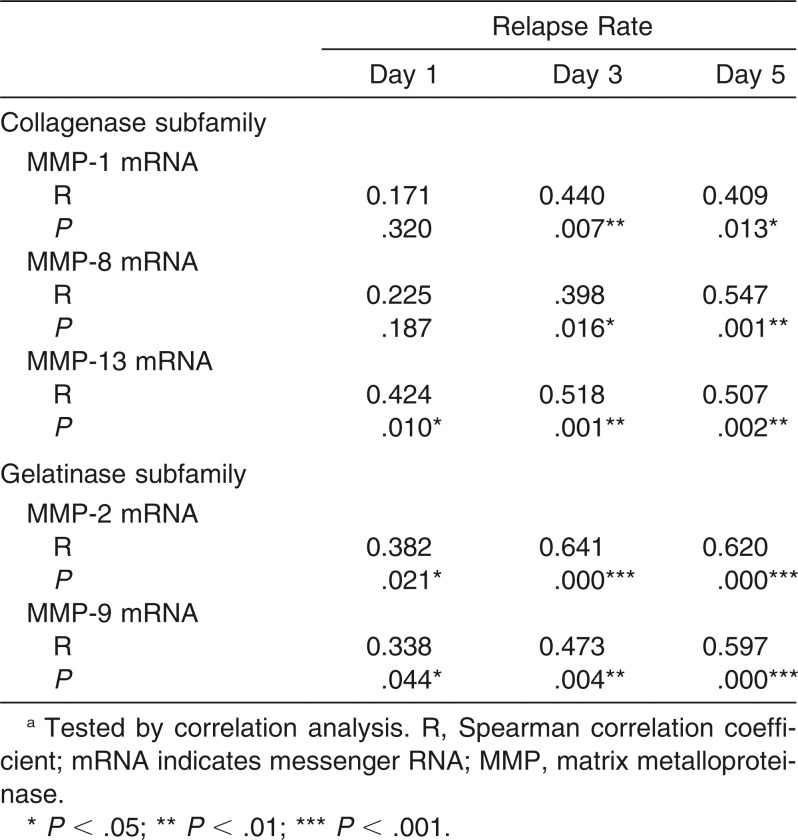
The converted relative values of mRNA expression levels of all the tested MMPs increased in each group in a time-dependent manner (Table 2). In comparison with group C, the mRNA expression of MMP-1, MMP-8, MMP-13, MMP-2, and MMP-9 were all stimulated in group L (P < .01) and inhibited in group D (P < .01), showing the greatest intergroup difference between groups L and D on day 5. On the other hand, group LD presented no significant difference from group C at all observation time points. A significant correlation was found between MMP gene expression and relapse rate: MMP-1 and MMP-8 showed a positive correlation at day 3 and 5; MMP-2, MMP-9, and MMP-13 showed positive correlations at days 1, 3, and 5 (Table 3).
Table 2.
Intergroup Comparison on the Relative Values of mRNA Expression of Each Tested MMP at Days 1, 3, and 5 of Relapsea
Table 3.
Correlation Between the Relapse Rate and mRNA Expression of Each MMP at Days 1, 3, and 5 of Relapsea
Immunohistochemical Findings
There were no pathologic tissue changes, such as inflammation, that may have affected the results of MMP expression in all specimens. The mesial side of each central incisor, converted from the tension side during the previous movement to the compression side during relapse, presented mixed formative and resorption findings for PDL and alveolar bone. PDL fibers were irregularly arranged or partially disconnected with surrounding dispersed hyalinization. Multinucleated osteoclast-like cells within the resorption lacunae were observed along the newly formed bone during the previous OTM (Figure 4).
Figure 4.
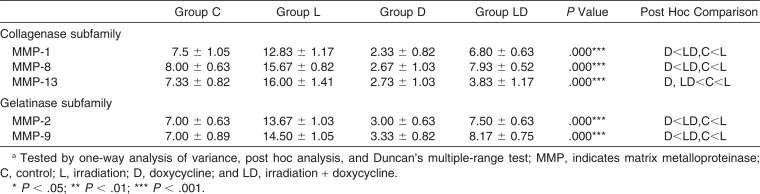
Immunohistochemical staining for experimental groups in the range of interest at day 5 of relapse, ×200. (A) Control group. (B) Irradiation group. (C) Doxycycline group. (D) Irradiation + doxycycline group. Solid arrow indicates direction of relapse movement; Rt, incisor root; PDL, periodontal ligament; Ab, alveolar bone.
The protein expression level of each MMP was evaluated by the number of immunopositive cells at day 5 after relapse (Table 4). Immunoreactivity was remarkable in PDL fibroblasts and multinucleated osteoclast-like cells (Figure 2C). Group L revealed the greatest recruitment of osteoclast-like cells as well as a greater ratio of immunoreactive cells for all the tested MMPs (Figure 4B), whereas group D showed very few reactive cells (Figure 4C). There were no significant differences in immunoreactive cell numbers between group LD (Figure 4D) and group C (Figure 4A), except in the case of MMP-13.
Table 4.
Intergroup Comparison of the Number of Immunoreactive Cells for Each Tested MMP in the Periodontal Ligament and Alveolar Bone Surface in the Direction of Tooth Relapse
DISCUSSION
In the present study, the combined effect of LLLT with doxycycline on both relapse rate and MMP expression in the PDL was proved to be antagonistic, although the stimulatory effect of LLLT and inhibitory effect of doxycycline were separately verified. Basically, relapse rate and MMP expression increased in a time-dependent manner and showed a positive correlation in all groups, thus strongly indicating that MMPs play an important role in PDL remodeling during initial relapse. In terms of relapse rate, the increment slope for 5 days in group LD was comparable with that in group C, which was significantly smaller than that in group L and greater than that in group D (Figure 3).
Doxycycline-induced inhibition of MMPs may have decreased the relapse rate by reducing the collagen turnover rate and delaying gelatin-like hyalinized tissue removal in the PDL. As for the stability enhancement, doxycycline might be applied when no fixed retainer is available. However, the repression of catabolic activity could disturb the biological balance and could cause harm to periodontal health after OTM. On the other hand, LLLT-induced biostimulation of collagenases and gelatinases increased the relapse rate in the absence of retainer while keeping the balance between collagen degradation and synthesis in an activated state. This could be inversely used to shorten the total retention period via faster tissue reorganization in the presence of a mechanical retainer, instead of being used for complete substitution of a conventional retainer. The present study was designed to determine whether the combined approach of LLLT with an MMP inhibitor would serve as a comprehensive approach to control the periodontal remodeling activity favorably for the purpose of a better postorthodontic retention. However, the photobiomodulating effect of LLLT directly acted on the periodontal cells instead of assisting the action of doxycycline, counterbalancing the tissue remodeling.
Interestingly, group LD showed significantly lower relapse rates and MMP expression levels than group L, whereas it showed a higher relapse rate and MMP expression levels than group D with no significance within day 3. Then, differences between LD and D groups became more remarkable and significant at day 5. The anti-MMP activity of systemically administered doxycycline seemed to be more potent than local photobiostimulation at the initial stages; nonetheless, the reverse situation might occur after day 5 in consideration of the greater increment of relapse rate and MMP expression in group L than in group D. Although the interactive mechanisms between the two modalities could not be clearly investigated, the direct inhibitory effect of doxycycline on MMP or pro-MMP activity17 and the indirect stepwise effect of LLLT on the overall remodeling activity18 seem to lead to a different total effect over time. The findings of the present study will provide a clue for the development of a novel biologic procedure targeting the MMP-dependent PDL remodeling in further studies.
The biologic effects of the two modalities must be dose-dependent. Our protocols for doxycycline administration and LLLT followed the previously reported therapeutic windows after confirming each effect in the pilot study using rats. Doxycycline was orally administered with a minimum effective dose of 5 mg/kg per day.16 The total energy dose of LLLT was set to 20 J/cm2 per day. Since the effect of LLLT on OTM is known to vary from acceleration to deceleration based on the energy dose,19 it is possible that LLLT at a certain dose may act synergistically with doxycycline administration.
Besides the catabolic activity of PDL tissue as a result of MMPs, various factors are related to the relapse. The effects of doxycycline and LLLT on the alveolar bone remodeling cannot be excluded to explain the ultimate results of relapse rate. Doxycycline was reported to show antiresorption activities in alveolar bone and root cementum associated with delayed OTM by inhibiting osteoclast recruitment and proliferation.16 On the other hand, many studies supported the accelerating effects of LLLT on bone resorptive and bone formative activities by the simultaneous activation of all the periodontal cells.18–20 Nevertheless, the present study is valuable in elucidating early PDL tissue response, which is considered to be more critical for immediate relapse than subsequent bone remodeling.
CONCLUSIONS
The combined effect of photobiomodulation by LLLT and an MMP inhibitor, doxycycline, resulted in no significant effects on relapse rate and MMP expression in the PDL by offsetting the effects of the two modalities.
Nonetheless, based on the result that the relapse rate and MMP expression showed a positive correlation in all treatment groups, this study suggests a basis for the development of a novel biologic procedure targeting the MMP-dependent PDL remodeling to control the relapse rates.
ACKNOWLEDGMENT
This work was supported by a National Research Foundation of Korea grant funded by the Korean government (No. 2010-0004873).
REFERENCES
- 1.Stamenkovic I. Extracellular matrix remodeling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi I, Onodera K, Nishimura M, Mitnai H, Sasano Y, Mitani H. Expression of genes for gelatinases and tissue inhibitors of metalloproteinases in periodontal tissues during orthodontic tooth movement. J Mol Histol. 2006;37:333–342. doi: 10.1007/s10735-006-9060-7. [DOI] [PubMed] [Google Scholar]
- 4.Verstappen J, Von den Hoff JW. Tissue inhibitors of metalloproteinases: their biological functions and involvement in oral disease. J Dent Res. 2006;85:1074–1084. doi: 10.1177/154405910608501202. [DOI] [PubMed] [Google Scholar]
- 5.Bolcato-Bellemin AL, Elkaim R, Abehsera A, Fausser JL, Haikel Y, Tenenbaum H. Expression of mRNAs encoding for alpha and beta integrin subunits, MMPs, and TIMPs in stretched human periodontal ligament and gingival fibroblasts. J Dent Res. 2000;79:1712–1716. doi: 10.1177/00220345000790091201. [DOI] [PubMed] [Google Scholar]
- 6.Bumann A, Carvalho RS, Schwarzer CL, Yen EHK. Collagen synthesis from human PDL cells following orthodontic tooth movement. Eur J Orthod. 1997;19:29–37. doi: 10.1093/ejo/19.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Bildt MM, Snoek-Van Beurden AMP, DeGroot J, Van El B, Kuijpers-Jagtman AM, Von den Hoff JW. Chemically modified tetracyclines stimulate matrix metalloproteinase-2 production by periodontal ligament cells. J Periodont Res. 2006;41:463–470. doi: 10.1111/j.1600-0765.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 8.Bildt MM, Henneman S, Maltha JC, Kuijpers-Jagtman AM, Von den Hoff JW. CMT-3 inhibits orthodontic tooth displacement in the rat. Arch Oral Biol. 2007;52:571–578. doi: 10.1016/j.archoralbio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Bildt MM, Bloemen M, Kuijpers-Jagtman AM, Von den hoff JW. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in gingival crevicular fluid during orthodontic tooth movement. Eur J Orthod. 2009;31:529–535. doi: 10.1093/ejo/cjn127. [DOI] [PubMed] [Google Scholar]
- 10.Hudson JB, Hatch N, Hayami T, et al. Local delivery of recombinant osteoprotegerin enhances postorthodontic tooth stability. Calcif Tissue Int. 2012;90:330–342. doi: 10.1007/s00223-012-9579-4. [DOI] [PubMed] [Google Scholar]
- 11.Golub LM, Wolff M, Roberts S, Lee HM, Leung M, Payonk GS. Treating periodontal disease by blocking tissue-destructive enzymes. J Am Dent Assoc. 1994;125:163–169. doi: 10.14219/jada.archive.1994.0261. [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Kang YG, Park JH, Kim EC, Park YG. Effects of low-intensity laser therapy on periodontal tissue remodeling during relapse and retention of orthodontically moved teeth. Lasers Med Sci. 2013;28:325–333. doi: 10.1007/s10103-012-1146-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Paek JH, Park KH, Kang SG, Park YG. Laser-aided supracrestal circumferential fiberotomy and low-level laser therapy effects on relapse of rotated teeth in beagles. Angle Orthod. 2010;80:385–390. doi: 10.2319/051609-268.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavish L, Perez L, Gertz SD. Low-level laser Irradiation modulates matrix metalloproteinase activity and gene expression in procine aortic smooth muscle cells. Lasers Surg Med. 2006;38:779–786. doi: 10.1002/lsm.20383. [DOI] [PubMed] [Google Scholar]
- 15.Kwak C, Kim SS, Park SH, et al. The expression of MMP-1, -8, and -13 mRNA in the periodontal ligament of rats during tooth movement with cortical punching. Korean J Orthod. 2008;38:187–201. [Google Scholar]
- 16.Marvagani M, Brundvik P, Selvig KA. Orthodontically induced root and alveolar bone resorption: inhibitory effect of systemic doxycycline administration in rats. Eur J Orthod. 2005;27:215–225. doi: 10.1093/ejo/cji015. [DOI] [PubMed] [Google Scholar]
- 17.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 18.Karu T, Pyatibrat L, Kalendo G. Irradiation with HE-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol. 1995;27:219–223. doi: 10.1016/1011-1344(94)07078-3. [DOI] [PubMed] [Google Scholar]
- 19.Seifi M, Shafeei HA, Daneshdoost S, Mir M. Effects of two types of low-level laser wave lengths (850 and 630 nm) on the orthodontic tooth movements in rabbits. Lasers Med Sci. 2007;22:261–264. doi: 10.1007/s10103-007-0447-9. [DOI] [PubMed] [Google Scholar]
- 20.Franzen TJ, Zahra SE, El-Kadi A, Vandevska-Radunovic V. The influence of low-level laser on orthodontic relapse in rats. Eur J Orthod. 2015;37:111–117. doi: 10.1093/ejo/cju053. [DOI] [PubMed] [Google Scholar]