Abstract
Glucocorticoids (GCs) have been the mainstay of immunosuppressive therapy in solid organ transplantation (SOT) for decades, due to their potent effects on innate immunity and tissue protective effects. However, some SOT centers are reluctant to administer GCs long-term because of the various related side effects. This review summarizes the advantages and disadvantages of GCs in SOT. PubMed and Scopus databases were searched from 2011 to April 2021 using search syntaxes covering “transplantation” and “glucocorticoids”. GCs are used in transplant recipients, transplant donors, and organ perfusate solution to improve transplant outcomes. In SOT recipients, GCs are administered as induction and maintenance immunosuppressive therapy. GCs are also the cornerstone to treat acute antibody- and T-cell-mediated rejections. Addition of GCs to organ perfusate solution and pretreatment of transplant donors with GCs are recommended by some guidelines and protocols, to reduce ischemia-reperfusion injury peri-transplant. GCs with low bioavailability and high potency for GC receptors, such as budesonide, nanoparticle-mediated targeted delivery of GCs to specific organs, and combination use of dexamethasone with inducers of immune-regulatory cells, are new methods of GC application in SOT patients to reduce side effects or induce immune-tolerance instead of immunosuppression. Various side effects involving different non-targeted organs/tissues, such as bone, cardiovascular, neuromuscular, skin and gastrointestinal tract, have been noted for GCs. There are also potential drug-drug interactions for GCs in SOT patients.
Keywords: Corticosteroids, Glucocorticoids, Solid organ transplantation, Liver, Kidney, Heart, Lung
Core Tip: Due to their potent immunosuppressive and anti-inflammatory effects, glucocorticoids (GCs) are widely used in solid organ transplantation (SOT). We review the current status of GC usage in SOT, including the different clinical uses in transplant recipients and donors, new strategies for targeted organ delivery of GCs, and enhancement of immune-tolerance vs immunosuppressive effects. Major concerns about GCs, such as their adverse effects on various organs and their potential drug-drug interactions in SOT patients, are also discussed.
INTRODUCTION
Glucocorticoids (GCs) have long been used as induction and maintenance immunosuppressive therapy, as well as treatment of acute allograft rejection in solid organ transplant (SOT) patients. However, complications of GCs make them undesirable for long-term use. Therefore, steroid sparing regimens have been used in different types of SOT[1-3].
French insurance data in 2014 showed that only 54% of patients who received kidney transplantation in 2012 were taking prednisolone[4]. A large cohort study on adult liver transplant patients who were transplanted between 2006 and 2014 showed that during 6 mo after transplantation approximately 43% of the liver transplant recipients were treated with three immunosuppressive drugs, including prednisolone, a calcineurin inhibitor (CNI), and mycophenolate/azathioprine, while 15.4% of the patients were on steroid sparing regimens; however, approximately 34% of the patients on triple therapy changed to a steroid sparing regimen between months 7 to 12 after liver transplantation[2]. It should be kept in mind that these data underestimate the number of patients who discontinued steroids because patients who received only tacrolimus have been categorized as antimetabolite sparing and not steroid sparing.
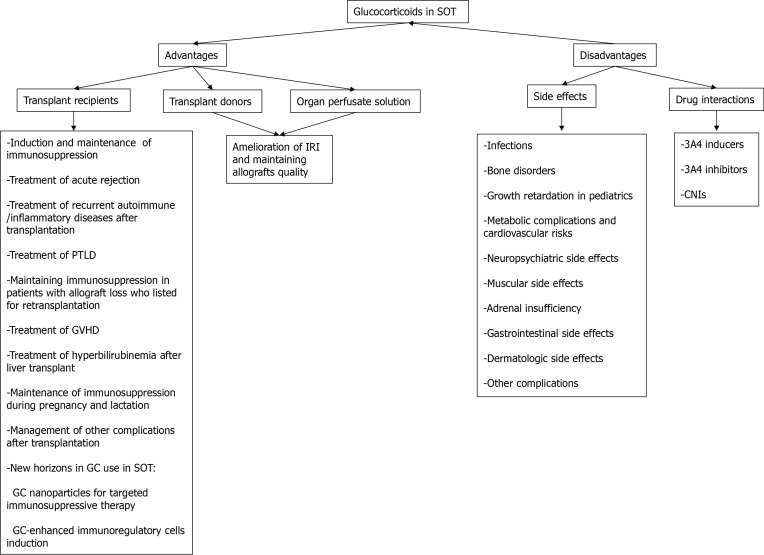
Regarding heart transplantation, a report from International Society of Heart and Lung Transplantation Registry on adult heart recipients who were transplanted between 2000 and 2008 indicated that long-term use of steroids (use for more than 5 years after transplantation) has declined over time from 60% in the year 2000 to 43% in the year 2008, and early GC withdrawal (discontinuation between 2 to 5 years after transplantation) has increased from 19% to 33% during these years[3]. Here, we review different advantages and disadvantages of GCs in SOT (Figure 1).
Figure 1.
Advantages and disadvantages of glucocorticoids in solid organ transplantation. CNI: Calcineurin inhibitor; GC: Glucocorticoid; GVHD: Graft vs host disease; IRI: Ischemia-reperfusion injury; PTLD: Post-transplant lymphoproliferative disorder; SOT: Solid organ transplantation.
DOCUMENT RETRIEVAL
PubMed and Scopus databases were searched from January 2011 to April 2021 using search syntaxes: (transplantation [Title/Abstract] OR transplant [Title/Abstract]) AND (corticosteroid* [Title/Abstract] OR glucocorticoid* [Title/Abstract] OR steroid* [Title/Abstract] OR prednisolone [Title/Abstract] OR prednisone [Title/Abstract] OR methylprednisolone [Title/Abstract] OR dexamethasone [Title/Abstract] OR hydrocortisone [Title/Abstract]). Articles’ references were reviewed for relevant publications.
MECHANISMS OF ACTIONS OF CORTICOSTEROIDS IN SOLID ORGAN TRANSPLANTATION
For many years, the adaptive immunity system (T and B cells) has been focused on preventing allograft rejection. However, the innate immune system (dendritic cells, phagocytes [monocytes, macrophages, neutrophils], and natural killer [NK] cells) also plays major roles in the peritransplant immunologic process. Innate immunity is activated peritransplant by donor brain death, ischemia-reperfusion injury (IRI), non-adherence to immunosuppressive therapy, and infections. Innate immune activation ultimately induces acute allograft rejection and chronic allograft damage[5]. GCs exert a wide range of anti-inflammatory and immunosuppressive impacts, mainly through their genomic and partly via their non-genomic effects. Their genomic effects, that usually have prolonged onset of action, are mediated by binding of GCs to their cytosolic receptors, entering the nucleus, and activating GC response elements that induce anti-inflammatory genes (transactivation) while repressing elements that induce expression of inflammatory factors, such as nuclear factor kappa-light-chain enhancer of activated B-cells (NF-kB) and activator protein-1 (AP1) (transrepression). Anti-inflammatory effects of GCs are related to both transactivation and transrepression effects, while their adverse effects mainly correlate to their transactivation impacts. Genomic effects usually depend on the cumulative dose over the duration of GC administration[1,5,6]. The non-genomic mechanism of GCs has been less known and is partly mediated by membrane receptors that modulate anti-inflammatory and anti-oxidant effects. Their non-genomic effects are of rapid onset, short duration of action, and happen with high or pulse doses (prednisolone doses of > 30 mg/day)[1,6,7].
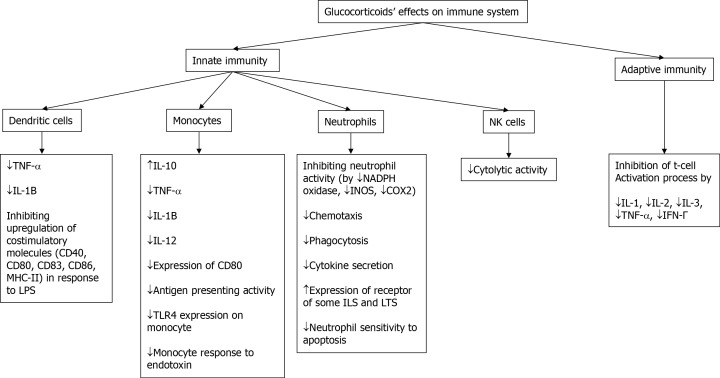
In the innate immunity system, GCs decrease the production of inflammatory cytokines (tumor necrosis factor-alpha [TNF-á] and interleukin [IL]-1β) in dendritic cells in response to CD40L and lipopolysaccharide (LPS). GCs also inhibit upregulation of costimulatory molecules (CD40, CD80, CD83, CD86 and MHC-II) in dendritic cells in response to LPS. In monocytes, GCs increase the expression of anti-inflammatory cytokines (IL-10) and repress production of inflammatory cytokines (TNF-α, IL-1β, IL-12), reduce expression of CD80 in response to inflammatory stimuli, impair monocyte antigen presenting activity, and down-regulate expression of TLR4 on the surface of monocytes, leading to a subsequent monocyte hypo-responsiveness to endotoxin. In neutrophils, GCs inhibit neutrophil activation (by reducing the expression of NADPH oxidase, inducible nitric oxide synthase [iNOS], and cyclooxygenase 2), reduce chemotaxis, phagocytosis and cytokine secretions, increase the expression of some receptors for ILs and proinflammatory leukotrienes (IL1R1, BLT1), and reduce neutrophil sensitivity to apoptosis that leads to increased neutrophil life span. GCs reduce NK cell cytolytic activity and increase their production of pro-inflammatory cytokines[8]. By repressing the expression of IL-1, IL-2, IL-3, TNF-α, and IFN-γ, the T-cell activation process (a part of adaptive immunity) is inhibited by GCs[5]. These mechanisms have been summarized in Figure 2. Considering the above-mentioned mechanisms, GCs have various advantages and disadvantages in SOT patients that are reviewed here.
Figure 2.
Effects of glucocorticoids on the immune system. COX2: Cyclooxygenase 2; IFN-γ: Interferon-gamma; IL: Interleukin; iNOS: Inducible nitric oxide synthase; LT: Leukotriene; NK: Natural killer; TNF-α: Tumor necrosis factor-alpha.
ADVANTAGES OF GCs IN SOT
GCs are administered pre-transplant to potential donors and organ perfusate solution to decrease IRI and preserve organs quality; moreover, GCs are given peri- and post-transplant to recipients as induction or maintenance immunosuppression, treatment of acute rejection, or for management of some post-transplant complications.
TRANSPLANT RECIPIENTS
GCs as induction and maintenance immunosuppressive therapy
GCs are commonly used as induction and maintenance immunosuppressive agents in SOT patients[1-3]. As maintenance immunosuppressive therapy, some centers are shifting toward steroid sparing maintenance immunosuppressive regimens by different steroid withdrawal or avoidance protocols[1-3]. Steroid sparing means rapid, early, or late steroid discontinuation (within 1 wk to several months after transplantation), while steroid avoidance refers to avoiding steroid use in regimens with or without initial high corticosteroid induction therapy[3,9-12]. Although old studies on steroid sparing regimens (GC minimization or discontinuation after 3 mo of transplant surgery) showed higher rates of acute rejection and graft loss, in those studies immunosuppressive regimens contained cyclosporine as a CNI[13]. Nowadays, induction therapies with thymoglobulin or IL2 receptor antagonists and new maintenance immunosuppressive regimens, such as tacrolimus instead of cyclosporine as CNI or mTOR inhibitors, in combination with low doses of CNIs and/or mycophenolate, provided the opportunity for successful steroid-sparing immunosuppression regimens[1,9-11,14,15]. Although, steroid sparing immunosuppressive regimens were used for low immunological risk patients, an analysis of 169479 renal transplant patients using the Scientific Registry of Transplant Recipients found that rapid discontinuation of steroids can be used in all adult and pediatric first kidney transplant recipients from either a deceased or living donor and in second kidney transplant recipients from a living donor or patients at risk for rejection or recurrence of underlying diseases without decreasing patients’ or graft survival rates. Rapid steroid withdrawal was only associated with worse graft survival[9] in adult patients after a second kidney transplantation from a deceased donor. Another systematic review and meta-analysis consisting of seven cohort studies that included high-risk kidney transplant patients, such as re-transplanted patients, African-American ethnicity, or recipients with panel reactive antibody (referred to as PRA) of 20% or more, found that acute rejection episodes and graft loss were comparable between patients maintained on steroids compared with steroid withdrawal or avoidance group. Steroid withdrawal was initiated within 1 wk after transplantation in many of these patients. Based on this meta-analysis, steroid withdrawal within 1 wk after transplantation was associated with significant reduced risk of patient death[10].
Steroid withdrawal regimens are used in most liver transplant patients. A Cochrane systematic review consisting of 1347 liver transplant patients revealed that early steroid withdrawal or steroid avoidance (excluding intraoperative GC use) have been beneficial in some patients, especially those at risk for hypertension or diabetes mellitus[11]. Although steroid avoidance after using a high intraoperative dose may be beneficial in liver transplant recipients, data showed that complete steroid avoidance (even avoiding intraoperative use) decreased patient and graft survival[12].
Most centers that perform simultaneous kidney and pancreas transplantations are also shifting toward steroid avoidance, or early or late steroid withdrawal[16].
Long-term GC therapy had been the cornerstone of immunosuppressive therapy in heart transplant patients. However, a report from the International Society of Heart and Lung Transplantation Registry on adult heart recipients who were transplanted between 2000 and 2008 showed that early or late GC withdrawal has increased among heart transplant patients. Compared to long-term steroid users (GC use for > 5 years after transplantation), 10-year patient survival was significantly higher among early (GC discontinuation between 2 years to 5 years after transplantation) or late (GC discontinuation after year 5 of transplant) steroid withdrawal (73%, 82% and 80%, respectively)[3]. Steroid discontinuation within 1 wk of transplantation has also been applied in low-risk heart transplant pediatric patients with acceptable 1-year outcomes[17].
Corticosteroids are usually a part of maintenance immunosuppression in lung transplant patients as well. Glucocorticoid receptor (GR) in lung epithelia is essential for lung development, and GCs are widely used to treat certain lung diseases[18]. It seems that there is a difference in lung transplant outcomes between patients with different variants of glucocorticoid-induced transcript 1 gene (GLCCI1) that modulates GC sensitivity. A study on 71 lung transplant recipients showed that compared with those with the CC variant (wild type allele), patients with the TT variant (homozygous for mutant allele) had lower total lung capacity and forced expiratory volume in 1 sec at 3 years after transplantation and also had significantly decreased chronic allograft dysfunction-free survival at year 3 after transplantation[19].
Despite available data regarding efficacy of steroid sparing regimens in SOT patients, systemic steroids are still used at least for several weeks in maintenance immunosuppression regimens, even in low immunologic transplant types, such as liver transplantation[20]. Budesonide is a synthetic corticosteroid with minimal systemic bioavailability of about 10% due to extensive first-pass hepatic metabolism that results in decreased side effects[21]. On the other hand, compared to methylprednisolone and prednisone, budesonide possesses strong local anti-inflammatory effect in the liver due to approximately 15-times higher affinity for GR[22]. A phase 2 clinical study in first liver transplant recipients compared budesonide (tapering from 9 mg to 3 mg over 12 wk) with prednisolone in the maintenance immunosuppressive regimen containing CNI and mycophenolate. Patients were followed for 2 years. Biopsy-proven acute cellular rejection was the same between the two groups (5% in each group), while post-transplant diabetes mellitus (PTDM) (0% vs 15%) and infection rates (0% vs 30%) were significantly lower in the budesonide-taking group[23].
Treatment of acute rejection
High doses of intravenous (methylprednisolone or dexamethasone) or oral (prednisolone or prednisone) GCs have been historically administered for the treatment of acute cellular and antibody-mediated rejections in different types of SOT[24-28]. Recently, a United States center retrospectively assessed the 6 mo outcomes of 29 pediatric liver transplant patients who were prescribed oral budesonide in an outpatient setting for treatment of biopsy-proven (19 patients) or presumed (based on blood biochemistry tests; 10 patients) mild to moderate acute cellular rejection. In these patients, budesonide was administered at daily doses of 6 mg to 9 mg for several weeks, tapering down thereafter. Only 3 patients needed to be switched to systemic GCs (methylprednisolone or prednisone). All other patients experienced significant decreases in liver transaminases without progressive graft injury or chronic allograft rejection[29].
Post-transplant malignancies
A main complication after SOT is post-transplant malignancies, including post-transplant lymphoproliferative disorders (PTLD). Immunosuppression reductions or changes are recommended in patients with PTLD. However, GCs are a basis of chemoimmunotherapy in some malignancies, including PTLD, and are usually kept in the immunosuppressive regimen of SOT recipients with PTLD[30]. Sometimes, under the umbrella of corticosteroids, other chemotherapeutic agents (with some safety concerns in SOT recipients) are administered. Although immune checkpoint inhibitors have increasingly been successful in treating multiple types of cancer, the risk of allograft rejection with these drugs in SOT patients is concerning[31]. A pilot study showed that immune checkpoint inhibitors, along with prophylactic steroids, may be a safe and effective treatment for some SOT patients with advanced cutaneous squamous cell carcinoma[32]. While a Danish historical cohort study revealed a tendency toward a higher occurrence of post-transplant cancer in patients treated at a kidney transplant center that applied a steroid-free immunosuppressive regimen compared to patients treated at centers that adhered to GC-containing immunosuppressive protocols[33], another Danish registry analysis on over 59000 patients found a standardized incidence risk of 1.32 (95% confidence interval [CI]: 1.09-1.59) for cutaneous squamous cell cancer among GC users; however, this increased risk was seen across all patients in that study, not just transplant patients[34].
Prevention or treatment of recurrent autoimmune diseases
Diverse de novo autoimmune diseases in different organs can happen after SOT and these cases are usually treated similarly to patients in the general population; this topic, however, is out of the scope of this review. Recurrent glomerulonephritis (GN) after renal transplantation is the fourth most common cause of allograft loss, with a reported recurrence rate of 2.6% to 50% and average graft loss risk of 8.4% over 10 years. Data from Australia and New Zealand Dialysis and Transplant Registry over 30 years reported that focal segmental glomeruosclerosis (FSGS), IgA nephropathy, membranous GN, and membranoproliferative GN (MPGN) showed recurrence after renal transplantation. Different risk factors have been reported for these GN recurrences. Regarding the role of GCs, when all GNs were included, multivariate analysis found baseline steroid use in maintenance immunosuppression had a protective effect (adjusted hazard ratio [HR]: 0.54; 95%CI: 0.37-0.76; P < 0.001). When FSGS and IgA nephropathy were analyzed separately, baseline steroid use was a protective factor only for transplant recipients with IgA nephropathy[35]. Another study also revealed that the rate of recurrence of IgA nephropathy after kidney transplantation was higher among patients with steroid withdrawal at any time after transplant[36]. There is a lack of evidence for treatment and outcomes of recurrent GN after transplantation. Recurrent GN after transplantation is usually managed similar to de novo cases in the general population, although sometimes with different protocols and responses. GCs are usually a part of GN management regimen[37,38]. Recurrent IgA nephropathy after renal transplantation is treated with GCs[39].
Autoimmune liver diseases (autoimmune hepatitis [AIH], primary biliary cirrhosis [PBC], and primary sclerosing cholangitis [PSC]) may recur after liver transplantation, with varying rates of 10% to 50%. Recurrence of PBC or PSC has not been associated with dose or duration of GC administration or discontinuation of GCs. Recurrent PBC is traditionally treated with ursodexycholic acid, with varying results. Recurrent PSC usually causes progressive allograft damage and requires repeat liver transplantation. Although overall dose and duration of GC treatment pre- and post-liver transplantation are not related to AIH recurrence, rapid weaning of GC after liver transplantation has been associated with higher AIH recurrence rate. AIH recurrence is usually treated with GCs[40,41].
Maintaining immunosuppression in patients with graft loss who listed for re-transplantation
Although maintenance of low-dose CNI after kidney allograft loss can decrease the development of donor-specific antibody and repress the rise in PRA in patients listed for repeat kidney transplantation, such effects were not observed with GCs[42]. Meanwhile, some clinicians continue low dose prednisolone in kidney recipients with graft loss more than 1 year after transplantation who are planned for repeat renal transplantation within 1 year[43].
Graft vs host disease (GVHD) after SOT
Although rare after SOT, GVHD may still occur. Case series show administration of methylprednisolone for treatment of GVHD in some SOT patients; however, there are GC-treatment refractory patients, with high mortality rate of 82%[44,45].
Hyperbilirubinemia after liver transplantation
Hyperbilirubinemia after liver transplantation is common and is sometimes due to early allograft dysfunction. A randomized controlled trial assessed the effect of low-dose steroid in combination with ursodeoxycholic acid in liver transplant patients. The control group received only ursodeoxycholic acid. Patients with hyperbilirubinemia due to biliary complications and acute rejection were excluded from the study. Both groups had comparable immunosuppressive regimens, donor and recipient characteristics, and time after transplantation surgery. The steroid group had significantly lower bilirubin concentration 1 d and 15 d after intervention was completed and had shorter hospital stay compared with the control group[46].
Pregnancy and lactation
GCs cross the placenta, but nearly 90% of the dose of prednisolone and methylprednisolone (and to lesser extent dexamethasone and betamethasone) is metabolized by placenta 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2) to an inactive metabolite. Although there have been concerns about oral-facial clefts, hypothalamus-pituitary-adrenal (HPA) axis dysfunction, or retarded growth in newborns from GC-taking mothers, the risk seems minimal unless there is a 11β-HSD2 dysfunction (e.g., due to preeclampsia in the mother). It is also possible that GCs may predispose pregnant women to hypertension and preeclampsia. Taken together, GCs in daily doses equivalent to less than 20 mg prednisolone are considered acceptable in pregnant women, and GCs are usually continued in transplanted mothers. GCs are also considered compatible with breast-feeding[47].
Management of other complications
The number of patients with pulmonary complications after hematopoietic stem cell transplantation (HSCT) is increasing, and some of these patients need lung transplants to survive. Steroid therapy is the current treatment for pulmonary complications in HSCT patients. A retrospective study that compared 9 patients on low-dose and 13 patients on high-dose GCs for post-HSCT pulmonary complications and before their lung transplantation showed that taking low-dose vs high-dose GCs before lung transplantation in these patients was associated with significantly fewer complications during the first year after lung transplantation and improved long-term survival[48].
New horizons of GCs use in SOT recipients
Targeted delivery of GCs to the affected organ is a favorable method to reduce GC side effects when used for treatment of inflammatory diseases and in SOT patients. After parenteral administration, nanoparticles largely translocate into the liver by passive targeting. Therefore, nanoparticle-mediated drug delivery would be a promising method for treatment of inflammatory liver diseases. In several studies, different nanoparticles have been used for transportation of dexamethasone, such as biodegradable polymers (PLGA, PLLA, PCL, cellulose, cyclodextrin, chitosan, polyglutamic acid, and lipids), inorganic materials, polymer micelles, liposome, and carbon nanotubes. Entrapment of dexamethasone in these nanoparticles resulted in prolonged and sustained release of dexamethasone, but premature release out of the target organ is an undesired consequence. To overcome this possibility, dexamethasone in concentrations up to 100 mg/mL in olive oil were encapsulated in core-shell silica nanocapsules. During an experimental study, these nanocapsules were internalized by non-parenchymal murine liver cells and resulted in suppression of inflammatory response of liver macrophages and a significant decrease in inflammatory cytokines. Pegylation of these nanocapsules led to good stability in plasma and controlled interaction with blood proteins[49].
With the hope of improving efficacy while decreasing side effects, another animal study compared liposomal encapsulated prednisolone vs conventional prednisolone in a murine model of acute renal allograft rejection. The liposomes were 100 nm phospholipid bilayer vesicles coated with polyethyleneglycol. These liposomes remained in blood for several days after intravenous injection. Liposomes prevent the encapsulated drug from diffusing over blood vessel endothelial cells and spreading throughout the body, while they are small enough to extravasate and accumulate in inflamed sites with increased vascular permeability, where macrophages and other phagocytic cells digest the vesicles and release the entrapped GC. The results of that animal study showed improved renal bioavailability of prednisolone, increased renal perfusion, and decreased cellular infiltrate in allograft by liposomal prednisolone compared with conventional prednisolone. In that study, liposomes were detected in other organs, such as liver, stomach, and intestine, but in much lower density than in the kidney allograft[50]. More animal studies are needed before clinical studies to bring these bench findings to the bedside.
Inducing immune tolerance and eliminating the need for long-term immunosuppressive therapy has been an old ideal in SOT. Modulating immunoregulatory cells represents a potential target for this purpose. Myeloid-derived suppressor cells (MDSCs) are novel immunoregulatory cells induced by granulocyte macrophage colony stimulating factor (GM-CSF). In an in vitro study, the combination of dexamethasone with GM-CSF was successful for enhanced production of the phenotype of MDSCs with enhanced in vitro immunosuppressive activity. Adoptive transfer of these MDSCs significantly enhanced expansion of regulatory T cells and prolonged heart allograft survival in a mouse model. Mechanistic studies showed that iNOS signaling was required for MDSCs in the control of the T cell response. GR signaling had a major role in the recruitment of transferred MDSCs into the allograft, through upregulating CXCR2 expression on MDSCs. These findings revealed that co-administration of dexamethasone and GM-CSF may be a new and applicable strategy for the induction of immune tolerance in SOT[51].
TRANSPLANT DONORS
The brain death process induces an inflammatory response in the donor. Increased intracranial pressure and decreased cerebral blood flow during the brain death process activate neurohormonal systems and the inflammatory cascade. Increased release of inflammatory cytokines, chemokines, and adhesion molecules leads to infiltration of T lymphocytes and macrophages into the organs[52]. This inflammatory response causes allograft injury that, in combination with IRI, increases the risk of initial allograft poor function[53]. There are two separate stages for IRI. Ischemia leads to cellular metabolic disturbances, glycogen consumption, lack of oxygen supply, and ATP depletion, which lead to initial parenchymal cell death. Reperfusion injury results from both metabolic disruptions and intense inflammatory response. IRI triggers inflammatory response mainly through innate immune response. Innate immune activation leads to increased production of cytokines, chemokines, and reactive oxygen species (ROS), and increased expression of adhesion molecules. Moreover, cross-talk between innate and adaptive immunity trigger an adaptive immune response that results in tissue infiltration by lymphocytes and monocytes, and graft rejection[54]. IRI is an important cause of early allograft dysfunction[54]. Therefore, several investigators have administered anti-inflammatory drugs to deceased donors to ameliorate IRI. Although animal[55,56] and small clinical[57] studies have shown that administering GCs to brain dead donors decreased IRI in kidney, heart, or liver grafts[55-57] and is recommended by organ procurement guidelines[58], the effect of pretreatment of brain dead donors with anti-inflammatory agents on long-term allografts outcomes are not promising[59,60]. A multicenter randomized controlled trial consisting 455 kidney transplant recipients from 306 deceased donors were followed for 5 years after transplantation. These deceased donors were randomized to receive 1 g of methylprednisolone or placebo before organ procurement. The incidence of biopsy-confirmed rejection (Banff > 1) at 3 mo after transplantation and 5-year graft survival and the mean estimated glomerular filtration rates were comparable between steroid and placebo groups[59]. In addition, a meta-analysis on methylprednisolone treatment of brain dead liver donors (two studies, 183 participants) showed no effect of the treatment on rates of acute rejection (Table 1)[61]. Interestingly, an animal study showed that pretreatment with methylprednisolone markedly prevented warm liver IRI in normal rats, but aggravated IRI in the steatotic livers of the diabetic Zucker rats with deficiency of the leptin receptor[62]. Leptin resistance is common in diabetes. Thus, more studies are needed to understand how deficiency in the leptin signaling may switch the GC action from protection to aggravation in liver IRI and thus affect GC action in SOT of liver and other organs in humans.
Table 1.
Effect of pretreatment of transplant donors with methylprednisolone on outcomes of solid organ transplantation
|
Type of the study
|
Type of SOT
|
Follow-up duration
|
Findings
|
| RCT[57] | Liver | 6 mo | Significant lower liver enzymes in GC vs placebo group at 1st and 10th d after transplantation; No difference in PNF rate between groups (2 of 50 patients in GC and 3 of 50 patients in the placebo group); Lower acute rejection during 6 mo in GC group (22% vs 36%; P < 0.05) |
| RCT[60] | Liver | Maximum 3 yr | No difference in liver enzymes between GC and placebo groups during 1st wk after transplantation; Acute rejection during 3 mo after transplantation was 24% in each group; 1 yr graft loss of 15% in GC and 24% in the placebo group (P = 0.41); Relative risk of acute rejection in GC vs placebo group: 1.02 (95%CI: 0.5-2.1; P = 1); Relative risk of mortality in GC vs placebo group: 0.63 (95%CI: 0.29-1.36; P = 0.31) |
| Meta-analysis of two above RCTs[61] | Liver | Maximum 6 mo | Risk ratio for incidence of acute rejection during 1 mo to 6 mo after transplantation: 0.72 (95%CI: 0.44-1.19; P = 0.2) |
| RCT[59] | Kidney | 5 yr | 3 mo BPAR: 10% in GC and 12% in placebo group (P = 0.468); 5 yr graft survival: 84% in GC and 82% in placebo group (P = 0.941); Mean eGFR at 5 yr: 47 mL/min/1.73 m2 in GC and 48 mL/min/1.73 m2 in placebo group (P = 0.756) |
BPAR: Biopsy-proven acute rejection; CI: Confidence interval; eGFR: Estimated glomerular filtration rate; GC: Glucocorticoid; PNF: Primary non-function; RCT: Randomized clinical trial; SOT: Solid organ transplantation.
The pro-inflammatory state induced by the brain death process also decreases the quality of lungs for donation. To preserve lung quality, methylprednisolone is administered to donors with various doses. To assess the dose-effect association between methylprednisolone and brain death lung inflammation, an animal study compared low (5 mg/kg), intermediate (12.5 mg/kg), and high (22.5 mg/kg) doses of methylprednisolone. All methylprednisolone doses decreased inflammatory cytokines, such as TNF-α, IL-6, and IL-1β. Intermediate and high doses of methylprednisolone also increased protective anti-inflammatory response as established by increased IL-10 expression. Macrophage chemotaxis was attenuated with all doses of methylprednisolone, while neutrophil chemotaxis was more evident with intermediate and high doses of methylprednisolone. Considering dose-related side effects of methylprednisolone, this study suggested the intermediate dose of methylprednisolone reduced brain death-induced inflammatory responses in donors’ lungs[63]. These findings need human studies before extrapolation to routine clinical use.
ORGAN PERFUSATE SOLUTIONS
Animal studies have shown decreased generation of pro-inflammatory cytokines, IRI, and donated tissues edema by adding (methyl)prednisolone to perfusate STEEN solutionTM and Perfadex® solution for heart and lung grafts[64,65]. Recently, normothermic ex vivo heart perfusion using the Transmedics organ care systemTM (OCS) has been used clinically for preservation of hearts donated after circulatory death. Based on the Transmedics OCS protocol, methylprednisolone is added to the perfusate solution to reduce IRI and preserve cardiac function[66].
DISADVANTAGES OF GCS IN SOT
The main drawback of GCs is their diverse adverse effects on various tissues. Side effects are usually related to genomic mechanism of action of these drugs, mainly transactivation ones; therefore, these side effects usually have prolonged onset and are associated with the cumulative dose of GC over the duration of its administration[1,5,6]. Another aspect that should be considered, especially in SOT patients, is drug interactions of GCs with other drugs in these patients. These aspects are briefly reviewed below.
SIDE EFFECTS
Infections
GCs increase the risk of bacterial, fungal and viral infections[6]. A multivariate regression analysis on data of 45164 kidney transplant recipients in 2000-2011 from the United States Renal Data System (USRDS) showed that a steroid-free immunosuppressive regimen was associated with reduced risk of pneumonia (adjusted HR: 0.89; P = 0.002) and sepsis (adjusted HR: 0.80; P < 0.001)[67]. A multicenter, case-control study on 988 episodes of Enterobacterales-induced blood stream infection among SOT patients showed that about 40% of these episodes are caused by extended-spectrum β-lactamase (ESBL)-producing organisms. Taking corticosteroid-containing immunosuppressive regimens was identified as a risk factor for ESBL-Enterobacterale-induced blood stream infection (adjusted odds ratio [OR]: 1.3; 95%CI: 1.03-1.65; P = 0.03)[68]. Nocardiosis is another bacterial infection reported among immunocompromised patients, such as SOT recipients. A retrospective study compared 60 adult patients who were hospitalized with nocardiosis to a group of 120 patients which had been randomly selected from among hospitalized patients with community-acquired pneumonia. Multivariable logistic regression analyses showed that immunosuppressive therapy was positively associated with nocardiosis (matched OR: 4.40; 95%CI: 2.25-8.62; P < 0.001). Among immunosuppressive therapy, GC therapy was a typical risk factor for nocardiosis (matched OR: 4.69; 95%CI: 2.45-8.99; P < 0.001), especially for pulmonary nocardiosis (matched OR: 5.90l 95%CI: 2.75-12.66; P < 0.001). The positive association between SOT and nocardiosis was mitigated following adjustment for GC administration in a multivariate model. The association between taking GC and developing nocardiosis was stronger in patients with chronic pulmonary disease (OR: 5.74; 95%CI: 2.75-12.66; P < 0.001) than in the pooled analysis of all nocardiosis cases[69]. Another analysis on 112 patients with nocardiosis, among which 67 were immunocompromised patients, showed that pulmonary nocardiosis among immunocompromised patients was significantly associated with taking high-dose GC. Immunocompromised patients showed more disseminated forms of infection, with the highest rate in SOT recipients, and had significantly higher mortality compared with immunocompetent patients[70].
Taking GCs is a risk factor for fungal infections, including mucormycosis[71] and invasive Aspergyllosis[72,73]. Although the American Society of Transplantation suggests re-initiation of Pneumocystis jirovecii pneumonia (PJP) prophylaxis with intensification of immunosuppression, such as treatment of acute rejection with GCs[74], the association between GC bolus for acute rejection and PJP remains controversial[75]. While a French case-control study exhibited GC bolus administration for acute rejection in kidney transplant patients as an independent factor correlated with PJP[76] and a Korean study showed that taking GCs is significantly associated with PJP[77], a meta-analysis found that GC injections for acute rejection did not increase the risk of PJP[75]. On the other hand, a retrospective case series showed that adding GCs to PJP treatment of non-human immunodeficiency virus (HIV)-infected patients (3 of 28 were SOT patients) was associated with lower mortality[78], while an older retrospective study comparing PJP-infected non-HIV patients with or without adjunctive steroid therapy (12 of 59 patients in the GC-taking group and 14 of 29 patients in no-GC group were SOT patients) found that GC use may not improve outcome of moderate to severe PJP in these patients[79].
Regarding viral infections, it has been reported that prednisolone daily doses of 10 mg or higher is associated with higher risk of respiratory viral infection[80]. Community-acquired viral respiratory infections (rhinovirus followed by coronavirus and respiratory syncytial virus) has been reported in approximately 25% of lung transplant recipients during the first year after transplantation, especially in those receiving nasal glucocorticoids[81]. Corticosteroid use is a risk factor for adenovirus infections, including urinary tract infection with this virus (OR = 3.86; 95%CI: 1.21-12.24; P = 0.02 for acquiring urinary tract infections)[82]. It has been reported that kidney transplant patients on maintenance GCs are more likely to be admitted with coronavirus disease-2019 (COVID-19)[83]. Some authors reported that one of the major risk factors associated with survival among kidney transplant patients infected with the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is receipt of prednisolone (OR: 5.98; 95%CI: 1.65-21.60; P < 0.01)[84].
BK (polyoma) virus infection is common during 6 mo after renal transplantation and may lead to BK virus associated nephropathy (BKVAN). There are case reports of BKVAN that resulted in native kidney failure in other types of SOT patients. A GC pulse for the treatment of acute allograft rejection has been reported in these patients before BKVAN[85]. Causality assessment needs more studies.
Regarding cytomegalovirus (CMV) infection, a retrospective study evaluated 71 SOT patients during the same time period; among them, 49 patients were tested for genotypic resistance CMV variants, while 22 were not because of no clinical suspicion for the resistance variants. This study compared the patients in the following three groups: group 1, patients with resistant CMV infections (defined as document of failure to reach at least 1 log10 decline in CMV DNA load after 2 wk of treatment with (val)ganciclovir, foscarnet, cidofovir, and at least 1 CMV resistant genotypic mutation); group 2, patients with refractory CMV infection (defined as documented failure to achieve 1 log10 or more decline in CMV DNA level after at least 2 wk of treatment with (val)ganciclovir); and group 3, no suspected CMV resistance and not tested for such. Results showed that patients in groups 1 and 2 were taking higher mean daily doses of prednisolone compared with patients in group 3 (10 mg a day or higher vs 5 mg daily); however, in the final model, daily GC dose was not a significant risk factor for resistant or refractory CMV infection[86]. An experimental study indicated that GCs activate the major immediate-early promoter (MIEP), which drives CMV gene expression. This GC effect is mediated via the GR pathway and leads to reactivation of latent CMV from primary monocytes. To investigate the clinical relevance of this experimental finding, the same researchers retrospectively analyzed data of liver transplant patients and found that taking prednisolone as baseline immunosuppression and/or methylprednisolone as augmented immunosuppression can trigger CMV reactivation in intermediate-risk patients (D+/R+) to the levels comparable with high-risk patients (D+/R-)[87].
One-year cumulative doses of 1830 mg or more of GCs has been associated with tuberculosis infection in patients with systemic lupus erythematosus (OR: 2.74; 95%CI: 1.26-5.98; P = 0.011)[88] that may be true for SOT patients as well.
Severe Sterongyloides stercolaris infection is associated with high morbidity and mortality among kidney transplant patients and is usually accompanied by gastrointestinal and respiratory symptoms. A multicenter cohort study consisting of 46 kidney transplant patients with severe Sterongyloides stercolaris infection and 92 matched control patients found that cumulative GC dose was an independent risk factor for severe Sterongyloides stercolaris infection (median [IQR] of doses of 73.32 [40.93-157.46] mg/kg in the case group vs 65.23 [32.05- 155.28] mg/kg in controls) (OR: 1.005; 95%CI: 1.001-1.009; P = 0.008)[89]. As seen, the calculated OR is approximately 1, which may not be of clinical importance despite statistical significance.
Although rare, visceral leishmaniasis may occur after SOT relating to general prevalence of this parasite in that geographic area. High-dose prednisolone within the preceding 6 mo has been associated with this infection in SOT patients[90].
Bone disorders
GCs antagonize the effects of vitamin D, decrease intestinal absorption of calcium, inhibit secretion of growth hormone, inhibit bone formation (by inhibiting osteoblasts differentiation and increasing their apoptosis), increase bone resorption (by enhancing osteoclasts formation), and finally lead to osteoporosis and an increased risk of fractures, especially in trabecular bones[6]. Chronic kidney disease-mineral bone disorder (CKD-MBD) after kidney transplantation is a mix of pre-existing disorders and new alterations. The final results are abnormal mineral metabolism (hypercalcemia and hypophosphatemia) and bone changes (high or, more commonly, low bone turnover disease), with consequences of decreased bone mineral density and increased risk of bone fractures[91]. Although not completely clarified, several factors play roles in post-transplant bone disorders, such as immunosuppressive treatment, especially corticosteroids, persistently high levels of PTH, vitamin D deficiency, and hypophosphatemia. Transplant recipients are at a four-fold higher risk of fracture compared with the general population. One of the most relevant risk factors is high-dose or prolonged GC therapy[91,92]. Kidney transplant recipients with early steroid withdrawal have shown higher bone mineral density in the lumbar spine and femoral neck and less osteopenia[93]. On the other hand, one study followed 36 renal transplant patients who continued low daily dose of 5 mg prednisolone from day 42 after transplantation onward for 1 year. None of these patients received any treatment for bone disorders. In addition to bone mass densitometry, novel bone quality parameters, including trabecular bone score and bone material strength index, were evaluated for these patients. Findings indicated a small decrease in bone mineral density in the femoral neck at 3 mo and in the lumbar spine at 12 mo after transplantation, while, no changes in trabecular bone score and bone material strength index were found, showing limited effects of low daily doses of GCs on bone[94]. Osteonecrosis of the hip is another side effect of corticosteroids in SOT patients[95]. Although some studies showed that the cumulative dose of methylprednisolone/prednisolone after kidney transplantation has been a risk factor for avascular osteonecrosis[96,97], a meta-analysis found little correlation between cumulative doses and duration of administration of methylprednisolone/prednisolone and avascular osteonecrosis of the femoral head[98].
Growth in pediatric transplant recipients
By inducing abnormal growth hormone secretion and response, GCs impair stature growth in children and prepubertal adolescence[6]. In contrast, GR in hepatocytes is essential for postnatal body growth by mediating the growth hormone signaling in mice[99]. New animal data also suggests that GCs decrease longitudinal bone growth by upregulation of fibroblast growth factor-23 (FGF23) and its receptor (FGF23R3) expression[100]. GC-induced growth retardation in pediatric transplant patients encouraged SOT teams to apply steroid minimization protocols in prepubertal kidney transplant patients with better bone health, growth outcome, and comparable allograft rejection rates[14,101,102].
Metabolic complications and cardiovascular risks
Obesity and metabolic syndrome with the three components of hyperglycemia, hyperlipidemia, and hypertension are common long-term side effects of GCs; these adverse effects increase atherogenesis and the risk of cardiovascular events[6]. Adipocyte GR deficiency promotes adipose tissue expandability and improves the metabolic profile during GC exposure[103]. In contrast, GR in cardiomyocytes is essential in cardio protection; deletion of cardiomyocyte GR increases mortality due to the development of spontaneous cardiac pathology in both male and female mice[104]. The mechanism of GC-induced hyperglycemia is insulin resistance followed by increased hepatic gluconeogenesis[1]. PTDM is associated with higher risk of mortality and graft loss[105]. PTDM incidence in SOT patients varies from 10% to 74% depending on the country and ethnicity of the patients and diagnostic criteria[105-107]. There are several risk factors for PTDM, such as viral infections, underlying kidney diseases, and different immunosuppressive drugs, that can confound causality assessment between steroid dose and duration and PTDM in SOT patients[1]. A Malaysian study of 168 patients without diabetes before transplantation showed the PTDM incidence was 17% up to 1 year after renal transplantation. In that study, the daily prednisolone dose was not associated with the development of PTDM[107]. Another 4-year follow-up study on 400 kidney transplant patients without history of diabetes before transplantation (96 patients on steroid-free and 304 patients on 5 mg/day prednisolone in immunosuppressive regimen) indicated that taking 5 mg daily prednisolone was associated with a small but not statistically significant increase in HbA1c and significantly higher risk of prediabetes (relative risk [RR]: 1.789; 95%CI: 1.007-3.040; P = 0.026) but not PTDM compared with a steroid withdrawal regimen. Although other components of the immunosuppressive regimen, such as the type of CNI (tacrolimus vs cyclosporine A), can affect PTDM risk, as in the multivariate analysis of Tillmann et al’s[108] study showed higher risk of prediabetes with long-term low-dose steroid, independent of the higher risk of tacrolimus inducing PTDM compared with cyclosporine. On the other hand, a meta-analysis on more than 22000 kidney transplant patients found that early steroid withdrawal during 1 wk after transplantation is associated with less PTDM risk (RR: 0.91; 95%CI: 0.37-0.97; P = 0.04)[10]. Association between new-onset hyperglycemia and GC-containing maintenance immunosuppression among liver transplant recipients is controversial[109,110]. While a Japanese retrospective analysis on 461 adult liver transplant recipients from living donors did not find any association between taking GC and PTDM[109], a randomized clinical trial on live donor liver transplant patients reported significantly higher incidences of PTDM among patients taking steroids vs steroid-free group at 3 mo and 6 mo follow-ups[110]. One confounding factor in data interpretation is the use of different diagnostic criteria for PTDM in different studies. For example, in the Toshima et al[111] study, fasting plasma glucose of 110 mg/dL or higher was used as a cut-off for PTDM definition[109], while based on the standard criteria of the American Diabetes Association, s plasma glucose level of 126 mg/dL or higher is defined as diabetes.
Hyperlipidemia is another known metabolic side effect of GCs. Increased total cholesterol, very low-density lipoprotein (VLDL) cholesterol, and triglyceride levels and decreased high-density lipoprotein (HDL)-cholesterol concentration have been reported with GCs, depending on the dose and duration of their administration[6]; however, a large United States cohort study showed beneficial effect of GCs on increasing HDL cholesterol among patients older than 65 years of age[112]. Wide ranges of mechanisms have been supposed for GC effects on the lipid profile, including increased activity of acetyl-Coenzyme A carboxylase and free fatty acids synthetase and enhanced hepatic synthesis of VLDL, inhibition of lipoprotein lipase, alteration in the insulin signaling pathway, and possible inhibition of the activity of 3-hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) reductase[6]. The latter mechanism can theoretically have positive effects on the lipid profile, which may explain some controversies regarding GC-induced lipid changes in the literature. Regarding organ transplant patients, a study on liver transplant recipients showed that taking maintenance GCs was an independent factor associated with hyperlipidemia but not with the two other components of metabolic syndrome (hyperglycemia and hypertension) in this patient population[109]. In contrast, another study that compared steroid-free vs steroid-taking immunosuppressive regimens in living donor liver transplant recipients found significantly higher incidences of all components of metabolic syndrome, including new-onset hyperglycemia, new-onset hypertension, and post-transplant hypertriglyceridemia among the steroid-taking group[110].
Although the effect of steroid withdrawal on hypertension after transplantation is controversial, a study on pediatric liver transplant patients that followed the patients with ambulatory blood pressure monitoring found that blood pressure improved, especially nocturnal hypertension, and the circadian rhythm of blood pressure was restored after GC discontinuation in these patients[113]. A Saudi study on adult kidney transplant patients found that patients on steroid sparing regimens had significantly lower weight gain and a non-statistically significant improvement in blood pressure and lipid control[114]. Different mechanisms have been reported for GC-induced arterial hypertension, including salt and water retention by activating renal mineralocorticoid receptor (MR) and regulating vascular activity by activating GR in endothelial and vascular smooth muscle cells. Interestingly, GR in vascular endothelial cells is required for dexamethasone-induced hypertension[115], while loss of endothelial GR increases hemodynamic instability, inflammation, and mortality in sepsis, and GR deficiency in endothelial cells prevents the therapeutic protection by dexamethasone after LPS treatment[116,117]. Hypertension is more common among patients taking daily doses of more than 20 mg prednisolone. Metabolic changes and hypertension increase atherogenesis and risk of cardiovascular events in patients taking long-term GC[6].
Neuropsychiatric side effects
Most immunosuppressive drugs, especially CNIs, glucocorticoids and mTOR inhibitors, can induce neurologic side effects. Sometimes the assessment of causality is hard and all drugs work together to manifest the side effect(s). Glucocorticoids easily pass the blood-brain barrier and reach all brain cells, which results in HPA axis suppression and neuropsychiatric and neurodegenerative side effects. Prolonged exposure to glucocorticoids in SOT patients and high GC doses and concentrations (e.g., during treatment of acute rejection) increase the risk of neuropsychiatric side effects because of structural remodeling in neurons, synoptic loss, and maladaptive alterations in glial function[118]. GCs cause different neurologic side effects, such as headache, tremor, seizure, stroke, and pseudotumor cerebri. GC-induced psychiatric adverse effects vary from minor mood changes and confusion, sleep disorders, anxiety to severe psychotic features[6,118].
Muscular side effects
GCs have catabolic effects on muscles, leading to muscular atrophy, cramping and progressive symmetrical muscle deficit. They can induce acute or chronic myopathy. Tendon rupture is a rare side effect of GCs[6]. High doses of GCs cause muscular atrophy via activating GR in the muscle[119].
Adrenal insufficiency
Suppression of the activity of the HPA axis and the subsequent adrenal insufficiency are well-known side effects of GCs. Adrenal insufficiency may be potentially life-threatening because of the risk of acute adrenal crisis. A study on renal transplant patients treated with oral prednisolone at daily doses of 5 to 7.5 mg for 6 mo or more found insufficient adrenal response to Synacthen in about 43% of the patients, which shows a high prevalence of adrenal insufficiency due to long-term low dose GCs in these patients[120].
In addition to a decrease in endogenous GC production, exogenous GC, such as prednisolone, may also enhance the activity of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), the enzyme that is responsible for regeneration of cortisol from the inactive metabolite, cortisone. A cohort study investigated this hypothesis in prednisolone-treated kidney transplant patients compared with healthy controls. The median daily dose of prednisolone in these patients was 10 mg (IQR of 7.5-10 mg). The 24-h urinary cortisol, cortisone, tetrahydrocorisol (THF), allotetrahydrocortisol (alloTHF), and tetrahydrocortisone (THE) were measured. The 24-h urinary excretion of cortisol and its metabolites were used as measures of endogenous glucocorticoid production, while (THF + alloTHF)/THE and cortisol/cortisone ratios were used as reflectors of 11β-HSD1 activity. Findings revealed that urinary cortisol and metabolite excretion were significantly lower (indicating reduced endogenous cortisol synthesis), while (THF + alloTHF)/THE and cortisol/cortisone ratios were significantly higher (indicating increased 11β-HSD1 activity) in kidney transplant recipients compared with healthy controls. Daily doses of prednisolone had a significant inverse association with reduced endogenous cortisol synthesis and significant and a positive association with markers of 11β-HSD1 activity. Such changes in endogenous GC production and regeneration were associated with increased risk of mortality in kidney transplant patients even after adjustment for confounders such as patients’ age, gender, estimated glomerular filtration rate, C-reactive protein, body surface area, and daily doses of prednisolone[121]. Some researchers found significant associations between HPA suppression and higher prevalence of metabolic syndrome and its individual components (central obesity, dyslipidemia, hypertension, and hyperglycemia) in kidney transplant patients taking prednisolone[122].
Gastrointestinal side effects
Gastrointestinal side effects of GCs include peptic ulcers, upper gastrointestinal bleeding, pancreatitis, diverticular perforation, and colonic malakoplakia (a chronic granulomatous disease)[123].
Immunosuppressive therapy after SOT may change gut microbiota and be associated with increased rates of overall and infection-related mortality, rates of all infections, including nosocomial infections, duration of infections, infections complications, rejection rates and graft loss. Some studies tried to differentiate the effect of different types of immunosuppressive drugs that are used in combination in SOT patients. Regarding corticosteroids, a study on liver-transplanted mice showed that prednisolone administration reduced the concentration of Bacteroidetes while increasing the concentration of Firmicutes in the feces. In that study, prednisolone, in combination with mycophenolate and tacrolimus, increased Escherichia coli colonization. Serial testing of fecal samples of kidney transplant recipients revealed that compared to those remaining on maintenance corticosteroid, patients with early GC withdrawal had numerically but not statistically significant lower Clostridiales and Erysipelotrichales[124].
Dermatologic effects
Cushingoid appearance, facial erythrosis, skin thinning, rosacea, acne that may rarely progress to nodulocystic transformation, impaired wound healing, purpura after minor trauma, hirsutism, and striae rubrae are dermatologic side effects of GCs that are usually dose- and treatment duration-dependent[6].
Other complications
GCs increase the risk of thrombosis due to endothelial damage and inducing hypercoaguable state and stasis[6]. Posterior subcapsular cataract and glaucoma are dose-related ophthalmologic side effects of GCs[6]. Hernia occurrence is common after liver transplantation and attributed to several factors, one of them being taking steroids[125].
A retrospective analysis on surgery complications of 382 patients with metabolic and bariatric surgery and prior history of SOT showed that while taking GCs are associated with a two-fold increase in overall morbidity, it did not contribute to morbidities related to bariatric surgery[126].
Pretransplant administration of GCs in patients with idiopathic pulmonary fibrosis may decrease graft survival after lung transplantation compared with GC-free patients[127]. Although concerns have arisen regarding airway anastomotic complications after lung transplantation in patients who were treated with GCs before transplantation, a retrospective study on 66 double-lung transplant recipients (40 used steroids prior to transplantation) found that early development of airway complications was not significantly higher in patients who took steroids before lung transplantation. In addition, in preoperative steroid users, the dose of steroid was not associated with the rate of post-transplant airway complications[128].
DRUG-DRUG INTERACTIONS
GCs are primarily metabolized by the CYP450 3A4 isoenzyme and are also substrates for the energy-dependent efflux pump P-glycoprotein[123,129]. GC metabolism may or may not be affected by CYP450 3A4 inhibitors, such as macrolide antibiotics, azole antifungal medications, and protease inhibitors. Studies have shown decreased clearance of methylprednisolone, but not prednisolone, with co-administration of CYP3A4 inhibitors[130,131]. CYP450 3A4 inducers (rifampin, carbamazepine, phenobarbital, phenytoin) can decrease GC’s serum levels[129]. GCs can induce CYP450 3A4/5 isoenzymes[129], and therefore increase the metabolism of CNIs (cyclosporine and tacrolimus) as the substrates of CYP450 3A4/5[132]. Clinical studies have shown a significant increase in dose-adjusted tacrolimus blood levels in patients on GC withdrawal regimens compared with patients taking GC-containing maintenance immunosuppression[132]. Interactions between GCs and tacrolimus are more seen in patients carrying the CYP3A5*1 allele[133]. GCs significantly contribute to inter-individual variability of apparent clearance of oral tacrolimus[134]. On the other hand, some studies reported that cyclosporine decreases prednisolone clearance by 25%-30% in kidney transplant patients[135], while others found no difference in dose-adjusted exposure of prednisolone when co-administered with cyclosporine or tacrolimus[136].
Possible need for therapeutic drug monitoring
Prednisolone is a standard component of most immunosuppressive protocols after SOT. Therapeutic drug level monitoring is not usually done for GCs. A study evaluated the pharmacokinetic characteristics of prednisolone, prednisone, and also cortisol and cortisone profiles, after treatment with prednisolone in adult kidney transplant recipients in the early 8-wk post-transplant period. Blood samples were obtained pre-dose and during a 24-h dose interval. Findings showed that renal transplant recipients experienced a relatively high prednisolone exposure, in parallel with strong suppression of endogenous cortisol profile as confirmed by a low evening-to-morning ratio of cortisol. A significant negative correlation (r = -0.83) between prednisolone area under the curve (AUC) 0-24 and morning cortisol concentrations was seen. AUC 0-24 of prednisolone and cortisol varied by three-fold and eighteen-fold, respectively, among patients. These results reveal large inter-individual variability in both prednisolone exposure and suppression of endogenous cortisol that signify a possible need for therapeutic drug monitoring of GCs[137].
CONCLUSION
GCs have been the mainstay for SOT for decades due to GC’s potent anti-inflammatory and immunosuppressive effects on the innate immunity and the significant tissue protective effects of GR on liver, kidney, and heart. In contrast, many of the side effects of GCs are on the non-target organs/tissues, such as bone, neuromuscular, adipose tissue, GI tract, and skin. Thus, specific delivery of GCs, via nanoparticles or transporter-mediated prodrugs, to the target organs of liver, kidney, and/or heart will enhance the efficacy and decrease the side effects of GCs in SOT. GCs’ side effects are generally associated with long-term use of high doses. It is noteworthy that most GCs activate both GR and MR. Recent studies indicate that some of the side effects of GCs on the liver, heart, kidney, and adipose tissues may be due to the activation of MR by GCs[138-141]. Therefore, GCs with higher selectivity for GR over MR, such as dexamethasone and budesonide, may have fewer side effects in SOT patients[23,142]. Additionally, GCs’ metabolic actions can be modulated by AMP-activated protein kinase (AMPK), a master regulator of energy metabolism. Activation of AMPK increased the phosphorylation of GR at serine-211 and reversed GC-induced hepatic steatosis and suppressed GC-mediated stimulation of glucose production in rats[143]. Interestingly, impaired AMPK activity was associated with steatotic graft injury in patients with living donor liver transplantation[144]. Thus, whether AMPK activators can ameliorate the metabolic side effects of GCs in SOT warrants investigation. In conclusion, approaches that enhance GC’s selectivity for GR, increase target tissue-specific delivery of GCs, and ameliorating the metabolic side effects of GCs will increase the efficacy and decrease the side effects of GCs in SOT.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review started: May 27, 2021
First decision: July 28, 2021
Article in press: November 3, 2021
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ssekandi AM S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
Contributor Information
Simin Dashti-Khavidaki, Department of Clinical Pharmacy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 14155, Iran.
Reza Saidi, Department of Surgery, SUNY Upstate Medical University, Syracuse, NY 13210, United States.
Hong Lu, Department of Pharmacology, SUNY Upstate Medical University, Syracuse, NY 13210, United States. luh@upstate.edu.
References
- 1.De Lucena DD, Rangel É B. Glucocorticoids use in kidney transplant setting. Expert Opini Drug Metab Toxicol. 2018;14:1023–1041. doi: 10.1080/17425255.2018.1530214. [DOI] [PubMed] [Google Scholar]
- 2.Nazzal M, Lentine KL, Naik AS, Ouseph R, Schnitzler MA, Zhang Z, Randall H, Dharnidharka VR, Segev DL, Kasiske BL, Hess GP, Alhamad T, McAdams-Demarco M, Axelrod DA. Center-driven and clinically driven variation in us liver transplant maintenance immunosuppression therapy: A national practice patterns analysis. Transplant Direct. 2018;4:e364. doi: 10.1097/TXD.0000000000000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldraich LA, Stehlik J, Cherikh WS, Edwards LB, Urban R, Dipchand A, Ross HJ. Duration of corticosteroid use and long-term outcomes after adult heart transplantation: A contemporary analysis of the international society for heart and lung transplantation registry. Clinical Transplant. 2018;32:e13340. doi: 10.1111/ctr.13340. [DOI] [PubMed] [Google Scholar]
- 4.Sitruk L, Couchoud C, Hourmant M, Tuppin P, Macher MA, Legeai C. Description of immunosuppressive maintenance treatments post kidney transplant through the national system of health insurance. Nephrol Ther. 2018;14:523–530. doi: 10.1016/j.nephro.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Zaza G, Leventhal J, Signorini L, Gambaro G, Cravedi P. Effects of antirejection drugs on innate immune cells after kidney transplantation. Front Immunol. 2019;10:2978. doi: 10.3389/fimmu.2019.02978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponticelli C, Glassock RJ. Prevention of complications from use of conventional immunosuppressants: A critical review. J Nephrol. 2019;32:851–870. doi: 10.1007/s40620-019-00602-5. [DOI] [PubMed] [Google Scholar]
- 7.Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O. Non-genomic effects of glucocorticoids: An updated view. Trend Pharmacol Sci. 2019;40:38–49. doi: 10.1016/j.tips.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eddy JL, Krukowski K, Janusek L, Mathews HL. Glucocorticoids regulate natural killer cell function epigenetically. Cell Immunol. 2014;290:120–130. doi: 10.1016/j.cellimm.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vock DM, Matas AJ. Rapid discontinuation of prednisone in kidney transplant recipients from at-risk subgroups: An optn/srtr analysis. Transplant Int. 2020;33:181–201. doi: 10.1111/tri.13530. [DOI] [PubMed] [Google Scholar]
- 10.Song TR, Jiang YM, Liu JP, Wang ZL, Zeng J, Huang ZL, Fan Y, Wang XD, Lin T. Steroid withdrawal or avoidance is safe in high-risk kidney transplants: A systematic review and meta-analysis. Kaohsiung J Med Sci. 2019;35:350–357. doi: 10.1002/kjm2.12064. [DOI] [PubMed] [Google Scholar]
- 11.Fairfield C, Penninga L, Powell J, Harrison EM, Wigmore SJ. Glucocorticosteroid-free versus glucocorticosteroid-containing immunosuppression for liver transplanted patients. Cochrane Database Syst Rev. 2015:CD007606. doi: 10.1002/14651858.CD007606.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Pelletier SJ, Nadig SN, Lee DD, Ammori JB, Englesbe MJ, Sung RS, Magee JC, Fontana RJ, Punch JD. A prospective, randomized trial of complete avoidance of steroids in liver transplantation with follow-up of over 7 years. HPB. 2013;15:286–293. doi: 10.1111/j.1477-2574.2012.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasiske BL, Chakkera HA, Louis TA, Ma JZ. A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol. 2000;11:1910–1917. doi: 10.1681/ASN.V11101910. [DOI] [PubMed] [Google Scholar]
- 14.Tönshoff B, Tedesco-Silva H, Ettenger R, Christian M, Bjerre A, Dello Strologo L, Marks SD, Pape L, Veldandi U, Lopez P, Cousin M, Pandey P, Meier M. Three-year outcomes from the cradle study in de novo pediatric kidney transplant recipients receiving everolimus with reduced tacrolimus and early steroid withdrawal. Ame J Transplant. 2021;21:123–137. doi: 10.1111/ajt.16005. [DOI] [PubMed] [Google Scholar]
- 15.Nanmoku K, Kurosawa A, Kubo T, Shinzato T, Shimizu T, Kimura T, Yagisawa T. Conversion from steroid to everolimus in maintenance kidney transplant recipients with posttransplant diabetes mellitus. Exp Clin Transplant. 2019;17:47–51. doi: 10.6002/ect.2017.0178. [DOI] [PubMed] [Google Scholar]
- 16.Montero N, Webster AC, Royuela A, Zamora J, Crespo Barrio M, Pascual J. Steroid avoidance or withdrawal for pancreas and pancreas with kidney transplant recipients. Cochrane Database Syst Revi. 2014:CD007669. doi: 10.1002/14651858.CD007669.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamour JM, Mason KL, Hsu DT, Feingold B, Blume ED, Canter CE, Dipchand AI, Shaddy RE, Mahle WT, Zuckerman WA, Bentlejewski C, Armstrong BD, Morrison Y, Diop H, Iklé DN, Odim J, Zeevi A, Webber SA. Early outcomes for low-risk pediatric heart transplant recipients and steroid avoidance: A multicenter cohort study (clinical trials in organ transplantation in children - CTOTC-04) J Heart Lung Transplant. 2019;38:972–981. doi: 10.1016/j.healun.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird AD, McDougall AR, Seow B, Hooper SB, Cole TJ. Glucocorticoid regulation of lung development: Lessons learned from conditional gr knockout mice. Mol Endocrinol. 2015;29:158–171. doi: 10.1210/me.2014-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto H, Sugimoto S, Tanaka S, Kurosaki T, Otani S, Yamane M, Taira N, Oto T, Toyooka S. A single-nucleotide polymorphism in a gene modulating glucocorticoid sensitivity is associated with the decline in total lung capacity after lung transplantation. Surg Today. 2019;49:268–274. doi: 10.1007/s00595-018-1717-9. [DOI] [PubMed] [Google Scholar]
- 20.Kim WR, Lake JR, Smit JM, Schladt DP, Skeans MA, Noreen SM, Robinson AM, MillerE , Synder JJ, Israni AK, Kasiske BL. Optn/srtr 2017 annual data report: Liver. Am J Transplant. 2019;19:184–283. doi: 10.1111/ajt.15276. [DOI] [PubMed] [Google Scholar]
- 21.Aberg F. Role of budesonide in liver transplantation. Transpl Immunol. 2014;30:178–179. doi: 10.1016/j.trim.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Zandieh I, Krygier D, Wong V, Howard J, Worobetz L, Minuk G, Witt-Sullivan H, Yoshida EM. The use of budesonide in the treatment of autoimmune hepatitis in canada. Can J Gastroenterol. 2008;22:388–392. doi: 10.1155/2008/509459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bari K, Shah SA, Kaiser TE, Cohen RM, Anwar N, Kleesattel D, Sherman KE. Safety and efficacy of budesonide for liver transplant immune suppression: Results of a pilot phase 2a trial. Liver Transpl. 2020;26:1430–1440. doi: 10.1002/lt.25837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan D. Kidney transplantation in adults: treatment of acute t-cell mediated (cellular) rejectionof therenal allograft; 2021. Database:uptodate. [cited 15 March 2021]. Available from: http://www.uptodate.com .
- 25.Djamali A. Kidney transplantation in adults: prevention and treatment of antibody-mediated rejection of the allograft. 2021. Database:uptodate. [cited 15 March 2021]. Available from: http://www.uptodate.com .
- 26.Cotler SJ. Liver transplantaion in adults: treatment of acute t cell mediated (cellular) rejection of the liver allograft. 2021. Database:uptodate. [cited 15 March 2021]. Available from: http://www.uptodate.com .
- 27.Eisen HJ. Heart transplantation in adults: treatment of acute allograft rejection. 2021. Database:uptodate. [cited 15 March 2021]. Available from: http://www.uptodate.com .
- 28.Pilewski J. Evaluation and treatment of acute lung transplant rejection. 2021. Database:uptodate. [cited 15 March 2021]. Available from: http://www.uptodate.com .
- 29.Chen J, Ferreira J, Martinez M, Lobritto S, Goldner D, Vittorio J. Role of budesonide for the treatment of rejection in pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 2020;71:388–392. doi: 10.1097/MPG.0000000000002784. [DOI] [PubMed] [Google Scholar]
- 30.Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, Marcus R, Parameshwar J, Ramsay A, Newstead C Haemato-oncology Task Force of the British Committee for Standards in Haematology and British Transplantation Society. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients-bcsh and bts guideline. Br J Haematol. 2010;149:693–705. doi: 10.1111/j.1365-2141.2010.08160.x. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen LS, Ortuno S, Lebrun-Vignes B, Johnson DB, Moslehi JJ, Hertig A, Salem JE. Transplant rejections associated with immune checkpoint inhibitors: A pharmacovigilance study and systematic literature review. Eur J cancer. 2021;148:36–47. doi: 10.1016/j.ejca.2021.01.038. [DOI] [PubMed] [Google Scholar]
- 32.Tsung I, Worden FP, Fontana RJ. A pilot study of checkpoint inhibitors in solid organ transplant recipients with metastatic cutaneous squamous cell carcinoma. Oncologist. 2021;26:133–138. doi: 10.1002/onco.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engberg H, Wehberg S, Bistrup C, Heaf J, Sørensen SS, Thiesson HC, Hansen JM, Svensson M, Green A, Marckmann P. Cancer risk and mortality after kidney transplantation: A population-based study on differences between danish centres using standard immunosuppression with and without glucocorticoids. Nephrol Dial Transplant. 2016;31:2149–2156. doi: 10.1093/ndt/gfw304. [DOI] [PubMed] [Google Scholar]
- 34.Sørensen HT, Mellemkjaer L, Nielsen GL, Baron JA, Olsen JH, Karagas MR. Skin cancers and non-hodgkin lymphoma among users of systemic glucocorticoids: A population-based cohort study. J Natl Cancer Inst. 2004;96:709–711. doi: 10.1093/jnci/djh118. [DOI] [PubMed] [Google Scholar]
- 35.Allen PJ, Chadban SJ, Craig JC, Lim WH, Allen RDM, Clayton PA, Teixeira-Pinto A, Wong G. Recurrent glomerulonephritis after kidney transplantation: Risk factors and allograft outcomes. Kidney Int. 2017;92:461–469. doi: 10.1016/j.kint.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Di Vico MC, Messina M, Fop F, Barreca A, Segoloni GP, Biancone L. Recurrent iga nephropathy after renal transplantation and steroid withdrawal. Clin Transplant. 2018;32:e13207. doi: 10.1111/ctr.13207. [DOI] [PubMed] [Google Scholar]
- 37.Hansrivijit P, Ghahramani N. Combined rituximab and plasmapheresis or plasma exchange for focal segmental glomerulosclerosis in adult kidney transplant recipients: A meta-analysis. Intl Urol Nephrol. 2020;52:1377–1387. doi: 10.1007/s11255-020-02462-6. [DOI] [PubMed] [Google Scholar]
- 38.Lim WH, Shingde M, Wong G. Recurrent and de novo glomerulonephritis after kidney transplantation. Front Immunol. 2019;10:1944. doi: 10.3389/fimmu.2019.01944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Infante B, Rossini M, Di Lorenzo A, Coviello N, Giuseppe C, Gesualdo L, Giuseppe G, Stallone G. Recurrence of immunoglobulin a nephropathy after kidney transplantation: A narrative review of the incidence, risk factors, pathophysiology and management of immunosuppressive therapy. Clin Kidney J. 2020;13:758–767. doi: 10.1093/ckj/sfaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faisal N, Renner EL. Recurrence of autoimmune liver disease after transplantation. World J Hepatol. 2015;7:2896–2905. doi: 10.4254/wjh.v7.i29.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czaja AJ. Diagnosis, pathogenesis, and treatment of autoimmune hepatitis after liver transplantation. Dig Dis Sci. 2012;57:2248–2266. doi: 10.1007/s10620-012-2179-3. [DOI] [PubMed] [Google Scholar]
- 42.Freist M, Bertrand D, Bailly E, Lambert C, Rouzaire PO, Lemal R, Aniort J, Büchler M, Heng AE, Garrouste C. Management of immunosuppression after kidney transplant failure: Effect on patient sensitization. Transplant Proceed. 2021;53:962–969. doi: 10.1016/j.transproceed.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Miller WM, Karus MA, Brennan DC. Kidney transplantation in adults: Management of the patient with a failed kidney transplant. 2021. Database:uptodate. [cited 15 March 2021]. Available from: http://www.uptodate.com .
- 44.Zhao XF, Lin DD, Li N, Wu JS, Guo QL, Wang L. Diagnosis and treatment of acute graft-versus-host disease after liver transplantation: A report of 11cases. Transpl Immunol. 2020;62:101307. doi: 10.1016/j.trim.2020.101307. [DOI] [PubMed] [Google Scholar]
- 45.Kanthasamy K, Chang MT, Kaur M. Graft-vs-host disease colitis after lung transplant. ACG case Rep J. 2019;6:e00287. doi: 10.14309/crj.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J, Yang L, Wu L, Zhao Q, Chen M, He X. Efficacy and safety of steroid therapy for posttransplant hyperbilirubinemia caused by early allograft dysfunction: A randomized controlled trial. Med Sci Monit. 2019;25:1936–1944. doi: 10.12659/MSM.915128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponticelli C, Moroni G. Fetal toxicity of immunosuppressive drugs in pregnancy. J Clin Med. 2018;7 doi: 10.3390/jcm7120552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugimoto S, Miyoshi K, Kurosaki T, Otani S, Yamane M, Kobayashi M, Oto T. Favorable survival in lung transplant recipients on preoperative low-dose, as compared to high-dose corticosteroids, after hematopoietic stem cell transplantation. Int J Hematol. 2018;107:696–702. doi: 10.1007/s12185-018-2417-3. [DOI] [PubMed] [Google Scholar]
- 49.Jiang S, Prozeller D, Pereira J, Simon J, Han S, Wirsching S, Fichter M, Mottola M, Lieberwirth I, Morsbach S, Mailänder V, Gehring S, Crespy D, Landfester K. Controlling protein interactions in blood for effective liver immunosuppressive therapy by silica nanocapsules. Nanoscale. 2020;12:2626–2637. doi: 10.1039/c9nr09879h. [DOI] [PubMed] [Google Scholar]
- 50.van Alem CMA, Schmidbauer M, Rong S, Derlin K, Schmitz J, Bräsen JH, Thorenz A, Chen R, Ruben JM, Winter EM, Schilperoort M, Kooijman S, Lalai RA, Metselaar JM, Klemann C, Meier M, van Kooten C, Gueler F, Rotmans JI. Liposomal delivery improves the efficacy of prednisolone to attenuate renal inflammation in a mouse model of acute renal allograft rejection. Transplantation. 2020;104:744–753. doi: 10.1097/TP.0000000000003060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y, Shen XF, Cao K, Ding J, Kang X, Guan WX, Ding YT, Liu BR, Du JF. Dexamethasone-induced myeloid-derived suppressor cells prolong allo cardiac graft survival through inos- and glucocorticoid receptor-dependent mechanism. Front Immunol. 2018;9:282. doi: 10.3389/fimmu.2018.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pratschke J, Wilhelm MJ, Kusaka M, Basker M, Cooper DK, Hancock WW, Tilney NL. Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation. 1999;67:343–348. doi: 10.1097/00007890-199902150-00001. [DOI] [PubMed] [Google Scholar]
- 53.De Vries DK, Lindeman JHN, Ringers J, Reinders M, Rabelink T, Schaapherder AFM. Donor brain death predisposes human kidney grafts to a proinflammatory reaction after transplantation. Am J Transplant. 2011;11:1064–1070. doi: 10.1111/j.1600-6143.2011.03466.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sayed Zeid AS, Sayed SS. A comparative study of the use of dexamethasone, n-acetyl cysteine, and theophylline to ameliorate renal ischemia-reperfusion injury in experimental rat models: A biochemical and immuno-histochemical approach. Saudi J Kidney Dis Transpl. 2020;31:982–997. doi: 10.4103/1319-2442.301203. [DOI] [PubMed] [Google Scholar]
- 56.Yang X, Wu X, Wu K, Yang D, Li Y, Shi J, Liu Y. Correlation of serum- and glucocorticoid-regulated kinase 1 expression with ischemia-reperfusion injury after heart transplantation. Pediatr Transplant. 2015;19:196–205. doi: 10.1111/petr.12417. [DOI] [PubMed] [Google Scholar]
- 57.Kotsch K, Ulrich F, Reutzel-Selke A, Pascher A, Faber W, Warnick P, Hoffman S, Francuski M, Kunert C, Kuecuek O, Schumacher G, Wesslau C, Lun A, Kohler S, Weiss S, Tullius SG, Neuhaus P, Pratschke J. Methylprednisolone therapy in deceased donors reduces inflammation in the donor liver and improves outcome after liver transplantation: A prospective randomized controlled trial. Ann Surg. 2008;248:1042–1050. doi: 10.1097/SLA.0b013e318190e70c. [DOI] [PubMed] [Google Scholar]
- 58.Kotloff RM, Blosser S, Fulda GJ, Malinoski D, Ahya VN, Angel L, Byrnes MC, DeVita MA, Grissom TE, Halpern SD, Nakagawa TA, Stock PG, Sudan DL, Wood KE, Anillo SJ, Bleck TP, Eidbo EE, Fowler RA, Glazier AK, Gries C, Hasz R, Herr D, Khan A, Landsberg D, Lebovitz DJ, Levine DJ, Mathur M, Naik P, Niemann CU, Nunley DR, O'Connor KJ, Pelletier SJ, Rahman O, Ranjan D, Salim A, Sawyer RG, Shafer T, Sonneti D, Spiro P, Valapour M, Vikraman-Sushama D, Whelan TP. Management of the potential organ donor in the icu: Society of critical care medicine/american college of chest physicians/association of organ procurement organizations consensus statement. Crit Care Med. 2015;43:1291–1325. doi: 10.1097/CCM.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 59.Reindl-Schwaighofer R, Kainz A, Jelencsics K, Heinzel A, Berlakovich G, Remport Á, Heinze G, Langer R, Oberbauer R. Steroid pretreatment of organ donors does not impact on early rejection and long-term kidney allograft survival: Results from a multicenter randomized, controlled trial. Am J Transplant. 2019;19:1770–1776. doi: 10.1111/ajt.15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matschek S, Wilflingseder J, Pones M, Kainz A, Bodingbauer M, Mühlbacher F, Langer RM, Gerlei Z, Oberbauer R. The effect of steroid pretreatment of deceased organ donors on liver allograft function: a blinded randomized placebo-controlled trial. J Hepatol. 2012;56:1305–1309. doi: 10.1016/j.jhep.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Erp AC, van Dullemen LFA, Ploeg RJ, Leuvenink HGD. Systematic review on the treatment of deceased organ donors. Transplant Rev (Orlando) 2018;32:194–206. doi: 10.1016/j.trre.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Saidi RF, Chang J, Verb S, Brooks S, Nalbantoglu I, Adsay V, Jacobs MJ. The effect of methylprednisolone on warm ischemia-reperfusion injury in the liver. Am J Surg. 2007;193:345–347; discussion 347-348. doi: 10.1016/j.amjsurg.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 63.Van Zanden JE, A’t Hart N, Ottens PJ, Liu B, Rebolledo RA, Erasmus ME, Leuvenink HGD. Methylprednisolone treatment in brain death-induced lung inflammation-a dose comparative study in rats. Front Pharmacol. 2021;12:587003. doi: 10.3389/fphar.2021.587003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paulus P, Holfeld J, Urbschat A, Mutlak H, Ockelmann PA, Tacke S, Zacharowski K, Reissig C, Stay D, Scheller B. Prednisolone as preservation additive prevents from ischemia reperfusion injury in a rat model of orthotopic lung transplantation. PloS one. 2013;8:e73298. doi: 10.1371/journal.pone.0073298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Messer SJ, Axell RG, Colah S, White PA, Ryan M, Page AA, Parizkova B, Valchanov K, White CW, Freed DH, Ashley E, Dunning J, Goddard M, Parameshwar J, Watson CJ, Krieg T, Ali A, Tsui S, Large SR. Functional assessment and transplantation of the donor heart after circulatory death. J Heart Lung Transplant. 2016;35:1443–1452. doi: 10.1016/j.healun.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Ardehali A, Esmailian F, Deng M, Soltesz E, Hsich E, Naka Y, Mancini D, Camacho M, Zucker M, Leprince P, Padera R, Kobashigawa J. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): A prospective, open-label, multicentre, randomised non-inferiority trial. Lancet. 2015;385:2577–2584. doi: 10.1016/S0140-6736(15)60261-6. [DOI] [PubMed] [Google Scholar]
- 67.Dharnidharka VR, Schnitzler MA, Chen J, Brennan DC, Axelrod D, Segev DL, Schechtman KB, Zheng J, Lentine KL. Differential risks for adverse outcomes 3 years after kidney transplantation based on initial immunosuppression regimen: A national study. Transplant Int. 2016;29:1226–1236. doi: 10.1111/tri.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anesi JA, Lautenbach E, Tamma PD, Thom KA, Blumberg EA, Alby K, Bilker WB, Werzen A, Tolomeo P, Omorogbe J, Pineles L, Han JH. Risk factors for extended-spectrum β-lactamase-producing enterobacterales bloodstream infection among solid-organ transplant recipients. Clin Infect Dis. 2021;72:953–960. doi: 10.1093/cid/ciaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Margalit I, Goldberg E, Ben Ari Y, Ben-Zvi H, Shostak Y, Krause I, Muhsen K. Clinical correlates of nocardiosis. Sci Rep. 2020;10:14272. doi: 10.1038/s41598-020-71214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinbrink J, Leavens J, Kauffman CA, Miceli MH. Manifestations and outcomes of nocardia infections: Comparison of immunocompromised and nonimmunocompromised adult patients. Medicine (Baltimore) 2018;97:e12436. doi: 10.1097/MD.0000000000012436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nucci M, Engelhardt M, Hamed K. Mucormycosis in south america: A review of 143 reported cases. Mycoses. 2019;62:730–738. doi: 10.1111/myc.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matthews H, Rohde H, Wichmann D, Kluge S. Invasive Pulmonary Aspergillosis. Deutsch Med Wochenschr. 2019;144:1218–1222. doi: 10.1055/a-0817-7432. [DOI] [PubMed] [Google Scholar]
- 73.Smolovic B, Vukcevic B, Muhovic D, Ratkovic M. Renal aspergillosis in a liver transplant patient: A case report and review of literature. World J Clin Cases. 2018;6:1155–1159. doi: 10.12998/wjcc.v6.i16.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fishman JA, Gans H. Pneumocystis jiroveci in solid organ transplantation: Guidelines from the american society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33:e13587. doi: 10.1111/ctr.13587. [DOI] [PubMed] [Google Scholar]
- 75.Permpalung N, Kittipibul V, Mekraksakit P, Rattanawong P, Nematollahi S, Zhang SX, Mehta Steinke S. A comprehensive evaluation of risk factors for pneumocystis jirovecii pneumonia in adult solid organ transplant recipients: A systematic review and meta-analysis. Transplantation. 2020 doi: 10.1097/TP.0000000000003576. [DOI] [PubMed] [Google Scholar]
- 76.Kaminski H, Belliere J, Burguet L, Del Bello A, Taton B, Poirot-Mazères S, Accoceberry I, Delhaes L, Visentin J, Gregori M, Iriart X, Charpentier E, Couzi L, Kamar N, Merville P. Identification of predictive markers and outcomes of late-onset pneumocystis jirovecii pneumonia in kidney transplant recipients. Clin Infect Dis. 2020:ciaa1611. doi: 10.1093/cid/ciaa1611. [DOI] [PubMed] [Google Scholar]
- 77.Lee HY, Choi SH, Kim T, Chang J, Kim SH, Lee SO, Kim MN, Sung H. Epidemiologic trends and clinical features of pneumocystis jirovecii pneumonia in non-hiv patients in a tertiary-care hospital in korea over a 15-year-period. Jpn J Infect Dis. 2019;72:270–273. doi: 10.7883/yoken.JJID.2018.400. [DOI] [PubMed] [Google Scholar]
- 78.Mundo W, Morales-Shnaider L, Tewahade S, Wagner E, Archuleta S, Bandali M, Chadalawada S, Johnson SC, Franco-Paredes C, Shapiro L, Henao-Martínez AF. Lower mortality associated with adjuvant corticosteroid therapy in non-hiv-infected patients with pneumocystis jirovecii pneumonia: A single-institution retrospective us cohort study. Open Forum Infect Dis. 2020;7:ofaa354. doi: 10.1093/ofid/ofaa354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moon SM, Kim T, Sung H, Kim MN, Kim SH, Choi SH, Jeong JY, Woo JH, Kim YS, Lee SO. Outcomes of moderate-to-severe pneumocystis pneumonia treated with adjunctive steroid in non-hiv-infected patients. Antimicrob Agents Chemother. 2011;55:4613–4618. doi: 10.1128/AAC.00669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samannodi M, Vaghefi-Hosseini R, Nigo M, Guevara EY, Hasbun R. Risk classification for respiratory viral infections in adult solid organ transplantation recipients. Transplant Proc. 2021;53:737–742. doi: 10.1016/j.transproceed.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 81.Mahan LD, Kanade R, Mohanka MR, Bollineni S, Joerns J, Kaza V, Torres F, La Hoz RM, Banga A. Characteristics and outcomes among patients with community-acquired respiratory virus infections during the first year after lung transplantation. Clin Transplant. 2021;35:e14140. doi: 10.1111/ctr.14140. [DOI] [PubMed] [Google Scholar]
- 82.Gu J, Su QQ, Zuo TT, Chen YB. Adenovirus diseases: a systematic review and meta-analysis of 228 case reports. Infection. 2021;49:1–13. doi: 10.1007/s15010-021-01588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santeusanio AD, Zendel A, Fenig Y, Mahamid A, Bhansali A, De Boccardo G, Delaney V, Farouk SS, Dunn D, Rana M, Florman S, Shapiro R. Kidney transplantation using lymphocyte depleting induction and standard maintenance immunosuppression at the height of the sars-cov-2 pandemic in new york city: A single-center experience. Clin Transplant. 2020;34:e14055. doi: 10.1111/ctr.14055. [DOI] [PubMed] [Google Scholar]
- 84.Willicombe M, Gleeson S, Clarke C, Dor F, Prendecki M, Lightstone L, Lucisano G, McAdoo S, Thomas D. Identification of patient characteristics associated with sars-cov-2 infection and outcome in kidney transplant patients using serological screening. Transplantation. 2021;105:151–157. doi: 10.1097/TP.0000000000003526. [DOI] [PubMed] [Google Scholar]
- 85.Crowhurst T, Nolan J, Faull R, Holmes M, Holmes-Liew CL. Bk virus-associated nephropathy in a lung transplant patient: Case report and literature review. BMC Infect Dis. 2020;20:600. doi: 10.1186/s12879-020-05292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sandkovsky U, Qiu F, Kalil AC, Florescu A, Wilson N, Manning C, Florescu DF. Risk factors for the development of cytomegalovirus resistance in solid organ transplantation: a retrospective case-control study. Transplant Proc. 2018;50:3763–3768. doi: 10.1016/j.transproceed.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 87.Van Damme E, Sauviller S, Lau B, Kesteleyn B, Griffiths P, Burroughs A, Emery V, Sinclair J, Van Loock M. Glucocorticosteroids trigger reactivation of human cytomegalovirus from latently infected myeloid cells and increase the risk for hcmv infection in d+r+ liver transplant patients. J Gen Virol. 2015;96:131–143. doi: 10.1099/vir.0.069872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.González-Naranjo LA, Coral-Enríquez JA, Restrepo-Escobar M, Muñoz-Vahos CH, Jaramillo-Arroyave D, Vanegas-García AL, Eraso R, Vásquez G, Jaimes F. Factors associated with active tuberculosis in colombian patients with systemic lupus erythematosus: a case-control study. Clin Rheumatol. 2021;40:181–191. doi: 10.1007/s10067-020-05225-x. [DOI] [PubMed] [Google Scholar]
- 89.Miglioli-Galvão L, Pestana JOM, Lopes-Santoro G, Torres Gonçalves R, Requião Moura LR, Pacheco Silva Á, Camera Pierrotti L, David Neto E, Santana Girão E, Costa de Oliveira CM, Saad Abboud C, Dias França J, Devite Bittante C, Corrêa L, Aranha Camargo LF. Severe strongyloides stercoralis infection in kidney transplant recipients: A multicenter case-control study. PLoS Negl Trop Dis. 2020;14:e0007998. doi: 10.1371/journal.pntd.0007998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clemente W, Vidal E, Girão E, Ramos AS, Govedic F, Merino E, Muñoz P, Sabé N, Cervera C, Cota GF, Cordero E, Mena A, Montejo M, López-Medrano F, Aguado JM, Fernandes P, Valerio M, Carratalá J, Moreno A, Oliveira J, Mourão PH, Torre-Cisneros J. Risk factors, clinical features and outcomes of visceral leishmaniasis in solid-organ transplant recipients: a retrospective multicenter case-control study. Clin Microbiol Infect. 2015;21:89–95. doi: 10.1016/j.cmi.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Wang C, Huo Y, Li X, Lin A, Hu Q, Xiong C, Deng Y. Factors related to bone metabolism in kidney transplant recipients. Mediators Inflamm. 2021;2021:6679095. doi: 10.1155/2021/6679095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torregrosa JV, Ferreira AC, Cucchiari D, Ferreira A. Bone mineral disease after kidney transplantation. Calcif Tissue Int. 2021;108:551–560. doi: 10.1007/s00223-021-00837-0. [DOI] [PubMed] [Google Scholar]
- 93.Batteux B, Gras-Champel V, Lando M, Brazier F, Mentaverri R, Desailly-Henry I, Rey A, Bennis Y, Masmoudi K, Choukroun G, Liabeuf S. Early steroid withdrawal has a positive effect on bone in kidney transplant recipients: a propensity score study with inverse probability-of-treatment weighting. Ther Adv Musculoskelet Dis. 2020;12:1759720x20953357. doi: 10.1177/1759720X20953357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pérez-Sáez MJ, Herrera S, Prieto-Alhambra D, Vilaplana L, Nogués X, Vera M, Redondo-Pachón D, Mir M, Güerri R, Crespo M, Díez-Pérez A, Pascual J. Maintenance low dose systemic glucocorticoids have limited impact on bone strength and mineral density among incident renal allograft recipients: a pilot prospective cohort study. Bone. 2018;116:290–294. doi: 10.1016/j.bone.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 95.Oya A, Umezu T, Ogawa R, Nishiwaki T, Niki Y, Nakamura M, Matsumoto M, Kanaji A. Short-term outcomes of total hip arthroplasty after liver transplantation. Arthroplast Today. 2021;8:11–14. doi: 10.1016/j.artd.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takao M, Abe H, Sakai T, Hamada H, Takahara S, Sugano N. Transitional changes in the incidence of hip osteonecrosis among renal transplant recipients. J Orthop Sci. 2020;25:466–471. doi: 10.1016/j.jos.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Felten R, Perrin P, Caillard S, Moulin B, Javier RM. Avascular osteonecrosis in kidney transplant recipients: risk factors in a recent cohort study and evaluation of the role of secondary hyperparathyroidism. PloS one. 2019;14:e0212931. doi: 10.1371/journal.pone.0212931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang J, Jing M, Yang X. Association between genetic polymorphisms and osteonecrosis in steroid treatment populations: a detailed stratified and dose-response meta-analysis. Biosci Rep. 2019;39 doi: 10.1042/BSR20190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tronche F, Opherk C, Moriggl R, Kellendonk C, Reimann A, Schwake L, Reichardt HM, Stangl K, Gau D, Hoeflich A, Beug H, Schmid W, Schutz G. Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Dev. 2004;18:492–497. doi: 10.1101/gad.284704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Delucchi Á, Toro L, Alzamora R, Barrientos V, González M, Andaur R, León P, Villanueva F, Galindo M, Las Heras F, Montecino M, Moena D, Lazcano A, Pinto V, Salas P, Reyes ML, Mericq V, Michea L. Glucocorticoids decrease longitudinal bone growth in pediatric kidney transplant recipients by stimulating the fgf23/fgfr3 signaling pathway. J Bone Miner Res. 2019;34:1851–1861. doi: 10.1002/jbmr.3761. [DOI] [PubMed] [Google Scholar]
- 101.McCaffrey J, Shenoy M. Acute rejection and growth outcomes in paediatric kidney allograft recipients treated with a corticosteroid minimisation immunosuppressive protocol. Pediatr Nephrol. 2021 doi: 10.1007/s00467-021-04948-6. [DOI] [PubMed] [Google Scholar]
- 102.Kusumi K, Shaikhkhalil A, Patel HP, Mahan JD. Promoting bone health in children and adolescents following solid organ transplantation. Pediatr Transplant. 2021;25:e13940. doi: 10.1111/petr.13940. [DOI] [PubMed] [Google Scholar]
- 103.Dalle H, Garcia M, Antoine B, Boehm V, Do TTH, Buyse M, Ledent T, Lamaziere A, Magnan C, Postic C, Denis RG, Luquet S, Feve B, Moldes M. Adipocyte glucocorticoid receptor deficiency promotes adipose tissue expandability and improves the metabolic profile under corticosterone exposure. Diabetes. 2019;68:305–317. doi: 10.2337/db17-1577. [DOI] [PubMed] [Google Scholar]
- 104.Cruz-Topete D, Oakley RH, Carroll NG, He B, Myers PH, Xu X, Watts MN, Trosclair K, Glasscock E, Dominic P, Cidlowski JA. Deletion of the cardiomyocyte glucocorticoid receptor leads to sexually dimorphic changes in cardiac gene expression and progression to heart failure. J Am Heart Assoc. 2019;8:e011012. doi: 10.1161/JAHA.118.011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: Causes, treatment, and impact on outcomes. Endocr Rev. 2016;37:37–61. doi: 10.1210/er.2015-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kgosidialwa O, Blake K, O'Connell O, Egan J, O'Neill J, Hatunic M. Post-transplant diabetes mellitus associated with heart and lung transplant. Ir J Med Sci. 2020;189:185–189. doi: 10.1007/s11845-019-02068-7. [DOI] [PubMed] [Google Scholar]
- 107.Guad RM, Taylor-Robinson AW, Wu YS, Gan SH, Zaharan NL, Basu RC, Liew CSL, Wan Md Adnan WAH. Clinical and genetic risk factors for new-onset diabetes mellitus after transplantation (NODAT) in major transplant centres in malaysia. BMC Nephrol. 2020;21:388. doi: 10.1186/s12882-020-02052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tillmann FP, Schmitz M, Rump LC, Quack I. Impact of low-dose steroids on hba1c levels and development of pre-diabetes and nodat in non-diabetic renal transplant recipients on long-term follow-up. Int Urol Nephrol. 2018;50:771–777. doi: 10.1007/s11255-017-1754-0. [DOI] [PubMed] [Google Scholar]
- 109.Toshima T, Yoshizumi T, Inokuchi S, Kosai-Fujimoto Y, Kurihara T, Yoshiya S, Mano Y, Takeishi K, Itoh S, Harada N, Ikegami T, Soejima Y, Shimokawa M, Maehara Y, Mori M. Risk factors for the metabolic syndrome components of hypertension, diabetes mellitus, and dyslipidemia after living donor liver transplantation. HPB (Oxford) 2020;22:511–520. doi: 10.1016/j.hpb.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 110.Kathirvel M, Mallick S, Sethi P, Thillai M, Durairaj MS, Nair K, Sunny A, Mathew JS, Varghese CT, Chandran B, Pillai Thankamony Amma BS, Menon RN, Balakrishnan D, Gopalakrishnan U, Surendran S. Randomized trial of steroid free immunosuppression with basiliximab induction in adult live donor liver transplantation (ldlt) HPB (Oxford) 2020:S1365–182x(20)31157-6. doi: 10.1016/j.hpb.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 111.ADA's standards of medical care in diabetes. Clinical diabetes. 2021;39:128. doi: 10.2337/cd21-pe01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi HK, Seeger JD. Glucocorticoid use and serum lipid levels in us adults: The third national health and nutrition examination survey. Arthritis Rheum. 2005;53:528–535. doi: 10.1002/art.21329. [DOI] [PubMed] [Google Scholar]
- 113.Höcker B, Weber LT, John U, Drube J, Fehrenbach H, Klaus G, Pohl M, Seeman T, Fichtner A, Wühl E, Tönshoff B. Steroid withdrawal improves blood pressure control and nocturnal dipping in pediatric renal transplant recipients: Analysis of a prospective, randomized, controlled trial. Pediatr Nephrol. 2019;34:341–348. doi: 10.1007/s00467-018-4069-1. [DOI] [PubMed] [Google Scholar]
- 114.Ahmad N, Khan TFT, Nadeem N, Fourtounas K. Steroid-sparing and steroid-based immunosuppression in kidney transplant: Is there a difference in outcomes and recipient comorbidities? Exp Clin Transplant. 2020;18:572–576. doi: 10.6002/ect.2020.0067. [DOI] [PubMed] [Google Scholar]
- 115.Goodwin JE, Zhang J, Gonzalez D, Albinsson S, Geller DS. Knockout of the vascular endothelial glucocorticoid receptor abrogates dexamethasone-induced hypertension. J Hypertens. 2011;29:1347–1356. doi: 10.1097/HJH.0b013e328347da54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goodwin JE, Feng Y, Velazquez H, Zhou H, Sessa WC. Loss of the endothelial glucocorticoid receptor prevents the therapeutic protection afforded by dexamethasone after lps. PloS one. 2014;9:e108126. doi: 10.1371/journal.pone.0108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goodwin JE, Feng Y, Velazquez H, Sessa WC. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc Natl Acad Sci U S A. 2013;110:306–311. doi: 10.1073/pnas.1210200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Faravelli I, Velardo D, Podestà MA, Ponticelli C. Immunosuppression-related neurological disorders in kidney transplantation. J Nephrol. 2021 doi: 10.1007/s40620-020-00956-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watson ML, Baehr LM, Reichardt HM, Tuckermann JP, Bodine SC, Furlow JD. A cell-autonomous role for the glucocorticoid receptor in skeletal muscle atrophy induced by systemic glucocorticoid exposure. Am J Physiol Endocrinol Metab. 2012;302:E1210–1220. doi: 10.1152/ajpendo.00512.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Valentin A, Borresen SW, Rix M, Elung-Jensen T, Sørensen SS, Feldt-Rasmussen U. Adrenal insufficiency in kidney transplant patients during low-dose prednisolone therapy: a cross-sectional case-control study. Nephrol Dial Transplant. 2020;35:2191–2197. doi: 10.1093/ndt/gfz180. [DOI] [PubMed] [Google Scholar]
- 121.Vulto A, Minović I, de Vries LV, Timmermans AC, van Faassen M, Gomes Neto AW, Touw DJ, de Jong MFC, van Beek AP, Dullaart RPF, Navis G, Kema IP, Bakker SJL. Endogenous urinary glucocorticoid metabolites and mortality in prednisolone-treated renal transplant recipients. Clin Transplant. 2020;34:e13824. doi: 10.1111/ctr.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Vries LV, de Jong WHA, Touw DJ, Berger SP, Navis G, Kema IP, Bakker SJL. Twenty-four hour urinary cortisol excretion and the metabolic syndrome in prednisolone-treated renal transplant recipients. Steroids. 2017;127:31–39. doi: 10.1016/j.steroids.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 123.Tielemans MM, van Boekel GAJ, van Gelder T, Tjwa ET, Hilbrands LB. Immunosuppressive drugs and the gastrointestinal tract in renal transplant patients. Transplant Rev (Orlando) 2019;33:55–63. doi: 10.1016/j.trre.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 124.Gibson CM, Childs-Kean LM, Naziruddin Z, Howell CK. The alteration of the gut microbiome by immunosuppressive agents used in solid organ transplantation. Transplant Infect Dis. 2021;23:e13397. doi: 10.1111/tid.13397. [DOI] [PubMed] [Google Scholar]
- 125.Garmpis N, Spartalis E, Schizas D, Patsouras D, Damaskos C, Spartalis M, Garmpi A, Nikiteas NI, Dimitroulis D. Incisional hernias post liver transplantation: Current evidence of epidemiology, risk factors and laparoscopic versus open repair. A review of the literature. In Vivo. 2019;33:1059–1066. doi: 10.21873/invivo.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fagenson AM, Mazzei M, Edwards MA. Does steroid use in transplant patients undergoing bariatric surgery independently impact outcomes? J Surg Res. 2020;254:294–299. doi: 10.1016/j.jss.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 127.Tuyls S, Verleden SE, Wuyts WA, Yserbyt J, Vos R, Verleden GM. Determinants of survival in lung transplantation patients with idiopathic pulmonary fibrosis: A retrospective cohort study. Transpl Int. 2019;32:399–409. doi: 10.1111/tri.13382. [DOI] [PubMed] [Google Scholar]
- 128.Kim HE, Paik HC, Kim SY, Park MS, Lee JG. Preoperative corticosteroid use and early postoperative bronchial anastomotic complications after lung transplantation. Korean J Thorac Cardiovasc Surg. 2018;51:384–389. doi: 10.5090/kjtcs.2018.51.6.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guengerich FP. Cytochrome p-450 3a4: Regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 130.Lebrun-Vignes B, Archer VC, Diquet B, Levron JC, Chosidow O, Puech AJ, Warot D. Effect of itraconazole on the pharmacokinetics of prednisolone and methylprednisolone and cortisol secretion in healthy subjects. Br J Clin Pharmacol. 2001;51:443–450. doi: 10.1046/j.1365-2125.2001.01372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fost DA, Leung DY, Martin RJ, Brown EE, Szefler SJ, Spahn JD. Inhibition of methylprednisolone elimination in the presence of clarithromycin therapy. J Allergy Clin Immunol. 1999;103:1031–1035. doi: 10.1016/s0091-6749(99)70175-2. [DOI] [PubMed] [Google Scholar]
- 132.Shihab FS, Lee ST, Smith LD, Woodle ES, Pirsch JD, Gaber AO, Henning AK, Reisfield R, Fitzsimmons W, Holman J. Effect of corticosteroid withdrawal on tacrolimus and mycophenolate mofetil exposure in a randomized multicenter study. Am J Transplant. 2013;13:474–484. doi: 10.1111/j.1600-6143.2012.04327.x. [DOI] [PubMed] [Google Scholar]
- 133.Hosohata K, Uesugi M, Hashi S, Hosokawa M, Inui K, Matsubara K, Ogawa K, Fujimoto Y, Kaido T, Uemoto S, Masuda S. Association between CYP3A5 genotypes in graft liver and increase in tacrolimus biotransformation from steroid treatment in living-donor liver transplant patients. Drug Metab Pharmacokinet. 2014;29:83–89. doi: 10.2133/dmpk.dmpk-13-rg-060. [DOI] [PubMed] [Google Scholar]
- 134.Al-Kofahi M, Oetting WS, Schladt DP, Remmel RP, Guan W, Wu B, Dorr CR, Mannon RB, Matas AJ, Israni AK, Jacobson PA. Precision dosing for tacrolimus using genotypes and clinical factors in kidney transplant recipients of european ancestry. J Clin Pharmacol. 2021 doi: 10.1002/jcph.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ost L. Impairment of prednisolone metabolism by cyclosporine treatment in renal graft recipients. Transplantation. 1987;44:533–535. [PubMed] [Google Scholar]
- 136.Bergmann TK, Isbel NM, Barraclough KA, Campbell SB, McWhinney BC, Staatz CE. Comparison of the influence of cyclosporine and tacrolimus on the pharmacokinetics of prednisolone in adult male kidney transplant recipients. Clin Drug Invest. 2014;34:183–188. doi: 10.1007/s40261-013-0162-1. [DOI] [PubMed] [Google Scholar]
- 137.Skauby RH, Gustavsen MT, Andersen AM, Bjerre A, Åsberg A, Midtvedt K, Vethe NT, Bergan S. Prednisolone and prednisone pharmacokinetics in adult renal transplant recipients. Ther Drug Monit. 2021;43:247–255. doi: 10.1097/FTD.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 138.Richardson RV, Batchen EJ, Denvir MA, Gray GA, Chapman KE. Cardiac gr and mr: From development to pathology. Trends Endocrinol Metab. 2016;27:35–43. doi: 10.1016/j.tem.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 139.Ferguson D, Hutson I, Tycksen E, Pietka TA, Bauerle K, Harris CA. Role of mineralocorticoid receptor in adipogenesis and obesity in male mice. Endocrinology. 2020;161 doi: 10.1210/endocr/bqz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65:257–263. doi: 10.1161/HYPERTENSIONAHA.114.04488. [DOI] [PubMed] [Google Scholar]
- 141.John K, Marino JS, Sanchez ER, Hinds Jr TD. The glucocorticoid receptor: Cause of or cure for obesity? Am J Physiol Endocrinol Metab. 2016;310:E249–257. doi: 10.1152/ajpendo.00478.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jacob KA, Leaf DE, Dieleman JM, van Dijk D, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, de Lange F, Boer C, Kluin J, Waikar SS, Dexamethasone for Cardiac Surgery Study G. Intraoperative high-dose dexamethasone and severe AKI after cardiac surgery. J Am Soc Nephrol. 2015;26:2947–2951. doi: 10.1681/ASN.2014080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nader N, Ng SS, Lambrou GI, Pervanidou P, Wang Y, Chrousos GP, Kino T. Ampk regulates metabolic actions of glucocorticoids by phosphorylating the glucocorticoid receptor through p38 mapk. Mol Endocrinol. 2010;24:1748–1764. doi: 10.1210/me.2010-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu J, Pang L, Ng KTP, Chiu TLS, Liu H, Liu X, Xu A, Lo CM, Man K. Compromised ampk-pgc1alpha axis exacerbated steatotic graft injury by dysregulating mitochondrial homeostasis in living donor liver transplantation. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004468. [DOI] [PubMed] [Google Scholar]