Abstract
BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can result in clinically significant multi-system disease including involvement in the kidney. The underlying histopathological processes were unknown at the start of the pandemic. As case reports and series have been published describing the underlying renal histopathology from kidney biopsies, we have started to gain an insight into the renal manifestations of this novel disease.
AIM
To provide an overview of the current literature on the renal histopathological features and mechanistic insights described in association with coronavirus disease 2019 (COVID-19) infection.
METHODS
A systematic review was performed by conducting a literature search in the following websites-‘PubMed’, ‘Web of Science’, ‘Embase’ and ‘Medline-ProQuest’ with the following search terms-“COVID-19 AND kidney biopsy”, “COVID-19 AND renal biopsy”, “SARS-CoV-2 AND kidney biopsy” and “SARS-CoV-2 AND renal biopsy”. We have included published data up until February 15, 2021, which includes kidney biopsies (native, transplant and postmortem) from patients with COVID-19. Data on clinical presentation, histopathological features, management and outcome was extracted from the reported studies.
RESULTS
The total number of biopsies reported on here is 288, of which 189 are postmortem, 84 native and 15 transplants. The results are varied and show underlying pathologies ranging from collapsing glomerulopathy and acute tubular injury (ATI) to anti-nuclear cytoplasmic antibody associated vasculitis and pigment nephropathy. There was variation in the specific treatment used for the various renal conditions, which included steroids, hydroxychloroquine, eculizumab, convalescent plasma, rituximab, anakinra, cyclophosphamide and renal replacement therapy, amongst others. The pathological process which occurs in the kidney following COVID-19 infection and leads to the described biopsy findings has been hypothesized in some conditions but not others (for example, sepsis related hypoperfusion for ATI). It is important to note that this represents a very small minority of the total number of cases of COVID-19 related kidney disease, and as such there may be inherent selection bias in the results described. Further work will be required to determine the pathogenetic link, if any, between COVID-19 and the other renal pathologies.
CONCLUSION
This report has clinical relevance as certain renal pathologies have specific management, with the implication that kidney biopsy in the setting of renal disease and COVID-19 should be an early consideration, dependent upon the clinical presentation.
Keywords: COVID-19, Histopathology, Kidney biopsy, Transplant, SARS-CoV-2
Core Tip: Coronavirus disease 2019 (COVID-19) affects multiple organ systems, including the kidneys resulting in acute kidney injury. Multiple pathologies and different mechanisms have been attributed to the pathogenesis of kidney disease in COVID-19. This systematic review aims to provide an overview of the histopathological findings reported in kidney biopsies associated with COVID-19 infection.
INTRODUCTION
The novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the disease coronavirus disease 2019 (COVID-19), was first identified in Wuhan, China in 2019; it has resulted in a global pandemic. The first cases were reported to the World Health Organization on December 31, 2019[1]. As of February 27, 2021, there were over 112 million cumulative cases and more than 2.5 million deaths worldwide[2]. The initial disease presentation is typically with respiratory symptoms[3], however, the multisystem effects of SARS-CoV-2 infection are now widely acknowledged and include cardiac, gastrointestinal tract, neurological, hematological and renal involvement[4-8]. It is recognized that patients with kidney dysfunction and COVID-19 have an increased risk of adverse outcomes[8,9]. A recent systematic review has shown an estimated incidence of acute kidney injury (AKI) of 10.0% in hospitalized patients with COVID-19[10]. Furthermore, within the United Kingdom, since September 1, 2020 and March 18, 2021, 3981 of 24542 (16.2%) patients with COVID-19 admitted to intensive care have required renal replacement therapy (RRT). Of these, 2633 (66.1%) died[11].
Various mechanisms of AKI secondary to COVID-19 have been proposed–from direct intrarenal infection to dysregulation of the renin-angiotensin-aldosterone system, to altered hemodynamic control, coagulation and cytokine homeostasis[12]. These proposed mechanisms require further validation with pathological correlation. An increasing number of case reports of patients with COVID-19, who have undergone kidney biopsies, are now published. The underlying pathology in these reports is varied and includes acute tubular injury (ATI) and collapsing glomerulopathy (CG) associated with high-risk apolipoprotein L1 (APOL1) alleles. Here, we provide a rapid clinical review of the current literature to help delineate the range of renal histopathological features associated with COVID-19. It is important to note that the case reports and series described in this review only represent a very small minority of the total number of cases of COVID-19 related kidney disease, and as such there may be inherent selection bias in the results described.
MATERIALS AND METHODS
Eligibility criteria
We included all research articles reporting histopathological findings in kidney biopsies from adult patients (> 18 years) with concurrent COVID-19 infection. These included native, transplant and postmortem kidney biopsies. We only included articles published in the English language. All studies published before February 15, 2021, were included in this review.
Search strategy and study selection
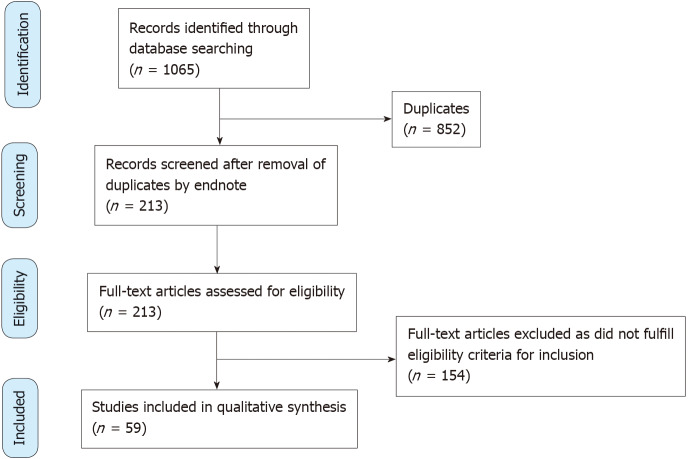
A systematic literature search was conducted by two independent authors (VJ and HW) in the following websites-‘PubMed’, ‘Web of Science’, ‘Embase’ and ‘Medline-ProQuest’. The search terms incorporated the following-“COVID-19 AND kidney biopsy”, “COVID-19 AND renal biopsy”, “SARS-CoV-2 AND kidney biopsy” and “SARS-CoV-2 AND renal biopsy”. The articles were screened by three authors (VJ, HW and RC) for relevance and duplicate publications were removed. Duplicate screening and eligibility check was performed by JS. The study selection was carried out as per the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guideline (Figure 1).
Figure 1.
PRISMA flow diagram.
Data extraction
Data including patient demographics (age, gender, ethnicity), co-morbidities, clinical presentation (COVID-19 and renal manifestations), kidney parameters at baseline (serum creatinine, serum albumin and proteinuria), time from COVID-19 diagnosis to kidney biopsy, management (COVID-19 and renal specific), indication for RRT and outcome (renal specific and all-cause outcomes) were extracted from each article. Data is illustrated as figures and tables.
Study registration
A pre-defined review protocol was registered at the PROSPERO international prospective register of systematic reviews, registration number CRD42020218048. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020218048.
RESULTS
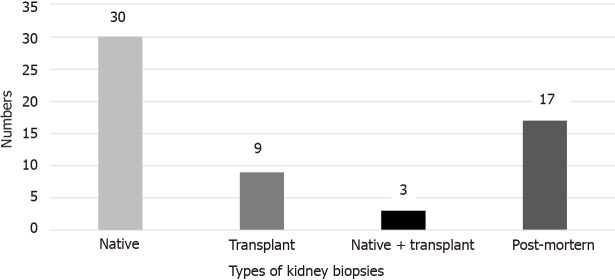
Our review identified a total of 59 studies reporting COVID-19 related histopathological diagnoses from kidney biopsy. Of these 59 studies, 30 reported on native kidney biopsies, 9 reported on transplant biopsies, 3 reported on a mixture of native and transplant kidney biopsies and 17 reported on post-mortem kidney biopsies (Figure 2). In total, there were 84 native biopsies, 15 transplant biopsies, and 189 post-mortem biopsies. Our review describes the presentation, management, and outcomes of the various pathologies. The various pathologies reported are listed in Supplementary Table 1.
Figure 2.
Number of studies describing the different types of kidney biopsy in patients with coronavirus disease 2019.
Native kidney biopsies
A list of all histopathological features reported in native kidney biopsy is illustrated in Figure 3.
Figure 3.

Native kidney biopsy histopathological features reported in association with coronavirus disease 2019.
Collapsing focal segmental glomerular sclerosis (CG)
CG was reported in 19 out of 30 native kidney biopsy studies which encompassed a total of 40 patients[13-30]. The median age of this cohort was 55 years with a predominance of males (80%) and black ethnicity (92.5%). A history of hypertension was reported in 30 patients and 11 patients had diabetes mellitus. APOL1 genetic mutation was reported in 10 patients. Non-resolving AKI and nephrotic range proteinuria (NRP) were the most common indications for kidney biopsy. The median time between COVID-19 positivity [as measured by polymerase chain reaction (PCR)] and kidney biopsy was 10 d. The most frequent treatment approach included steroids and hydroxychloroquine. There were 27 patients who needed RRT, of which 8 became dialysis independent on discharge, and 2 died. Table 1 illustrates the studies that demonstrated CG in kidney biopsy and their characteristics.
Table 1.
Native kidney biopsy outcomes of collapsing glomerulopathy in coronavirus disease 2019 cases
|
Ref.
|
Age
|
Sex
|
Ethnicity
|
Comorbidities
|
Renal Px
|
Baseline creatinine (mg/dL)
|
Presentation creatinine (mg/dL)
|
Presentation proteinuria (g/day)
|
Presentation albumin (g/L)
|
Treatment received
|
Outcome (renal and survival)
|
RRT needed
|
Time to biopsy
|
Haematuria
|
|
| Kudose et al[13] | 46 | M | B | Obesity | AKI, NS | 1.1 | 12.5 | 5.8 | 3.1 | Tocilizumab/Steroids | DD | Yes | - | < 10 | |
| Kudose et al[13] | 62 | M | B | HTN, prostate cancer, CKD | AKI, NS | 2 | 10.7 | 12.1 | 3.1 | None | DI | No | - | < 10 | |
| Kudose et al[13] | 62 | M | B | HTN, DM, prostate Cancer | AKI, NRP | 1 | 11.6 | 19 | 2.4 | HCQ, Steroids | DI | No | - | - | |
| Kudose et al[13] | 57 | M | B | HTN, hepatitis C, CKD | AKI, NRP | 1.1 | 4.9 | 6.2 | 2.5 | None | DI | No | - | < 10 | |
| Kudose et al[13] | 61 | M | B | HTN, obesity | AKI, NRP | Normal | 15 | 9 | 2.5 | - | DD | Yes | - | - | |
| Sharma et al[14] | 77 | F | B | HTN | AKI | 1 | 8.15 | 1.5 | - | HCQ, Steroids | DI | Yes | - | No | |
| Wu et al[16] | 63 | M | B | HTN, DM | - | 1.3 | 4.9 | 12.7 | - | - | DD | Yes | - | < 10 | |
| Wu et al[16] | 64 | F | B | HTN, DM | - | 1.5 | 4.2 | 4.6 | - | - | DI | No | - | Negative | |
| Wu et al[16] | 65 | F | B | HTN, DM | - | 1.3 | 2.9 | 13.6 | - | - | Died | Yes | - | Negative | |
| Wu et al[16] | 44 | M | B | - | - | 1.4 | 11.4 | 25 | - | - | DD | Yes | - | 50-100 | |
| Wu et al[16] | 37 | M | B | - | - | 1 | 9 | - | - | - | Died | Yes | - | < 10 | |
| Wu et al[16] | 56 | M | B | HTN | - | 1.2 | 6.7 | 3.6 | - | - | DI | Yes | - | > 100 | |
| Akilesh et al[17] | 46 | M | B | HTN | AKI, NS | - | 8.7 | 13.7 | - | - | DD | Yes | 2 wk | No | |
| Akilesh et al[17] | 60 | F | B | HTN | AKI, NRP | - | 5.7 | 21 | - | - | - | - | 4 wk | No | |
| Akilesh et al[17] | 58 | F | B | HTN | AKI, NS | - | 10.2 | 20 | - | - | DD | Yes | - | - | |
| Akilesh et al[17] | 44 | M | H | - | AKI, NRP | - | 12 | 11.4 | - | - | DD | Yes | 6 wk | No | |
| Akilesh et al[17] | 58 | M | B | - | AKI, NRP | - | 11.3 | 4 | - | - | DI | Yes | Day 4 | Yes | |
| Akilesh et al[17] | 47 | M | B | HTN | AKI, TMA | - | 6.6 | 7.6 | - | - | DD | Yes | Day 25 | Yes | |
| Akilesh et al[17] | 63 | F | B | HTN | AKI, NRP, TMA | - | 6 | 20 | - | - | DD | Yes | Day 10-14 | Yes | |
| Gupta et al[18] | 71 | M | I | HTN, DM | AKI, NS | 1.19 | 4.49 | 18.46 | 2 | Steroid (Prednisolone 60mg OD) | DD | Yes | 1st-D6, 2nd 2 mo | No | |
| Gupta et al[18] | 54 | M | B | HTN, DM | AKI, NS | 1.08 | 4.67 | 16 | 1.6 | None | - | No | Day 30 | No | |
| Noble et al[43] | 54 | M | B | HTN, obesity | AKI, NRP | 125 | 6.54 | 4.08 | - | None | DI | Yes | Day 16 | Yes | |
| Kissling et al[19] | 63 | M | B | HTN | AKI, NRP | - | 1.2 | 5 | - | None | DI | No | Day 8 | - | |
| Magoon et al[21] | 28 | M | B | - | AKI | - | 0.99 | 2 | - | None | DI | Yes | Day 7-34 | Yes | |
| Magoon et al[21] | 56 | M | B | HTN, CKD | AKI, NRP | - | 3.17 | 21 | - | None | DI | Yes | - | Yes | |
| Gaillard et al[20] | 79 | M | B | HTN, MGUS, CKD | AKI, NRP | - | 2.55 | 11.4 | 2.9 | Dexamethasone, lopinavir/ritonavir, PLEX | DD | Yes | Day 5 | No | |
| Sharma et al[15] | 67 | M | B | HTN, DM | AKI, NRP | 1 | 2.2 | 3.2 | - | HCQ/steroids | DD | Yes | Day 8-8/52 | < 10 | |
| Sharma et al[15] | 49 | M | B | HTN | AKI | 0.95 | 4.85 | 2.59 | - | HCQ/steroids | DD | Yes | > Day 4 | < 10 | |
| Nlandu et al[22] | 48 | M | B | HTN, DM | AKI, NS | 0.72 | 15.9 | 18 | - | Chloroquine, azithromycin, vitamin C | DI | Yes | Day 30 | No | |
| Deshmukh et al[23] | 42 | M | I | - | NS | - | 1 | 8 | 'hypoalbuminaemia' noted | Ramipril | - | No | Day 24 | Yes | |
| Kadosh et al[24] | 56 | M | B | CKD | AKI, NRP | - | 1.86 (peak 7.78) | 1.97 (peak 7.35) | - | MMF and steroids stopped, azithromycin, nitozaxonide | DI | No | > Day 7 | - | |
| Coutourier et al[25] | 53 | M | B | HTN | AKI, NRP | 1.02 | 1.89 (peak 2.20) | 5.64 (peak 18.7) | 1.3 (day 3) | Oseltamivir, HCQ, chloroquine, azithromycin | DI | No | Day 3-11 | No | |
| Couturier et al[25] | 53 | M | B | HTN, Hepatitis B | AKI, NRP | 1.35 | 5.34 (peak 6.01) | 1.5 (peak 2.65) | - | - | - | No | > Day 7 | No | |
| Larsen et al[26] | 44 | M | B | HTN, DM, CKD | AKI, NRP | 1.4 | 4 | 3.9 (peak 25) | 2.5 | None | DD | Yes | Day 8 | Yes | |
| Malhotra et al[27] | 64 | M | B | HTN, DM, CKD, HIV on HAART | AKI, NRP | - | 2.3 | 2.74 | - | Solumedrol, zinc, Vitamin C, Oxitris Filter | DD | Yes | Day 11 | Yes | |
| Izzedine et al[28] | 49 | F | B | CKD, heart transplant, type 2 diabetes, HTN, obesity | AKI, NS | 1.78 | 2.39 | 6.6 | 1.7 | - | DI | Yes | Day 8 | < 10 | |
| Izzedine et al[28] | 38 | F | B | CKD, SLE, HTN, obesity | AKI, NS | 14.64 | 11.7 | - | 1.9 | - | DI | No | - | < 10 | |
| Laboux et al[29] | 47 | M | B | HTN | AKI | 0.8 | 30.3 | 1.2 | 2.5 | Dialysis | DI | Yes | Day 30 | - | |
| Malik et al[30] | 57 | M | B | - | AKI, NS | - | 2.0 then 3.4 | 14.9 | 3.4 | Antibiotics, oseltamivir, oxygen | DD | Yes | - | - | |
| FSGS with podocytopathy | |||||||||||||||
| Akilesh et al[17] | 59 | M | B | HTN, DM | AKI, NRP | - | 11.9 | > 12 | - | Unknown | - | Unknown | Day 11 | - | |
AKI: Acute kidney injury; B: Black; CKD: Chronic kidney disease; DM: Diabetes mellitus; DD: Dialysis dependent at hospital discharge; DI: Dialysis independent at hospital discharge; F: Female; H: Hispanic; HAART: Highly active antiretroviral therapy; HCQ: Hydroxychloroquine; HIV: Human immunodeficiency virus; HTN: Hypertension; I: Indian; M: Male; MGUS: Monoclonal gammopathy of undetermined significance; MMF: Mycophenolate mofetil; NRP: Nephrotic range proteinuria; NS: Nephrotic syndrome; PLEX: Plasma exchange; SLE: Systemic lupus erythematosus; TMA: Thrombotic microangiopathic anemia; FSGS: Focal Segmental Glomerulosclerosis.
ATI
ATI was the second most frequent pathological process described in the kidney biopsies of patients with COVID-19 infection (observed in 14 patients over 6 studies)[13,14,17,31-33]. Of these 14 patients, 10 had a history of hypertension and five were diabetic. Nine (64%) were male with a median age of 60.5 years. AKI was the main presenting feature in all of these cases with three patients also reporting NRP. Eleven patients needed dialysis of which four remained dialysis dependent on discharge, and two patients died in hospital. Lenti et al[31] reported the presence of viral particles in endothelial and tubuloepithelial cells from the kidney biopsy of one patient[31]; this patient did not require dialysis and was discharged from hospital after 15 d. Table 2 illustrates the studies which describe ATI on kidney biopsy in association with COVID-19.
Table 2.
Native kidney biopsy outcomes of acute tubular injury and necrosis in coronavirus disease 2019 cases
|
Ref.
|
Age
|
Sex
|
Ethnicity
|
Comorbidities
|
Renal presentation
|
Baseline creatinine (mg/dL)
|
Presentation Creatinine (mg/dL)
|
Presentation proteinuria (g/day)
|
Presentation albumin (g/L)
|
Treatment received
|
Outcome (renal and survival)
|
RRT needed
|
Time to biopsy
|
Haematuria
|
| Sharma et al[14] | 62 | M | Hispanic | T2DM | AKI and proteinuria | - | 1.2 | 3 | - | Steroid, HCQ, anakinra, plasma | Died | Yes | - | Yes |
| Sharma et al[14] | 69 | M | Hispanic | HTN | AKI, proteinuria, anti-cardiolipin positive | - | 0.9 | 2.4 | - | Steroid, HCQ, anakinra, plasma | Died | Yes | - | Yes |
| Sharma et al[14] | 76 | F | Caucasian | T2DM, HTN | Severe AKI and Proteinuria | - | 1 (peak 4.4) | 0.9 | - | None | DI | No | - | No |
| Sharma et al[14] | 59 | M | Black | HTN, CCF | AKI, Proteinuria and raised K:L ratio | - | 4.5 (peak 6) | 2.8 | - | None | DI | No | - | Yes |
| Sharma et al[14] | 69 | F | Black | HTN, Hyperlipidaemia | AKI NRP | - | 1.9 | 7.6 | - | Steroids | DD | Yes | - | No |
| Kudose et al[13] | 43 | F | Black | T2DM, HLD, streptococcal infection, obesity (BMI 52.5) | AKI | - | 3.5 (peak 6.7) | 1 | - | None | DD | Yes | - | Yes |
| Kudose et al[13] | 67 | M | Caucasian | HTN, Gout, Obese | AKI on CKD | - | 5.7 | 0.3 | - | Tocilizumab, HCQ, azithromycin | DD | Yes | - | Yes |
| Kudose et al[13] | 51 | M | Black | HTN, AF, HLD, CVA, BPH | AKI on CKD | 1.8 | 4.8 | 0.5 | - | HCQ | DI | No | - | Yes |
| Akilesh et al[17] | 34 | F | Caucasian | HTN, T2DM | AKI NS | - | 1.2 | 7 | - | - | DI | No | Day 4 | No |
| Akilesh et al[17] | 67 | F | Hispanic | HTN | AKI | - | 1.4 | 1 | - | - | DI | No | Day 5 | Yes |
| Lenti et al[31] | 25 | M | Caucasian | - | AKI NS | - | 3.8 | 0.48 | - | - | - | - | - | - |
| Rossi et al[32] | 49 | M | Caucasian | Obesity | AKI | - | - | - | - | HCQ, Lopinavir/Ritonavir | DI | Required when in hospital | - | - |
| Papadimitriou et al[33] | 52 | M | - | HIV, HTN, coronary artery disease, Factor V deficiency | AKI | Normal | 7.5 | 1.85 | - | - | DD | Yes | Day 10 | - |
| Papadimitriou et al[33] | 64 | M | - | AF, hyperlipidaemia, gout | AKI | 1 | 1.4 | - | - | I&V, IV heparin then apixaban (AF), 4 units blood following haematemesis, meropenem (E. coli in sputum), RRT day 22 to 33, MRSA > linezolid | DI | Yes | Day 84 | - |
AKI: Acute kidney injury; AF: Atrial fibrillation; BPH: Benign prostatic hypertrophy; CCF: Congestive cardiac failure; CKD: Chronic kidney disease; CVA: Cerebrovascular accident; DD: Dialysis dependent at hospital discharge; DI: Dialysis independent at hospital discharge; DSA: Donor specific antibodies; HCQ: Hydroxychloroquine; HIV: Human immunodeficiency virus; HLD: Hyperlipidaemia; HTN: Hypertension; I&V: Intubated and ventilated; K:L ratio: Kappa:lambda light chain ratio; RRT: Renal replacement therapy; T2DM: Type 2 diabetes mellitus.
Thrombotic microangiopathy
Thrombotic microangiopathy (TMA) was observed in eight patients in three studies. Sharma et al[14] reported two cases presenting with TMA and severe AKI requiring dialysis in association with COVID-19 infection[14]. The first patient had a background of gemcitabine treatment for cervical malignancy. She had no COVID-19 respiratory symptoms and was noted to be Coombs immunoglobulin (Ig) G positive. She was managed with steroids and rituximab for suspected Autoimmune Hemolytic Anemia and gemcitabine induced TMA. The second patient had severe COVID-19 respiratory manifestations requiring mechanical ventilation. There were signs of alternative pathway activation (low factor H, raised serum CBb and C5b-9). She was given treatment with tocilizumab, steroids, anakinra, convalescent plasma and eculizumab. Unfortunately, both patients died.
Akilesh et al[17] described five patients with histological findings of TMA on light microscopy[17]. All five patients had hypertension and AKI with biochemical features of TMA, prompting a kidney biopsy. Three of these patients also had histopathological features consistent with concurrent collapsing Focal Segmental Glomerulosclerosis (FSGS). Two patients were noted to have had gemcitabine treatment for underlying malignancy. All five required dialysis and only one patient recovered renal function without needing further dialysis. Management was supportive for all except one patient who received plasma exchange and eculizumab, though she remains dialysis dependent (Supplementary Table 2).
Antinuclear cytoplasmic antibody associated vasculitis
In a case series of 10 COVID-19 positive patients who underwent kidney biopsy for AKI, Sharma et al[14] reported one patient (64-year-old Black male) with a positive myeloperoxidase (MPO) antibody in which his kidney biopsy demonstrated crescentic glomerulonephritis (GN), supporting a diagnosis of antinuclear cytoplasmic antibody (ANCA) associated vasculitis[14]. Electron microscopy features and immunostaining were negative for viral RNA particles. The same patient was reported as one of two cases by Uppal et al[34] in which COVID-19 was managed with oxygen support, tocilizumab, and convalescent plasma[34]. When his COVID-19 re-test became negative, the patient was initiated on methylprednisolone and rituximab; his renal function recovered back to baseline and further dialysis was not required.
A second case reported by Uppal et al[34] presenting with AKI, hematuria and proteinuria with concomitant COVID-19 infection, had proteinase 3 (PR3) ANCA positivity[34]. Kidney biopsy features were consistent with crescentic or focal segmental necrotizing GN. A skin biopsy of this patient, who had a new-onset skin rash, revealed leukocytoclastic vasculitis. The patient received hydroxychloroquine treatment alongside methylprednisolone and rituximab, achieving good outcomes: Reduction in PR3 from 57.3 units/mL to 28.8 units/mL and improvement in serum creatinine from 4.0 mg/dL to 2.0 mg/dL. Moeinzadeh et al[35] described another case of a 25-year-old male diagnosed with PR3 ANCA vasculitis who presented with AKI and pulmonary hemorrhage[35]. He was managed with methylprednisolone, plasma exchange, cyclophosphamide and hydroxychloroquine. The patient’s renal function stabilized on these treatments, and he avoided the need for acute dialysis.
Jalalzadeh et al[36] described a 46 year old female with a background of scleroderma and type 2 diabetes, who presented with respiratory and abdominal symptoms[36]. She had been diagnosed with COVID-19 six months previously. She had a significant AKI with proteinuria and was found to have a raised MPO titer at 161.8 units. She was managed with captopril (concern for potential scleroderma renal crisis), and methylprednisolone for 3 d. She did not require RRT and was discharged home. Kidney biopsy revealed a crescentic GN with 45 out of 48 glomeruli globally sclerosed.
Anti-glomerular basement membrane (anti-GBM) disease
Kudose et al[13] reported a case of anti-GBM disease in a COVID-19 positive patient who presented with pulmonary infiltrates on chest X-ray and severe AKI[13]. Kidney biopsy revealed crescentic GN alongside ATI with microcyst formation and interstitial infiltrates. The patient was managed with steroids, cyclophosphamide, and plasma exchange without an improvement in renal function; he was initiated on dialysis therapy.
Prendecki et al[37] described eight patients who presented with positive anti-GBM serology[37]. Though none of these patients had positive results for COVID-19 PCR, four patients tested positive for SARS-CoV-2 IgM. All eight patients reported non-specific prodromal symptoms although only five reported respiratory symptoms and/or diarrhea. None of the patients had pulmonary manifestations. Of the four patients with positive findings for SARS-CoV-2 IgM, crescentic linear IgG was reported in the kidney biopsy for two patients. With a confirmed histological diagnosis of anti-GBM disease, these two patients were treated with steroids, cyclophosphamide, rituximab and plasma exchange. One patient achieved complete recovery of renal function.
IgA vasculitis
Suso et al[38] described a case of a 78-year-old man who had COVID-19 associated respiratory failure along with extremely high IL-6 levels (177 pg/mL)[38]. He received treatment with hydroxychloroquine, lopinavir/ritonavir, dexamethasone, ceftriaxone, azithromycin, and tocilizumab. The patient presented three weeks later with a triad of arthralgia, cutaneous vasculitis and haematoproteinuria. Kidney biopsy was performed and showed crescentic manifestations in two of the seven glomeruli and mesangial IgA deposits, raising the possibility of IgA vasculitis as a result of COVID-19. The patient was treated with methylprednisolone followed by rituximab. He improved clinically with a reduction in proteinuria, and on discharge his creatinine improved to 1.4 mg/dL (baseline 0.78 mg/dL).
Huang et al[39] described a 65-year-old Chinese female who presented with headache, myalgia, fatigue, dark colored urine and flank pain[39]. She had haematoproteinuria. Kidney biopsy showed 16 glomeruli, 5 of which were globally sclerosed, with 2+ IgA staining on immunofluorescence. Electron microscopy showed mesangial electron dense deposits. She was treated with methylprednisolone for 3 d and oseltamivir for 5 d. Dialysis was not required, and she was discharged home.
Lupus nephritis
Kudose et al[13] described a case of a 27-year-old African female with a previous diagnosis of class II lupus nephritis who presented with COVID-19 associated respiratory failure[13]. On presentation, she displayed clinical features of nephrotic syndrome and AKI. Kidney biopsy revealed histopathological features of Class IV/Class V lupus nephritis. The patient was managed with steroids following kidney biopsy. She deteriorated clinically and died from multi-organ failure on her 6th day of hospital admission.
Minimal change disease
Kudose et al[13] described a single case of minimal change disease (MCD) in a young African-Caribbean patient with a homozygous G1 APOL1 variant presenting to hospital with nephrotic syndrome[13]. This patient went into partial remission following treatment with steroids in addition to azithromycin and hydroxychloroquine for COVID-19. Akilesh et al[17] presented another case of MCD in an elderly Caucasian female who presented with nephrotic syndrome[17]. The patient had a kidney biopsy six weeks after a positive COVID-19 PCR test. A full remission was achieved within four weeks after receiving high-dose steroid management.
Membranous nephropathy
Two reported cases of membranous nephropathy (MN) in association with COVID-19 infection were identified[13]. In these cases, both patients had NRP. The first patient had immunohistochemistry positive for phospholipase A2 receptor (PLA2R) on kidney biopsy staining and was treated with tacrolimus. He remained COVID-19 positive, although a reduction in proteinuria was noted. The second patient had previous cervical neoplasm and did not have PLA2R antibodies but had positive serum anti-dsDNA and antinuclear antibody were identified. The patient was not initiated on any active treatment and is currently under nephrology follow-up without the need for dialysis.
Oxalate nephropathy
Three cases of oxalate nephropathy in patients with positive COVID-19 status were identified in our review[27,40]. All three patients presented with AKI and received vitamin C in high doses as management for sepsis-related acute respiratory distress syndrome. Kidney biopsy showed calcium oxalate monohydrate crystals on hematoxylin and eosin staining, which were birefringent under polarized light. The scanning electron microscope and X-ray spectrometry analyses confirmed the presence of calcium oxalate monohydrate crystals. None of the patients required dialysis treatment and all three were discharged after clinical recovery.
Post infectious GN
Akilesh et al[17] reported a case of post infectious GN in a 69-year-old Caucasian female presenting with AKI and NRP[17]. She had a background history of diabetes mellitus and recurrent E. coli urinary tract infection. Kidney biopsy revealed the presence of subepithelial deposits, granular C3 staining, advanced changes related to diabetic nephropathy and severe ATI. The patient improved clinically but remained dialysis dependent.
Pigment nephropathy
Pigment nephropathy was reported in two case reports[13,14]. In both cases, the patients presented with ATI, with raised creatinine kinase levels and myoglobinuria secondary to rhabdomyolysis. The kidney histopathology showed pigment cast and was positive for myoglobin immunohistochemistry. Both patients required dialysis; one patient achieved complete recovery whilst the other deteriorated and died during hospitalization.
Atypical hemolytic uremic syndrome
There was one case report of atypical hemolytic uremic syndrome (aHUS) that reactivated post COVID-19 infection[41]. A 28-year-old Caucasian female who had previously been diagnosed with aHUS aged 3 presented with fever, dysphagia and headache. She had an AKI along with proteinuria. She was managed with eculizumab, did not require RRT and was discharged with a creatinine of 2 mg/dL.
Granulomatous interstitial nephritis
A 62-year-old Caucasian male presented with cough, fever and myalgia[42]. He had an AKI with non-NRP. He required critical care admission and was treated with hydroxychloroquine and continuous veno-venous hemofiltration for 38 d. Kidney biopsy was performed 32 d post admission and showed 34 mostly normal glomeruli with multiple non-caseating granulomas consistent with granulomatous interstitial nephritis. He survived to discharge and remained dialysis independent.
Transplant biopsies
We have also identified case reports highlighting histopathological changes amongst transplant recipients in the setting of COVID-19 infection[13,17,43-51] (Figure 4 and Table 3). Two cases of CG have been reported in transplant biopsies in addition to severe ATI changes, both in patients of African-Caribbean origin and presenting with AKI and NRP[43,47]. The antiproliferative agent (mycophenolate mofetil) was withheld in both cases. Whilst one patient recovered renal function, the other remained dialysis dependent. In one case the donor was found to have low risk APOL1 variant with G2 heterozygosity[47], a risk factor for CG, whilst the other case did not have genetic testing but on in-situ hybridization, viral RNA was detected in the tubulo-epithelial cell[43].
Figure 4.

Transplant kidney biopsy histopathological features reported in association with coronavirus disease 2019.
Table 3.
Transplant kidney biopsy findings in coronavirus disease 2019 cases
|
Ref.
|
Age
|
Sex
|
Ethnicity
|
Comorbidities
|
Renal Presentation
|
Baseline creatinine (mg/dL)
|
Presentation creatinine (mg/dL)
|
Presentation proteinuria (g/day)
|
Presentation albumin (g/L)
|
Treatment received
|
Outcome (renal and survival)
|
RRT needed
|
Time to biopsy
|
Haematuria
|
|
| T-cell mediated rejection | |||||||||||||||
| Kudose et al[13] | 54 | F | Caucasian | IgA Nephropathy, Donor Specific Ab +ve, HTN, obesity | AKI | 1.7 | 2.6 | 0.2 | - | Steroids, Tocilizumab, thymoglobulin, IVIG | DI | No | - | Yes | |
| ABMR | |||||||||||||||
| Akilesh et al[17] | 47 | F | Black | HIV-associated Nephropathy, Deceased Donor Tx 2015, Vascular Rejection Post-Tx, HTN | AKI | - | 1.63 | 2 | - | Renal transplantation, 5-MTP, PLEX IVIG | - | - | 6 wk | No | |
| Akilesh et al[17] | 54 | M | Asian | Chronic Transplant Glomerulopathy, C4d –ve, HTN, T2DM | AKI with Proteinuria | 1.9 | 5.2 | 3 | - | Regular MMF withheld, regular tacrolimus dose reduced, steroids | DI | No | 6 wk | No | |
| Abuzeineh et al[44] | 54 | M | Black | Diabetic nephropathy, Tx, HTN | AKI | 1.4 | 2.6 | - | - | IVF, MMF discontinued, NHF oxygen, antibiotics, antifungals, tocilizumab | DI | No | 73 d | - | |
| Acute tubular injury | |||||||||||||||
| Akilesh et al[17] | 42 | M | Hispanic | Live Donor Tx 2019, HTN | AKI | 1.27 | 1.53 | 0.15 | - | - | DI | No | 7 wk | No | |
| Kudose et al[13] | 54 | F | Hispanic | ADPKD, Deceased Donor Tx 2020, HTN | AKI | 2.5 | 2.9 | 0.2 | - | None | DI | No | - | - | |
| Westhoff et al[50] | 69 | M | - | Diabetic nephropathy | AKI | 1.1 | 2.2 | - | - | IV hydrocortisone, tacrolimus and MMF held, HCQ, levetiracetam | DI | No | 14 d | - | |
| FSGS | |||||||||||||||
| Doevelaar et al[45] | 35 | M | Black | Deceased donor Tx 2019 | AKI, Normothermic Regional Perfusion | - | 1.7 | 3.29 | - | Steroids (Hydrocortisone 200 mg/d) | DI | No | 34 d | - | |
| Oniszczuk et al[48] | 49 | M | Black | Renovascular disease, deceased donor Tx 2020 | AKI, Nephrotic Syndrome | 1.47 | 2.17 | 3.27 | 2.7 | Steroids, ACE Inhibitor | DI | No | 2 wk | - | |
| Yamada et al[51] | 49 | F | Black | Pre-eclampsia, Live donor Tx 1995 (from sibling) | AKI, Normothermic Regional Perfusion | 1.6 | 3.4 | 6.3 | 3.8 at diagnosis with COVID-19 | ACE Inhibitor, Steroids (Prednisolone 60 mg with quick wean due to side effects) | DI | No | 5 d | - | |
| Collapsing FSGS | |||||||||||||||
| Noble et al[43] | 45 | M | Black | Malignant HTN, Obesity (BMI 42.6), Live Donor Tx 2016 | AKI, Nephrotic syndrome | 3.22 | 4.69 | 1.09 | - | MMR withheld on admission, restarted after 14 d. Steroid (Prednisolone dose doubled from 10 mg OD to 20mg OD) | DD | Yes | 12 d | Yes | |
| Lazareth et al[47] | 29 | M | Black | Urinary Schistosomiasis, Deceased Donor Tx 2015, Previous ABMR in Jan 2020 | AKI, Nephrotic Syndrome | 3.18 | 6.06 | 0.49 | 2.8 | MMF withheld temporarily | DI | No | 2 d | - | |
| Transplant infarction | |||||||||||||||
| Kudose et al[13] | 22 | M | Black | Membranous Nephropathy PLA2R +ve, Deceased Donor Tx 2018, HTN | AKI | - | 9.4 | - | - | Tocilizumab, HCQ, Azithromycin | DD | Yes | - | - | |
| Webb et al[49] | 49 | M | - | Chronic glomerulonephritis, HTN, DCD renal transplant 2001 with subsequent ABMR, CMV | AKI | 0 | 2.03 | - | - | Nasal high flow oxygen, prednisolone, enoxaparin, ertapenem | DD | Yes | 27 d | - | |
| TMA | |||||||||||||||
| Jespersen et al[46] | 49 | F | - | FSGS | AKI | - | 2.81 | - | - | Supportive | DI | No | > 22 d | - | |
5-MTP: 5-methoxytryptophan; Ab: Antibody; ABMR: Antibody mediated rejection; ADPKD: Autosomal dominant polycystic kidney disease; AKI: Acute kidney injury; CMV: Cytomegalovirus; DCD: Donor after circulatory death; DD: Dialysis dependent at hospital discharge; DI: Dialysis independent at hospital discharge; FSGS: Focal and segmental glomerulosclerosis; HCQ: Hydroxychloroquine; HIV: Human immunodeficiency virus; HTN: Hypertension; IVF: Intravenous fluids; IVIG: Intravenous immunoglobulin; MMF: Mycophenolate mofetil; NHF: Nasal high flow; PLA2R: Phospholipase 2 receptor antibody; PLEX: Plasma exchange; T2DM: Type 2 diabetes mellitus; Tx: Transplant.
Recurrence of FSGS was reported in two cases in association with COVID-19 infection. The first case report describes a patient who had a second recurrence of FSGS (16 wk post-transplant) in the setting of COVID-19 infection and resolved spontaneously with viral clearance[45]. The second case presented with AKI and nephrotic syndrome five weeks post-transplant in a patient with high risk homozygous G2 APOL1 variant[48]. This patient was treated with steroids and renin-angiotensin-aldosterone system (RAAS) inhibition with improvement of renal parameters.
Yamada et al[51] describe a case in which the renal presentation was with AKI and NRP. The biopsy showed minimal change disease[51]. The patient was treated with high dose steroids and partial remission was achieved. Both the recipient and donor were homozygous for high risk G1 APOL1 variant and biopsy was taken five days after admission for COVID-19 infection.
There were 2 cases of isolated ATI in patients presenting with AKI[13,17]. These patients did not receive any specific treatment and were discharged with good renal outcomes, with the creatinine of one returning to baseline (around 1.3 mg/dL) shortly after biopsy[17].
Kudose et al[13] describe one case of a patient with grade 2A T-cell mediated rejection (TCMR) one-month post-transplant[13]. The patient had positive donor specific antibodies (DSA) and was treated with steroids, tocilizumab and thymoglobulin. Her creatinine stabilized and she was discharged. In the same case series, there was a case of transplant cortical infarction with the patient remaining dialysis dependent. A further case of transplant infarction was described by Webb et al[49]. A 49-year-old male presented with respiratory symptoms and AKI. He was managed with oxygen, steroids, low molecular weight heparin and ertapenem. He required dialysis and were subsequently discharged home. Kidney biopsy showed almost complete infarction of the renal cortical parenchyma with no viable glomeruli seen.
A case of de-novo DSA positivity was reported by Akilesh et al[17] in a patient presenting with AKI six weeks post COVID-19 infection[17]. The patient had active antibody mediated rejection (AMR) in the biopsy and was managed with steroids, plasma exchange, intravenous immunoglobulin, and rituximab. Another case of chronic active AMR was described in a patient who developed AKI and proteinuria on a background of known transplant glomerulopathy[17]. The biopsy showed signs of activity with C4 complement component (C4d) positivity and TMA. There were also mesangial changes with IgA deposition, which may indicate concurrent IgA changes. The primary kidney disease was unknown. Abuzeineh et al[44] reported a case of a 54-year-old male with a renal transplant from 2015 who presented with AKI and was managed with fluids, oxygen, antibiotics, antifungals and tocilizumab[44]. Subsequent transplant biopsy revealed AMR which was managed with intravenous immunoglobulin.
Jespersen et al[46] describe one patient who underwent a transplant kidney biopsy which showed TMA[46]. The patient presented with abdominal pain without any respiratory symptoms. Computerized tomography (CT) scan confirmed acute pancreatitis and they developed hemolytic anemia with worsening kidney function. Management was supportive; RRT was not needed, and they were discharged home.
Westhoff et al[50] described a case of kidney allograft infiltration with SARS-CoV-2. The patient had received a pancreas-kidney transplant 13 years prior and presented with SARS-CoV-2-pneumonitis, new insulin requirement, renal transplant AKI and high tacrolimus levels likely secondary to diarrhoea. Immunosuppression was rationalised to steroid monotherapy. Kidney transplant biopsy revealed mild ATI and mononuclear cell inflammation, in addition to SARS-CoV-2 spike protein RNA present in the interstitium and tubular epithelial cells, demonstrated via in-situ hybridisation. However, serum RT-PCR remained negative. This patient later developed neurological complications with cerebrospinal fluid positive for SARS-CoV-2 PCR requiring admission to intensive care. The patient made an excellent recovery and was discharged with complete resolution of renal transplant AKI and partial pancreatic graft recovery. Post-mortem studies have suggested that kidney viral tropism likely occurs due to viraemia and perfusion of the kidney with infected blood. However, kidney infiltration despite negative serum testing suggests a possible alternative pathophysiological mechanism.
Post-mortem biopsies
We identified 17 reports describing post-mortem kidney biopsies[52-68]. The most common findings were ATI, arterionephrosclerosis[64] and FSGS[52,55,67], with other findings including renal vein thrombus[52], pigment cast nephropathy[67], IgA staining[64,67], IgG humps on EM consistent with post-infectious GN[67], chronic interstitial nephritis[52,54,60], nodular diabetic glomerulosclerosis[57] and oxalosis[56,59] (Figure 5). The findings are summarized in Table 4.
Figure 5.
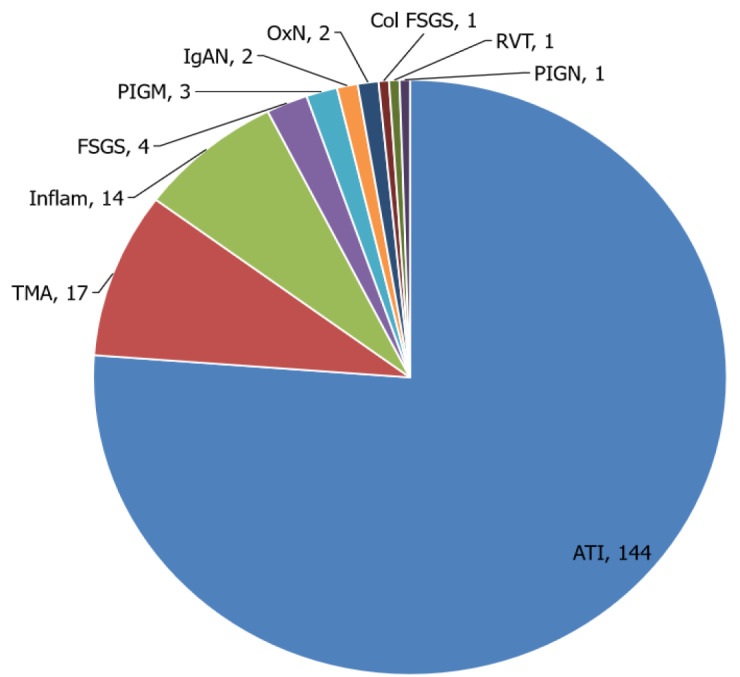
Postmortem kidney biopsy histopathological features reported in association with coronavirus disease 2019.
Table 4.
Post-mortem kidney biopsy findings in coronavirus disease 2019 cases
|
Ref.
|
Number in series
|
Number with kidney histology
|
Age-median (range)
|
Gender (male), n (%)
|
Comorbidities, n (%)
|
AKI, n (%)
|
RRT, n (%)
|
Covid treatment, n (%)
|
Pathological findings, n (%)
|
| Bradley et al[52] | 14 | 14 | 73.5 (42-84) | 6 | Diabetes 5, HTN 9, CKD 5 | 6 (1 at presentation) | - | ATI 11, FSGS 1, chronic inflammation 1, renal vein thrombus 1 | |
| Su et al[67] | 26 | 26 | 69 (39-87) | 19 | Diabetes 3, HTN 11, CKD 2 | - | 5 (6 no record) | Arbidol 10, Ribavirin 2, Lopinavir/ritonavir 5, steroids 15 | ATI 26, TMA 1, FSGS 2, pigment nephropathy 3, IgA 1, pyelonephritis 2, PIGN 1 |
| Santoriello et al[64] | 42 | 31 (11 excluded due to autolysis) | 71.5 (38-97) | 29 | Diabetes 17, HTN 30, CKD 8 | 31 (94)-stage (38.1) | 8 (36) | Plaquenil 36, steroids 22 (61% combination), tocilizumab 6 (17%) | ATI 31, TMA 6, collapsing FSGS 1, chronic inflammation 27, IgA 1 |
| Farkash et al[55] | 1 | 1 | 53 | - | - | 1 | 1 | HCQ, IL-6 blinded trial | ATI 1 |
| Werion et al[68] | 49 | 6 | 64 (54-74) | 34 | Diabetes 10, HTN 23, CKD 7 | 11 (22) | 2 (4) | HCQ 48 (98%), azi 7 (14%), steroids 7 14%), IL-7: 8 (16.5%), tocilizumab 1 (2%) | ATI 5, FSGS 1, chronic inflammation 2 |
| Lax et al[60] | 11 | 11 | N/A | 8 | Diabetes 5, HTN 8 | 6 (54.5) | - | Azi/HCQ 2 | ATI 11, chronic inflammation 2 |
| Golmai et al[56] | 12 | 12 | 75 (49-92) | 10 | Diabetes 4, HTN 9, CKD 1 | 9 | 8 | Tocilizumab/HCQ/steroids 7, HCQ/steroids 4 | ATI 9, oxalosis 1 |
| Falasca et al[54] | 18 | 9 | 76.5 (27-92) | 12 | Diabetes 4, HTN 4 , CKD 2 | - | - | Chronic inflammation 12 | |
| Schurink et al[65] | 21 | 21 | 68 (41-78) | 16 | Diabetes 1 | 15 (71); 10 stage 3 | 5 | Chloroquine 10 (48%), antiviral 4 (19%), steroids 5 (24%) | ATI 12, TMA 1 |
| Hanley et al[58] | 10 | 9 | 73 (IQR 52-79) | 7 | HTN 4 | - | - | - | ATI 9, TMA 5 |
| Rapkiewicz et al[62] | 7 | 7 | 60 (44-65) | 3 | Diabetes 5, HTN 6, CKD 1 | - | - | Azi/HCQ 2, Azi/HCQ/Tocilizumab 2, Azi/HCQ/Anakinra | ATI 7, TMA 1 |
| González Pessolani et al[57] | 4 | 2 | 78 | 2 | Diabetes 1, HTN 1 | 2 | - | ATI 2, TMA 1 | |
| Jacobs et al[59] | 1 | 1 | 78 | 1 | HTN 1 | 1 | 1 | Azi/HCQ/steroids | ATI 1, Oxalosis 1 |
| Sekulic et al[66] | 2 | 2 | (54-81) | 2 | Diabetes 2, HTN 2, CKD 1 | 2 | - | Remdesivir 1 | ATI 2 |
| Remmelink et al[63] | 17 | 17 | 72 (62-77) | 12 | Diabetes 9, HTN 10, CKD 3 | 15 | - | HCQ 15, Steroids 2, Lopinavir/ritonavir 2, Remdesivir 2, Oseltamivir 1 | - |
| Brook et al[53] | 5 | 3 | 75 (58-82) | 1 | Diabetes 2, HTN 3, CKD 1 | - | - | - | ATI 3 |
| Menter et al[61] | 21 | 17 | 76 (53-96) | 17 | Diabetes 7, HTN 21, CKD 4 | - | - | - | ATI 14, TMA 2 |
AKI: Acute kidney injury; ATI: Acute tubular injury; Azi: Azithromycin; CKD: Chronic kidney disease; FSGS: Focal and segmental glomerulosclerosis; HCQ: Hydroxychloroquine; HTN: Hypertension; IgA: Immunoglobulin A; IL-6: Interleukin-6; PIGN: Post infectious glomerulonephritis; RRT: Renal replacement therapy; TMA: Thrombotic microangiopathy.
Farkash et al[55] reported isometric tubular vacuolization on light microscopy, these corresponded to coronavirus like particles in the tubular epithelial cells noted in electron microscopy[55]. Remmelink et al[63] have reported positive viral RNA on PCR from renal samples of 10 of their 14 cases[63]. In the cases series by Su et al[67] three patients showed nucleocapsid SARS-CoV-2 positivity on in situ hybridization in tubuloepithelial cells[67].
Treatments received varied and included steroids, azithromycin, tocilizumab, hydroxychloroquine and anakinra. One of the significant limitations of the postmortem series is autolysis which often occurs resulting in many samples being excluded.
DISCUSSION
A wide range of histopathological findings were reported in the kidney biopsies of patients in association with COVID-19 infection. CG appears as the dominant histopathology amongst glomerular diseases, being observed in 40 out of 84 native kidney biopsies. In non-COVID-19 patients, CG is a distinct and aggressive variant of FSGS more commonly observed in African-Caribbean ethnic groups. It is characterized by glomerular tuft collapse in segmental or global distributions, where there is concurrent hypertrophy and hyperplasia of the overlying podocytes[69-71]. Recent reports highlighted the significance of podocytopathy as the major histopathological manifestation of COVID-19 induced glomerular disease[72]. CG is commonly associated with various infections and inflammatory conditions, such as human immunodeficiency virus (HIV) and systemic lupus erythematous[73]. Current opinion on COVID-19 associated CG suggests its pathogenesis as a multifactorial process. Direct viral podocyte invasion is supported by electron microscopy findings of coronavirus particles within the cytoplasm of podocytes in native and post-mortem biopsies. Suggestions of CG secondary to cytokine release from systemic COVID-19 manifestations have been proposed, particularly in those with APOL1 high risk genotype and African-Caribbean ethnicity[13-22,24-29]. Basic-science studies have shown viral infections stimulating interferon production which in turn encourages APOL1 gene expression[74].
Izzedine et al[28] argue in favor of a direct causal link between SARS-CoV-2 infection and the occurrence of CG and have suggested using the term COVIDAN (in a similar way to HIVAN for HIV associated nephropathy)[28]. Homozygosity for APOL1 risk alleles (G1/G2) confers significantly increase risk for CG[16]. This suggests kidney injury caused by SARS-CoV-2 is likely to manifest in different ways in different individuals depending on genetic risk (for example CG or direct viral mediated ATI).
ATI is another frequently reported pathological finding in the kidney biopsies described in this review. It most frequently causes frank epithelial necrosis with cellular debris in the tubular lumen[75]. Unsurprisingly, the hemodynamic compromise associated with COVID-19 related sepsis syndrome is thought to be the primary contributing factor to the development of ATI. ATI has also been reported to manifest through direct invasion of SARS-CoV-2 particles in renal tubular epithelium and podocytes via the angiotensin-converting enzyme 2 (ACE2) inhibitor pathway, causing AKI[12]. Intrarenal injury through the ACE2 pathway leads to mitochondrial dysfunction and progresses to acute tubular necrosis[12]. It should be recognized that biopsy findings of ATI are common even when there are other intrarenal pathologies, given the effects of SARS-CoV-2 particles on direct tubular injury.
A hypercoagulable state has been observed involving various organ complications in patients with COVID-19, of which there were several presentations of TMA. However, whether TMA is directly caused by COVID-19 in the majority of published cases remains uncertain. Many of the patients described have multiple co-morbidities, increasing their risk of coagulopathy. Increasing evidence supports the role of COVID-19 in contributing to procoagulatory interactions with the endothelial system[76]. TMA occurs where endothelial dysfunction and destruction is caused by pathological stimulation of immune cells, which leads to activation of the micro-thrombotic pathway and complement activity[75].
There have been reports of anti-GBM disease in patients with COVID-19. It has been hypothesized that respiratory insults from COVID-19 may expose the cryptic target of the Goodpasture antigen, leading to widespread pulmonary injury in the alveolar capillary membranes and glomerular basement membrane injury seen in anti-GBM nephritis[13,37]. However, coincidental associations remain a distinct possibility.
As with the other histopathologies described in COVID-19-associated kidney biopsies, vasculitis often presents with AKI. The pathophysiological mechanism of vasculitis induced by the SARS-CoV-2 virus remains elusive due to the scarce number of available kidney biopsies, though AKI within this context is believed to have been caused by glomerular hypoperfusion and tubular necrosis leading to fibrinoid necrosis in the arterial wall of small intrarenal vessels[38,39,77]. Neutrophil extracellular trap (NET) formation is well known to be part of the innate inflammatory process of SARS-CoV-2 infection. The inflammatory state of SARS-CoV-2 may in turn affect immunotolerance and lead to ANCA antibody formation. It is postulated that NET formation could be the source of presentation of MPO or PR3 antigen[34]. There is also the possibility that vasculitis in association with COVID-19 is a co-incidental finding, given the tens of millions of people who have acquired COVID-19.
Toxic nephropathies such as oxalate and pigment nephropathy have surprisingly had multiple descriptions in patients with COVID-19 disease (given the relative rarity of these presentations in non-COVID-19 disease). The mechanisms for how SARS-CoV-2 infection directly causes these conditions are unclear, and the authors of the original reports attribute these cases to their conventional pathophysiology[13,14,27,40].
Other forms of GN associated with COVID-19 are mainly reported as individual cases at present and will require further corroboratory reports to help establish if there is indeed an association with COVID-19 and to explain what the pathophysiological mechanism may be.
The findings from post-mortem biopsies are consistent with live patient biopsy reports in that there is a wide range of histopathological processes observed in patients with COVID-19. Whilst ATI was seen in all post-mortem series, there were also a number of other pathologies observed. This provides additional support to the hypothesis that a kidney biopsy should be considered in more patients with COVID-19 associated AKI.
As this review draws on case reports and case series, we have been limited in only being able to perform a qualitative analysis. In addition, whilst this data provides useful insights, we must remember that the vast majority of patients with AKI do not undergo a kidney biopsy and so there may be inherent selection bias in those cases presented here. Furthermore, there was significant autolysis observed in the postmortem series resulting in a lot of the data being excluded from analysis.
It is also likely that some of the rarer glomerulonephritidies, such as lupus nephritis and ANCA associated vasculitis, are coincidental and simply occur concomitantly with COVID-19 infection rather than as a direct consequence of it.
Nevertheless, we believe this provides an up-to-date, substantial insight into the underlying renal histopathological processes occurring in patients with COVID-19, given the number and range of case reports and series[78,79]. This has clinical relevance as for many of these conditions the AKI may not recover with standard management. As such, clinicians should pay careful attention to features of GN, and ensure that patients are followed up in the outpatient setting. In view of the significant number of histopathological findings reported in association with COVID-19 infection, we would recommend an early kidney biopsy where appropriate (e.g. unresolved AKI, proteinuria, positive immunology tests) at the safest possible time which can guide the management approach.
CONCLUSION
This review summarizes 59 published case reports and series which describe the histopathology of native, transplant and post-mortem kidney biopsies in patients with COVID-19. In addition to expected ATI, there were many other histopathological processes observed in association with COVID-19, with CG being prominent. There was significant variation in ethnicity, presentation creatinine and proteinuria, requirement for RRT and outcomes. This suggests that COVID-19 may cause multiple different effects in the kidney. Whilst the underlying pathological processes of ATI and CG resulting from COVID-19 can be hypothesized based on our current understanding of kidney disease, further work is required to determine what, if any, is the link between COVID-19 and some of the other processes described. It is a distinct possibility that many of the rarer glomerulopathies occurred coincidentally with COVID-19 infection. The need for kidney biopsy should be carefully considered in patients presenting with COVID-19 and kidney disease.
ARTICLE HIGHLIGHTS
Research background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can result in clinically significant multi-system disease, including involvement in the kidney. A wide range of histopathological findings have been reported in kidney biopsies in association with coronavirus disease 2019 (COVID-19) infection.
Research motivation
Renal dysfunction in COVID-19 infection is reported in association with multiple pathologies. However, the mechanism behind these pathologies is not well understood.
Research objectives
This systematic review was conducted to provide an overview of the current literature on the renal histopathological features and mechanistic insights described in association with COVID-19 infection.
Research methods
A systematic review was performed by conducting a literature search in the following websites-‘PubMed’, ‘Web of Science’, ‘Embase’ and ‘Medline-ProQuest’ with the following search terms- “COVID-19 AND kidney biopsy”, “COVID-19 AND renal biopsy”, “SARS-CoV-2 AND kidney biopsy” and “SARS-CoV-2 AND renal biopsy”. Data on presentation, histological features, management and outcome was extracted from the reported studies.
Research results
Our review identified a total of 59 studies reporting COVID-19 related histopathological diagnoses from kidney biopsy. Of these 59 studies, 30 reported on native kidney biopsies, nine reported on transplant biopsies, three reported on a mixture of native and transplant kidney biopsies and 17 reported on postmortem kidney biopsies. In total, there were 84 native biopsies, 15 transplant biopsies, and 189 postmortem biopsies. Many histopathological features were described, including acute tubular injury (ATI), collapsing focal segmental glomerular sclerosis, thrombotic microangiopathy and vasculitis.
Research conclusions
Many other histopathological processes were observed in association with COVID-19 in addition to the expected ATI, highlighting the need for an early kidney biopsy.
Research perspectives
Whilst the underlying pathological processes of a few conditions developing due to COVID-19 infection can be hypothesized based on our current understanding of kidney disease, further work is required to determine what, if any, is the link between COVID-19 and some of the other processes described.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest and no financial ties to declare.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review started: March 31, 2021
First decision: July 29, 2021
Article in press: October 31, 2021
Specialty type: Transplantation
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nooripour R, Patel L, Ulaşoğlu C S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Vishnu Jeyalan, Department of Renal Medicine, Royal Preston Hospital, Preston PR2 9HT, United Kingdom.
Joshua Storrar, Department of Renal Medicine, Salford Care Organisation, Northern Care Alliance NHS Foundation Trust, Salford M6 8HD, United Kingdom; Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PL, United Kingdom.
Henry H L Wu, Department of Renal Medicine, Royal Preston Hospital, Preston PR2 9HT, United Kingdom.
Arvind Ponnusamy, Department of Renal Medicine, Royal Preston Hospital, Preston PR2 9HT, United Kingdom.
Smeeta Sinha, Department of Renal Medicine, Salford Care Organisation, Northern Care Alliance NHS Foundation Trust, Salford M6 8HD, United Kingdom; Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PL, United Kingdom.
Philip A Kalra, Department of Renal Medicine, Salford Care Organisation, Northern Care Alliance NHS Foundation Trust, Salford M6 8HD, United Kingdom; Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PL, United Kingdom.
Rajkumar Chinnadurai, Department of Renal Medicine, Salford Care Organisation, Northern Care Alliance NHS Foundation Trust, Salford M6 8HD, United Kingdom; Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PL, United Kingdom. rajkumar.chinnadurai@srft.nhs.uk.
References
- 1.World Health Organization. Pneumonia of unknown cause. 2020. [cited 27 February 2021]. Available from: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/
- 2.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. [cited 27 February 2021]. Available from: https://covid19.who.int/?gclid=CjwKCAiAqJn9BRB0EiwAJ1SztaDZX6XhnL9tmEp0weSVA_KvmX3mJ8nAxXXR0jS7dSWfo813v3PYURoCVcEQAvD_BwE .
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akhmerov A, Marbán E. COVID-19 and the Heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, Sultan M, Easton A, Breen G, Zandi M, Coles JP, Manji H, Al-Shahi Salman R, Menon DK, Nicholson TR, Benjamin LA, Carson A, Smith C, Turner MR, Solomon T, Kneen R, Pett SL, Galea I, Thomas RH, Michael BD CoroNerve Study Group. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He W, Chen L, Yuan G, Fang Y, Chen W, Wu D, Liang B, Lu X, Ma Y, Li L, Wang H, Chen Z, Li Q, Gale RP. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J Am Soc Nephrol. 2020;31:1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunutsor SK, Laukkanen JA. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52:345–353. doi: 10.1080/07853890.2020.1790643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, Tang Y, Huang Q, Fu S, Li X, Lin B, Xu A, Chen J. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrol. 2021;22:52. doi: 10.1186/s12882-021-02244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ICNARC ICNARC report on COVID-19 in critical care: England, Wales and Northern Ireland 19 March 2021. 2021. [cited 27 March 2021]. Available from: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports .
- 12.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, Swaminathan S COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group. Acute Kidney Injury in COVID-19: Emerging Evidence of a Distinct Pathophysiology. J Am Soc Nephrol. 2020;31:1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, Canetta P, Ratner LE, Marasa M, Gharavi AG, Stokes MB, Markowitz GS, D'Agati VD. Kidney Biopsy Findings in Patients with COVID-19. J Am Soc Nephrol. 2020;31:1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, Khanin Y, Madireddy V, Larsen CP, Jhaveri KD, Bijol V Northwell Nephrology COVID-19 Research Consortium. COVID-19-Associated Kidney Injury: A Case Series of Kidney Biopsy Findings. J Am Soc Nephrol. 2020;31:1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR. COVID-19-Associated Collapsing Focal Segmental Glomerulosclerosis: A Report of 2 Cases. Kidney Med. 2020;2:493–497. doi: 10.1016/j.xkme.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, Chughtai A, Xie L, Gimenez JM, Sandow TA, Lusco MA, Yang H, Acheampong E, Rosales IA, Colvin RB, Fogo AB, Velez JCQ. AKI and Collapsing Glomerulopathy Associated with COVID-19 and APOL 1 High-Risk Genotype. J Am Soc Nephrol. 2020;31:1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akilesh S, Nast CC, Yamashita M, Henriksen K, Charu V, Troxell ML, Kambham N, Bracamonte E, Houghton D, Ahmed NI, Chong CC, Thajudeen B, Rehman S, Khoury F, Zuckerman JE, Gitomer J, Raguram PC, Mujeeb S, Schwarze U, Shannon MB, De Castro I, Alpers CE, Najafian B, Nicosia RF, Andeen NK, Smith KD. Multicenter Clinicopathologic Correlation of Kidney Biopsies Performed in COVID-19 Patients Presenting With Acute Kidney Injury or Proteinuria. Am J Kidney Dis. 2021;77:82–93.e1. doi: 10.1053/j.ajkd.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta RK, Bhargava R, Shaukat AA, Albert E, Leggat J. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol. 2020;21:326. doi: 10.1186/s12882-020-01970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, Lhopitallier L, Sadallah S, Fakhouri F. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaillard F, Ismael S, Sannier A, Tarhini H, Volpe T, Greze C, Verpont MC, Zouhry I, Rioux C, Lescure FX, Buob D, Daugas E. Tubuloreticular inclusions in COVID-19-related collapsing glomerulopathy. Kidney Int. 2020;98:241. doi: 10.1016/j.kint.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magoon S, Bichu P, Malhotra V, Alhashimi F, Hu Y, Khanna S, Berhanu K. COVID-19-Related Glomerulopathy: A Report of 2 Cases of Collapsing Focal Segmental Glomerulosclerosis. Kidney Med. 2020;2:488–492. doi: 10.1016/j.xkme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nlandu YM, Makulo JR, Pakasa NM, Sumaili EK, Nkondi CN, Bukabau JB, Beya FK, Nseka NM, Lepira FB. First Case of COVID-19-Associated Collapsing Glomerulopathy in Sub-Saharan Africa. Case Rep Nephrol. 2020;2020:8820713. doi: 10.1155/2020/8820713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deshmukh S, Zhou XJ, Hiser W. Collapsing glomerulopathy in a patient of Indian descent in the setting of COVID-19. Ren Fail. 2020;42:877–880. doi: 10.1080/0886022X.2020.1811122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadosh BS, Pavone J, Wu M, Reyentovich A, Gidea C. Collapsing glomerulopathy associated with COVID-19 infection in a heart transplant recipient. J Heart Lung Transplant. 2020;39:855–857. doi: 10.1016/j.healun.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couturier A, Ferlicot S, Chevalier K, Guillet M, Essig M, Jauréguiberry S, Collarino R, Dargelos M, Michaut A, Geri G, Roque-Afonso AM, Zaidan M, Massy ZA. Indirect effects of severe acute respiratory syndrome coronavirus 2 on the kidney in coronavirus disease patients. Clin Kidney J. 2020;13:347–353. doi: 10.1093/ckj/sfaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing Glomerulopathy in a Patient With COVID-19. Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malhotra V, Magoon S, Troyer DA, McCune TR. Collapsing Focal Segmental Glomerulosclerosis and Acute Oxalate Nephropathy in a Patient With COVID-19: A Double Whammy. J Investig Med High Impact Case Rep. 2020;8:2324709620963635. doi: 10.1177/2324709620963635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izzedine H, Brocheriou I, Arzouk N, Seilhean D, Couvert P, Cluzel P, Pha M, Le Monnier O, Varnous S, Andreelli F, Amoura Z, Mathian A. COVID-19-associated collapsing glomerulopathy: a report of two cases and literature review. Intern Med J. 2020;50:1551–1558. doi: 10.1111/imj.15041. [DOI] [PubMed] [Google Scholar]
- 29.Laboux T, Gibier JB, Pottier N, Glowacki F, Hamroun A. COVID-19-related collapsing glomerulopathy revealing a rare risk variant of APOL1: lessons for the clinical nephrologist. J Nephrol. 2021;34:373–378. doi: 10.1007/s40620-020-00935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik IO, Ladiwala N, Chinta S, Khan M, Patel K. Severe Acute Respiratory Syndrome Coronavirus 2 Induced Focal Segmental Glomerulosclerosis. Cureus. 2020;12:e10898. doi: 10.7759/cureus.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenti MV, Gregorini M, Borrelli de Andreis F, Rampino T, D'Ambrosio G, Verga L, Vanoli A, Mengoli C, Ravetta V, Paulli M, Di Sabatino A. Acute kidney injury caused by COVID-19 in a patient with Crohn's disease treated with adalimumab. J Clin Pathol. 2021;74:540–542. doi: 10.1136/jclinpath-2020-206912. [DOI] [PubMed] [Google Scholar]
- 32.Rossi GM, Delsante M, Pilato FP, Gnetti L, Gabrielli L, Rossini G, Re MC, Cenacchi G, Affanni P, Colucci ME, Picetti E, Rossi S, Parenti E, Maccari C, Greco P, Di Mario F, Maggiore U, Regolisti G, Fiaccadori E. Kidney Biopsy Findings in a Critically Ill COVID-19 Patient With Dialysis-Dependent Acute Kidney Injury: A Case Against "SARS-CoV-2 Nephropathy". Kidney Int Rep. 2020;5:1100–1105. doi: 10.1016/j.ekir.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papadimitriou JC, Drachenberg CB, Kleiner D, Choudhri N, Haririan A, Cebotaru V. Tubular Epithelial and Peritubular Capillary Endothelial Injury in COVID-19 AKI. Kidney Int Rep. 2021;6:518–525. doi: 10.1016/j.ekir.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uppal NN, Kello N, Shah HH, Khanin Y, De Oleo IR, Epstein E, Sharma P, Larsen CP, Bijol V, Jhaveri KD. De Novo ANCA-Associated Vasculitis With Glomerulonephritis in COVID-19. Kidney Int Rep. 2020;5:2079–2083. doi: 10.1016/j.ekir.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moeinzadeh F, Dezfouli M, Naimi A, Shahidi S, Moradi H. Newly Diagnosed Glomerulonephritis During COVID-19 Infection Undergoing Immunosuppression Therapy, a Case Report. Iran J Kidney Dis. 2020;14:239–242. [PubMed] [Google Scholar]
- 36.Jalalzadeh M, Valencia-Manrique JC, Boma N, Chaudhari A, Chaudhari S. Antineutrophil Cytoplasmic Antibody-Associated Glomerulonephritis in a Case of Scleroderma After Recent Diagnosis With COVID-19. Cureus. 2021;13:e12485. doi: 10.7759/cureus.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prendecki M, Clarke C, Cairns T, Cook T, Roufosse C, Thomas D, Willicombe M, Pusey CD, McAdoo SP. Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020;98:780–781. doi: 10.1016/j.kint.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suso AS, Mon C, Oñate Alonso I, Galindo Romo K, Juarez RC, Ramírez CL, Sánchez Sánchez M, Mercado Valdivia V, Ortiz Librero M, Oliet Pala A, Ortega Marcos O, Herrero Berron JC, Silvestre Torner N, Alonso Riaño M, Pascual Martin A. IgA Vasculitis With Nephritis (Henoch-Schönlein Purpura) in a COVID-19 Patient. Kidney Int Rep. 2020;5:2074–2078. doi: 10.1016/j.ekir.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y, Li XJ, Li YQ, Dai W, Shao T, Liu WY, Han M, Xu G, Liu L. Clinical and pathological findings of SARS-CoV-2 infection and concurrent IgA nephropathy: a case report. BMC Nephrol. 2020;21:504. doi: 10.1186/s12882-020-02163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontana F, Cazzato S, Giovanella S, Ballestri M, Leonelli M, Mori G, Alfano G, Ligabue G, Magistroni R, Cenacchi G, Antoniotti R, Bonucchi D, Cappelli G. Oxalate Nephropathy Caused by Excessive Vitamin C Administration in 2 Patients With COVID-19. Kidney Int Rep. 2020;5:1815–1822. doi: 10.1016/j.ekir.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ville S, Le Bot S, Chapelet-Debout A, Blancho G, Fremeaux-Bacchi V, Deltombe C, Fakhouri F. Atypical HUS relapse triggered by COVID-19. Kidney Int. 2021;99:267–268. doi: 10.1016/j.kint.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szajek K, Kajdi ME, Luyckx VA, Fehr TH, Gaspert A, Cusini A, Hohloch K, Grosse P. Granulomatous interstitial nephritis in a patient with SARS-CoV-2 infection. BMC Nephrol. 2021;22:19. doi: 10.1186/s12882-020-02213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noble R, Tan MY, McCulloch T, Shantier M, Byrne C, Hall M, Jesky M. Collapsing Glomerulopathy Affecting Native and Transplant Kidneys in Individuals with COVID-19. Nephron. 2020;144:589–594. doi: 10.1159/000509938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abuzeineh M, Tariq A, Rosenberg A, Brennan DC. Chronic Active Antibody-Mediated Rejection Following COVID-19 Infection in a Kidney Transplant Recipient: A Case Report. Transplant Proc. 2021;53:1202–1206. doi: 10.1016/j.transproceed.2020.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doevelaar AAN, Hölzer B, Seibert FS, Bauer F, Stervbo U, Rohn BJ, Zgoura P, Schenker P, Vonbrunn E, Amann K, Viebahn R, Babel N, Westhoff TH. Lessons for the clinical nephrologist: recurrence of nephrotic syndrome induced by SARS-CoV-2. J Nephrol. 2020;33:1369–1372. doi: 10.1007/s40620-020-00855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jespersen Nizamic T, Huang Y, Alnimri M, Cheng M, Chen LX, Jen KY. COVID-19 Manifesting as Renal Allograft Dysfunction, Acute Pancreatitis, and Thrombotic Microangiopathy: A Case Report. Transplant Proc. 2021;53:1211–1214. doi: 10.1016/j.transproceed.2020.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazareth H, Péré H, Binois Y, Chabannes M, Schurder J, Bruneau T, Karras A, Thervet E, Rabant M, Veyer D, Pallet N. COVID-19-Related Collapsing Glomerulopathy in a Kidney Transplant Recipient. Am J Kidney Dis. 2020;76:590–594. doi: 10.1053/j.ajkd.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oniszczuk J, Moktefi A, Mausoleo A, Pallet N, Malard-Castagnet S, Fourati S, El Karoui K, Sahali D, Stehlé T, Boueilh A, Verpont MC, Matignon M, Buob D, Grimbert P, Audard V. De Novo Focal and Segmental Glomerulosclerosis After COVID-19 in a Patient With a Transplanted Kidney From a Donor With a High-risk APOL1 Variant. Transplantation. 2021;105:206–211. doi: 10.1097/TP.0000000000003432. [DOI] [PubMed] [Google Scholar]
- 49.Webb C, Davidson B, Jones ESW, Wearne N, Chetty DR, Blom D, Barday Z. COVID-19-Associated Graft Loss From Renal Infarction in a Kidney Transplant Recipient. Kidney Int Rep. 2021;6:1166–1169. doi: 10.1016/j.ekir.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westhoff TH, Seibert FS, Bauer F, Stervbo U, Anft M, Doevelaar AAN, Rohn BJ, Winnekendonk G, Dittmer U, Schenker P, Vonbrunn E, Amann K, Viebahn R, Babel N. Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient. Am J Transplant. 2020;20:3216–3220. doi: 10.1111/ajt.16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada M, Rastogi P, Ince D, Thayyil A, Adela Mansilla M, Smith RJH, Kuppachi S, Thomas CP. Minimal Change Disease With Nephrotic Syndrome Associated With Coronavirus Disease 2019 After Apolipoprotein L1 Risk Variant Kidney Transplant: A Case Report. Transplant Proc. 2020;52:2693–2697. doi: 10.1016/j.transproceed.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brook OR, Piper KG, Mercado NB, Gebre MS, Barouch DH, Busman-Sahay K, Starke CE, Estes JD, Martinot AJ, Wrijil L, Ducat S, Hecht JL. Feasibility and safety of ultrasound-guided minimally invasive autopsy in COVID-19 patients. Abdom Radiol (NY) 2021;46:1263–1271. doi: 10.1007/s00261-020-02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E, Antinori A, Petrosillo N, Marchioni L, Biava G, D'Offizi G, Palmieri F, Goletti D, Zumla A, Ippolito G, Piacentini M, Del Nonno F. Postmortem Findings in Italian Patients With COVID-19: A Descriptive Full Autopsy Study of Cases With and Without Comorbidities. J Infect Dis. 2020;222:1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farkash EA, Wilson AM, Jentzen JM. Ultrastructural Evidence for Direct Renal Infection with SARS-CoV-2. J Am Soc Nephrol. 2020;31:1683–1687. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golmai P, Larsen CP, DeVita MV, Wahl SJ, Weins A, Rennke HG, Bijol V, Rosenstock JL. Histopathologic and Ultrastructural Findings in Postmortem Kidney Biopsy Material in 12 Patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31:1944–1947. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.González Pessolani T, Muñóz Fernández de Legaria M, Elices Apellániz M, Salinas Moreno S, Lorido Cortés MDM, García Sánchez S. Multi-organ pathological findings associated with COVID-19 in postmortem needle core biopsies in four patients and a review of the current literature. Rev Esp Patol. 2021;54:275–280. doi: 10.1016/j.patol.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacobs W, Lammens M, Kerckhofs A, Voets E, Van San E, Van Coillie S, Peleman C, Mergeay M, Sirimsi S, Matheeussen V, Jansens H, Baar I, Vanden Berghe T, Jorens PG. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020;7:3772–3781. doi: 10.1002/ehf2.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Remmelink M, De Mendonça R, D'Haene N, De Clercq S, Verocq C, Lebrun L, Lavis P, Racu ML, Trépant AL, Maris C, Rorive S, Goffard JC, De Witte O, Peluso L, Vincent JL, Decaestecker C, Taccone FS, Salmon I. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, Barasch J, Radhakrishnan J, D'Agati V, Markowitz G. Postmortem Kidney Pathology Findings in Patients with COVID-19. J Am Soc Nephrol. 2020;31:2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schurink B, Roos E, Radonic T, Barbe E, Bouman CSC, de Boer HH, de Bree GJ, Bulle EB, Aronica EM, Florquin S, Fronczek J, Heunks LMA, de Jong MD, Guo L, du Long R, Lutter R, Molenaar PCG, Neefjes-Borst EA, Niessen HWM, van Noesel CJM, Roelofs JJTH, Snijder EJ, Soer EC, Verheij J, Vlaar APJ, Vos W, van der Wel NN, van der Wal AC, van der Valk P, Bugiani M. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, Harding CV, Gilmore H, Sadri N. Molecular Detection of SARS-CoV-2 Infection in FFPE Samples and Histopathologic Findings in Fatal SARS-CoV-2 Cases. Am J Clin Pathol. 2020;154:190–200. doi: 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Werion A, Belkhir L, Perrot M, Schmit G, Aydin S, Chen Z, Penaloza A, De Greef J, Yildiz H, Pothen L, Yombi JC, Dewulf J, Scohy A, Gérard L, Wittebole X, Laterre PF, Miller SE, Devuyst O, Jadoul M, Morelle J Cliniques universitaires Saint-Luc (CUSL) COVID-19 Research Group. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98:1296–1307. doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albaqumi M, Barisoni L. Current views on collapsing glomerulopathy. J Am Soc Nephrol. 2008;19:1276–1281. doi: 10.1681/ASN.2007080926. [DOI] [PubMed] [Google Scholar]
- 70.Albaqumi M, Soos TJ, Barisoni L, Nelson PJ. Collapsing glomerulopathy. J Am Soc Nephrol. 2006;17:2854–2863. doi: 10.1681/ASN.2006030225. [DOI] [PubMed] [Google Scholar]
- 71.Detwiler RK, Falk RJ, Hogan SL, Jennette JC. Collapsing glomerulopathy: a clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int. 1994;45:1416–1424. doi: 10.1038/ki.1994.185. [DOI] [PubMed] [Google Scholar]
- 72.Shetty AA, Tawhari I, Safar-Boueri L, Seif N, Alahmadi A, Gargiulo R, Aggarwal V, Usman I, Kisselev S, Gharavi AG, Kanwar Y, Quaggin SE. COVID-19-Associated Glomerular Disease. J Am Soc Nephrol. 2021;32:33–40. doi: 10.1681/ASN.2020060804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laurinavicius A, Hurwitz S, Rennke HG. Collapsing glomerulopathy in HIV and non-HIV patients: a clinicopathological and follow-up study. Kidney Int. 1999;56:2203–2213. doi: 10.1046/j.1523-1755.1999.00769.x. [DOI] [PubMed] [Google Scholar]
- 74.Ossina NK, Cannas A, Powers VC, Fitzpatrick PA, Knight JD, Gilbert JR, Shekhtman EM, Tomei LD, Umansky SR, Kiefer MC. Interferon-gamma modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J Biol Chem. 1997;272:16351–16357. doi: 10.1074/jbc.272.26.16351. [DOI] [PubMed] [Google Scholar]
- 75.Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24:360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181–1189. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD. Pathophysiology and Pathology of Acute Kidney Injury in Patients With COVID-19. Adv Chronic Kidney Dis. 2020;27:365–376. doi: 10.1053/j.ackd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma P, Ng JH, Bijol V, Jhaveri KD, Wanchoo R. Pathology of COVID-19-associated acute kidney injury. Clin Kidney J. 2021;14:i30–i39. doi: 10.1093/ckj/sfab003. [DOI] [PMC free article] [PubMed] [Google Scholar]