Abstract
Caspase 8 plays an essential role in the execution of death receptor-mediated apoptosis. To determine the localization of endogenous caspase 8, we used a panel of subunit-specific anti-caspase 8 monoclonal antibodies in confocal immunofluorescence microscopy. In the human breast carcinoma cell line MCF7, caspase 8 predominantly colocalized with and bound to mitochondria. After induction of apoptosis through CD95 or tumor necrosis factor receptor I, active caspase 8 translocated to plectin, a major cross-linking protein of the three main cytoplasmic filament systems, whereas the caspase 8 prodomain remained bound to mitochondria. Plectin was quantitatively cleaved by caspase 8 at Asp 2395 in the center of the molecule in all cells tested. Cleavage of plectin clearly preceded that of other caspase substrates such as poly(ADP-ribose) polymerase, gelsolin, cytokeratins, or lamin B. In primary fibroblasts from plectin-deficient mice, apoptosis-induced reorganization of the actin cytoskeleton, as seen in wild-type cells, was severely impaired, suggesting that during apoptosis, plectin is required for the reorganization of the microfilament system.
Apoptosis is essential for development and homeostasis of the organism (60). It is a morphologically and biochemically distinct form of cell death that can be triggered by a wide range of internal and external signals (for a review, see reference 70). Recent studies demonstrated that a subfamily of the tumor necrosis factor receptor (TNF-R) superfamily, the death receptors, constitute an important system which can induce apoptosis (for a review, see reference 48). Among this death receptor family, CD95 (also called APO-1 or Fas) is one of the best-characterized members, especially with regard to intracellular signaling events. Apoptosis mediated by CD95 involves activation of a cascade of cysteine proteases, the caspases (45). In the CD95 system, caspase 8 (also called FLICE, Mach, or Mch5) (4, 9, 43), the most receptor-proximal caspase, is recruited to CD95 through the adapter molecule FADD (Mort1) (5, 8). This results in activation of caspase 8 by proteolytic cleavage into the prodomain containing two death effector domains (DEDs) and two active subunits, p18 and p10 (39, 56). We have recently shown that caspase 8 can be activated in two ways. Most of caspase 8 is activated at the CD95 receptor in type I cells and at the mitochondria in type II cells (55). Caspase 8 was also found to be essential for other death receptors such as TNF-RI, TRAIL-RI, and DR3 (25, 68).
Activation of caspase 8 and other caspases located more downstream in the pathway results in cleavage of various death substrates. These protein targets include various intermediate filament (IF) proteins (7, 16, 29). Thereby, apoptosis signaling profoundly affects the integrity of the cytoskeleton and consequently the cellular structure as a whole. Activation of caspases is also responsible for the specific nuclear changes characteristic for apoptosis involving activation of the endonuclease CAD (DFF40) (33, 53) and translocation of the DNA binding protein DEDD from cytoplasm to the nucleus (59).
The only reported substrates of caspase 8 so far are caspase 3 (61), BID, a BH3 domain-containing member of the Bcl-2 family (18, 30, 34), and RIP (31). During CD95-mediated apoptosis, caspase 3 and BID are required to propagate the caspase-only signal in type I cells and the mitochondrion-dependent signal of type II cells, respectively (55). Most data suggest that the major function of caspase 8 is to act as an initiator caspase at the top of the caspase cascade. However, its role at the mitochondria is unclear. To characterize the role of endogenous caspase 8 in apoptosis in more detail, we monitored the active subunits of caspase 8 in CD95 and TNF-α-sensitive MCF7-Fas breast carcinoma cells after induction of apoptosis by confocal immunofluorescence microscopy using monoclonal antibodies (MAbs) specific for individual subdomains of caspase 8 (56). In untreated MCF7-Fas cells, caspase 8 was located mostly at the mitochondria. Upon inducing apoptosis through CD95 or TNF-R, most of active caspase 8 translocated to plectin, a protein that cross-links members of all three filament systems of the cytoskeleton responsible for maintaining cellular integrity (71). During apoptosis induced by a variety of stimuli, this translocation resulted in complete and cell-wide cleavage of plectin in vivo. We provide evidence for a dual role of caspase 8: (i) as an initiator caspase that is essential during death receptor-mediated apoptosis to start the caspase cascade and (ii) as an effector caspase that cleaves plectin prior to any other tested cytoskeletal substrate of classical effector caspases such as caspase 3. This may ensure a hierarchical cleavage of structural key proteins involved in the morphological changes during apoptosis. Plectin seems to be important for these morphological changes since in fibroblasts from plectin-deficient mice, the typical reorganization of the actin cytoskeleton during CD95-mediated apoptosis was completely blocked.
MATERIALS AND METHODS
Immunofluorescence microscopy.
Cells were plated on glass coverslips at a confluency of 20% and were allowed to become adherent overnight. After being washed three times with phosphate-buffered saline (PBS) containing 1 mM MgCl2 (PBS-MgCl2), the cells were fixed with methanol-acetone (1:1) at −20°C for 15 min. The coverslips were allowed to dry, rehydrated with PBS-MgCl2, and incubated for 45 min with a fluorescein isothiocyanate (FITC)-labeled monoclonal antibody (MAb) against caspase 8: C1 (immunoglobulin G1a [IgG1]), C5 (IgG2a), or N2 (IgG1) (55). The anti-caspase 8 MAbs were labeled as described elsewhere (38). After three washes with PBS-MgCl2, coverslips were dehydrated in 100% ethanol for a few seconds, dried, and then were mounted on glass slides. For costaining, the coverslips were incubated with the primary antibody (antiplectin [guinea pig IgG]), antimitochondrial [concentrated anti-human mitochondrial antigen, mouse IgG1; BioGenex, San Ramon, Calif.], anti-cytochrome c [mouse IgG1; Pharmingen], or anti-protein disulfide isomerase [PDI] [rabbit polyclonal antibody]) for 45 min. After washing, the anti-caspase 8 antibody C5 and the secondary antibodies (goat anti-mouse IgG1, phycoerythrin [PE] labeled [Sigma]; goat-anti guinea pig IgG, Texas red labeled [Sigma], and goat-anti rabbit, PE labeled) were applied simultaneously. Photographs for colocalization were obtained by confocal microscopy (LSM 310; Zeiss, Jena, Germany). For actin staining of fibroblasts derived from wild-type and plectin-deficient mice, cells were grown on glass cover slides at a confluency of 70%. The slides were washed with PBS-MgCl2 and subsequently fixed with 2% formaldehyde in PBS. After being washed twice with 50 mM ammonium chloride and once with PBS-MgCl2, the cells were permeabilized with 0.1% NP-40 in PBS. After a wash with PBS-MgCl2 actin fibers were stained with Texas red-labeled phalloidin (Sigma) for 20 min, then washed three times with PBS-MgCl2, and dehydrated as described above.
Generation of antiplectin antisera.
For generating an antiserum against the C terminus of the plectin molecule (anti-plectin-C), a partial cDNA coding for the carboxy-terminal sixth repeat domain of human plectin (GenBank accession no. H23127; American Type Culture Collection, Manassas, Va.) was cloned into plasmid pET21a (Novagen, Madison, Wis.) using the unique restriction sites NotI and HindIII. The recombinant protein was generated and purified as described for recombinant vimentin (19). The recombinant polypeptide was purified by DEAE-Sepharose ion-exchange chromatography. Relevant fractions were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and pooled, and an aliquot was subjected to protein microsequencing. The recombinant protein spans amino acids 4376 to 4684, as verified by the sequencing of tryptic peptide fragments representing the amino- and carboxy-terminal peptides (20 and 18 amino acids, respectively) as well as four internal peptides (18 to 47 amino acids in length). The plectin polypeptide was desalted into PBS, and two guinea pigs were immunized using conventional procedures. Guinea pig antibodies specific for the amino-terminal domain (anti-plectin-N) of human plectin were obtained by immunization with a synthetic peptide covering amino acids 587 to 601 (RLLFNDVQTLKDGRH [32]) and coupled via a C-terminally added cysteine to keyhole limpet hemocyanin as recently described for other peptides (41).
IF preparation and cell lysates for Western blot detection.
Triton X-100/high-salt-insoluble cell fractions (IF pellet) were prepared from various cell lines and subjected to SDS-PAGE (6% gel) as described elsewhere (21). Using this method, plectin was enriched such that it could easily be detected by Coomassie blue staining. For Western blot detection of cytokeratin 8 (CK8), CK18, caspase 8, and its active subunit p18, cellular lysates equivalent to 5 × 105 cells were prepared using a Triton X-100-containing lysis buffer (30 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, small peptide inhibitors, 1% Triton X-100 [Serva], 10% glycerol [39]) and separated by SDS-PAGE (12% gel).
Western blot analysis.
After electrophoresis, proteins were transferred to Hybond nitrocellulose membrane (Amersham). For plectin Western blotting, we used a borate buffer system allowing a quantitative transfer of plectin (20). The membrane was blocked with 5% milk in PBS-Tween (PBS with 0.05% [vol/vol] Tween 20 [Serva]) for 1 h, washed with PBS-Tween, and incubated with the anti-caspase 8 antibody C15, anti-FADD antibody (Transduction Laboratories), antimitochondrial antibody, anti-cytochrome c antibody (Pharmingen), anti-CK8 (KS8.17.2), anti-CK18 (KS18.174) (10), anti-lamin B (Promega), and antigelsolin (Sigma) antibodies or the anti-plectin-C or anti-plectin-N antibody. The blots were washed with PBS-Tween and developed with horseradish peroxidase-coupled goat anti-mouse IgG2b, IgG2a, IgG1 (1:5,000 in 5% milk with PBS-Tween), or goat anti-guinea pig IgG (1:5,000 in 5% milk with PBS-Tween). After a wash with PBS-Tween, blots were developed by the chemiluminescence method as specified by the manufacturer (NEN).
Construction of a recombinant plectin fragment.
To generate a protein fragment corresponding to a carboxy-terminal segment of plectin's rod domain, rat plectin cDNA (bp 6703 to 7731, according to GenBank/EMBL/DDBJ database entry X59601 [73]) was amplified by PCR with EcoRI-tailed primers (upper, 5′-CCG GAA TTC AAG CTT GAG GCC CGG GAG CAG GCA GAA CGT GAG-3′; lower, 5′-GGC GAA TTC CTG GAT CTC GAG AGT CTG CAC-3′) using a cDNA clone as template (rat plectin is 95% identical to human plectin). The amplified fragment was subcloned into the unique EcoRI site of the bacterial expression vector pJD1 (46), a derivative of pET-15b (Novagen), thereby enabling expression of a protein bearing an amino-terminal His tag. Asp-to-Ala point mutations of the putative caspase cleavage sites were introduced by using standard PCR protocols. Amplified fragments were then exchanged with the corresponding part of the wild-type construct, using the two internal XhoI sites. Recombinant fragments were expressed in Escherichia coli BL21(DE3) and purified from inclusion bodies by solubilization in 6 M urea–500 mM NaCl–20 mM Tris-HCl (pH 7.9) (binding buffer) containing 5 mM imidazole, followed by affinity binding to His-Bind metal chelation resin as specified by the manufacturer (Novagen). Bound proteins were eluted from affinity columns using 500 mM imidazole in binding buffer. Subsequently, the proteins were dialyzed against decreasing concentrations of urea (4 and 2 M urea in dialysis buffer [20 mM HEPES, 100 mM NaCl, 10 mM dithiothreitol {DTT}, 1 mM EDTA {pH 7.2}] for 1 h and finally against dialysis buffer without urea for 2 h.
In vitro plectin cleavage by DISC-bound caspase 8.
SKW6.4 cells (2 × 108) were either first treated with anti-CD95 (anti-APO-1; 2 μg/ml) for 5 min at 37°C and then lysed (stimulated condition) or first lysed and then supplemented with anti-CD95 (2 μg/ml) (unstimulated condition) as described previously (39). Triton X-100 solubilization of cellular proteins was done as described above. The lysate was incubated with 60 μl of a protein A-Sepharose suspension (Sigma) for at least 3 h at 4°C. The protein A-Sepharose beads with immunoprecipitated death-inducing signal complex (DISC) were resuspended in 2× buffer A {50 mM HEPES (pH 7.4), 10 mM DTT, 100 mM NaCl, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 10% sucrose), added to the IF pellet resuspended in 1 mM Tris-HCl (pH 9.0), and incubated overnight at 4°C. The cleavage reaction was stopped by addition of standard reducing sample buffer containing 5 M urea. After boiling for 3 min at 95°C, the samples were subjected to SDS-PAGE (6% gel).
In vitro plectin cleavage by recombinant caspases.
Recombinant caspases 3, 6, 7, 8, and 10 were added to IF pellets resuspended in cleavage buffer (20 mM HEPES, 100 mM NaCl, 10 mM DTT, 1 mM EDTA, 0.1% CHAPS, 10% sucrose [pH 7.2]) or to 4 μg of the recombinant plectin fragment. The active concentrations of all caspases were 10 μM for incubation with the IF pellets, 40 μM for cleavage of the wild-type recombinant plectin fragment, and 60 μM for cleavage of mutant plectin fragments. After incubation for 8, 12, and 36 h at 4°C or 24 h at room temperature (for the cleavage of recombinant plectin fragments), samples were subjected to SDS-PAGE (6% or 15% gel) and subsequent Western blotting with the anti-plectin-C antiserum or staining with Coomassie brilliant blue.
In vitro binding assay of caspase 8 to mitochondria.
Mitochondria of MCF7-Fas cells were isolated as described elsewhere (67). One microliter of in vitro-transcribed-translated [35S]caspase 8/a or 2.5 μl of [35S]FADD (39) was incubated with mitochondria equivalent to 100 μg of protein in 100 μl of 10 mM KH2PO4 (pH 7.2)–0.3 mM mannitol–0.5 mg of bovine serum albumin per ml for 10 min at 37°C. The supernatant was kept, and mitochondria were washed once with 500 μl of the above buffer. Mitochondria were directly boiled in standard reducing sample buffer and loaded onto an SDS–12% polyacrylamide gel; 20 μl of a fivefold-concentrated reducing sample buffer was added to the supernatant and half of the sample was analyzed by SDS-PAGE (12% gel) and autoradiographed.
Subcellular fractionation of MCF7-Fas cells.
MCF7-Fas cells (5 × 107) were stimulated with anti-CD95 (2 μg/ml) for the indicated time periods and washed with PBS. Subcellular fractionation was performed as described previously (67).
Apoptosis assay.
For quantification of cell death in MCF7-Fas cells, cells were plated on CELLocate coverslips (square size, 175 μm; Eppendorf) and stimulated with anti-CD95 (2 μg/ml) or TNF-α (20 ng/ml with 1 μg of cycloheximide [CHX] per ml). Nonapoptotic cells which were still adherent were counted after different periods of time. Percentage of apoptosis was determined as follows: (adherent cells at time point 0 − adherent cells at time point x/adherent cells at time point 0) × 100. For assessment of cell death in Jurkat cells and primary fibroblasts from wild-type and plectin knockout mice, DNA fragmentation was quantified as described previously (49).
Caspase activity assay.
MCF7-Fas or Jurkat cells were stimulated and lysed with a Triton X-100-containing lysis buffer as described above. The cell lysates were incubated with 40 μM amino trifluoromethyl coumarin (ATC)-labeled caspase-specific peptides (zVDVAD-AFC for caspase 2, zDEVD-AFC for caspases 3 and 7, zVEID-AFC for caspase 6, zIETD-AFC for caspase 8, and Ac-LEHD-AFC for caspase 9; Bachem) in cleavage buffer and incubated for 1 h at 37°C. Caspase activities were determined fluorometrically using a fluorescence plate reader. Values obtained with unstimulated cells were taken as background and subtracted from those obtained with stimulated cells.
Preparation of fibroblasts from plectin-deficient mice.
Primary fibroblasts from wild-type and plectin-deficient mice (2) were cultivated from mouse skin explants as previously described (12). Fibroblasts derived from wild-type or plectin-deficient mice were negative for CD95 surface expression as determined by flow cytometry (data not shown). Therefore, all fibroblasts were resistant to CD95-mediated apoptosis as quantified by DNA fragmentation (data not shown). To upregulate CD95, cells were treated with gamma interferon (IFN-γ; Boehringer Mannheim) for different periods of time. Maximal surface expression of CD95 was achieved by incubating cells with 500 U IFN-γ per ml for 36 h, resulting in an increase of surface CD95-positive cells from 4% (untreated) to 86% (IFN-γ treated). Sensitivity to CD95-mediated apoptosis was assessed by treatment with 10 μg of anti-CD95 (Jo2; Pharmingen) per ml, 10 ng of protein A (Sigma) per ml, and 1 μg of CHX (Sigma) per ml. DNA fragmentation was quantified as described previously (49).
RESULTS
Intracellular redistribution of caspase 8 upon CD95 triggering.
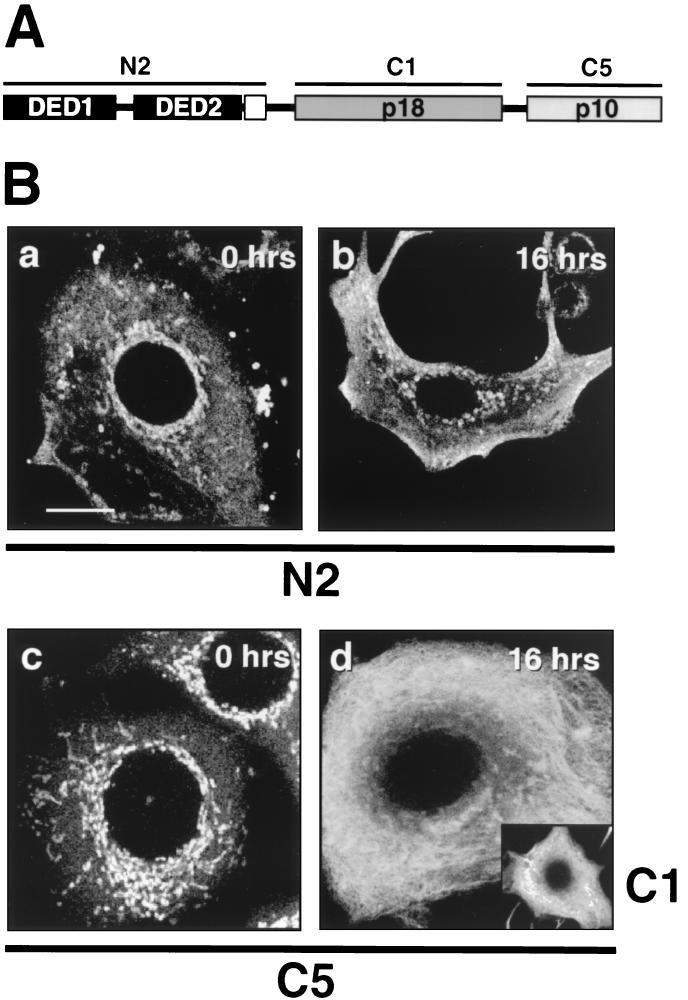
The subcellular localization of caspase 8 in MCF7-Fas cells was determined by confocal immunofluorescence microscopy using a panel of MAbs specific for individual subdomains of caspase 8 (56) (Fig. 1A). In untreated MCF7-Fas cells, caspase 8 was preferentially detected in rod-shaped structures in the perinuclear cytoplasm, as visualized with FITC-labeled MAbs against the caspase 8 prodomain (N2 [Fig. 1B-a]) and the active subunits p10 (C5 [Fig. 1B-c]) and p18 (C1) (data not shown). In addition, a less pronounced cytoplasmic staining was observed.
FIG. 1.
Localization of caspase 8 cleavage products in MCF7-Fas cells during CD95-mediated apoptosis. (A) Binding specificities of the anti-caspase 8 MAbs. N2 recognizes the caspase 8 prodomain containing the two DEDs. C1 and C5 are directed against the active subunits of caspase 8, p18 and p10, respectively. (B) MCF7-Fas cells were left untreated (0 h) or treated with anti-CD95 (2 μg/ml) for 16 h and subjected to laser scanning immunofluorescence microscopy using anti-caspase 8 MAbs C5, C1, and N2, all directly labeled with FITC. Bar, 10 μm. The specificity of the procaspase 8 staining was confirmed by using unlabeled primary antibodies and FITC-coupled secondary antibodies. These indirect immunofluorescence stainings gave results similar to those obtained by the direct immunofluorescence (data not shown). In addition, stainings of caspase 8-deficient Jurkat cells (provided by J. Blenis, Boston, Mass.) (25) with the anti-caspase 8 antibody C5 were negative (data not shown). Furthermore, direct immunofluorescence with an isotype-matched FITC-labeled control antibody resulted in only background staining (data not shown).
After triggering of CD95 by treating MCF7-Fas cells for 16 h with the stimulating anti-CD95 MAb, the staining pattern observed with the antibodies against the active subunits p10 and p18 of caspase 8 (C5 and C1 [Fig. 1B-d and inset]) changed completely. Rod-like structures, characteristic of unstimulated cells, disappeared and were replaced by a fine extensive meshwork. Remarkably, mostly the active subunits of caspase 8 in the CD95-sensitive MCF7-Fas cells redistributed to the reticular pattern upon anti-CD95 treatment. In contrast, this staining was not observed with the antibody against the caspase 8 prodomain (N2 [Fig. 1B-b]). Similar to procaspase 8 in untreated cells, the caspase 8 prodomain stayed mostly colocalized with rod-shaped structures. Statistical evaluation of the cell morphology of MCF7-Fas cells using light microscopy revealed that the number of apoptotic (detached) cells after anti-CD95 treatment increased with the number of cells exhibiting the reticular staining pattern. After 8 h, 30% (±7%) of the cells were apoptotic, and 30% (±6%) showed a network-like pattern. After 16 h, the number of apoptotic cells increased by 40% (±7%) to a total of 70%, and the number of cells exhibiting a reticular staining increased by 38% (±9%) to 68%. After 24 h, all cells had detached and undergone apoptosis (data not shown). Therefore, this analysis strongly indicates that the network-like distribution of active caspase 8 was characteristic for MCF7-Fas cells shortly before they start to exhibit morphological changes typical for apoptosis and detach.
Procaspase 8 shows a mitochondrial distribution in MCF7 cells.
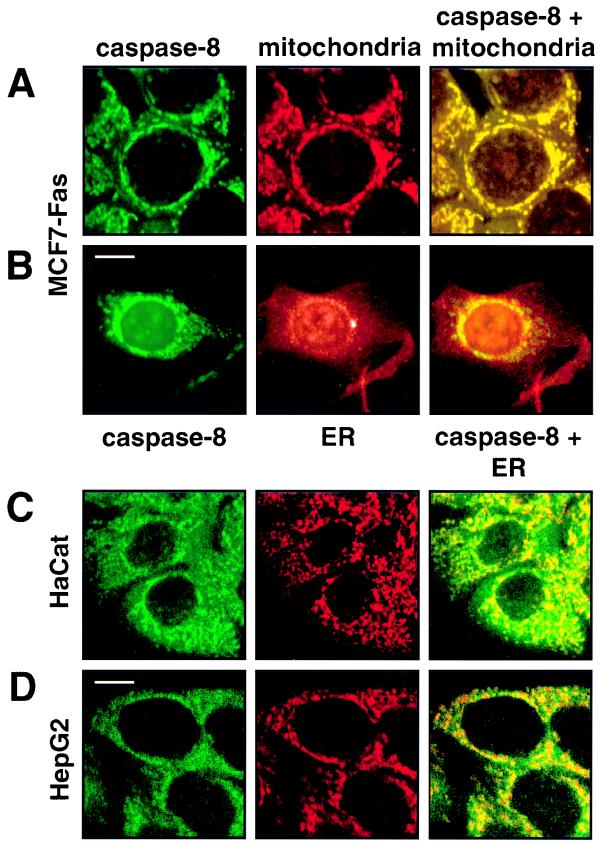
In unstimulated MCF7-Fas cells, procaspase 8 colocalized with rod-like structures. These structures were shown to be mitochondria, since double stainings obtained with the anti-caspase 8 MAb C5 and a mitochondrion-specific MAb were virtually identical (Fig. 2A). These colocalization results were also confirmed by using an anti-cytochrome c MAb to specifically stain mitochondria (data not shown). Since a previous report had suggested that caspase 8 is localized in the endoplasmic reticulum (ER) (44), we tested whether in MCF7 cells caspase 8 would in part colocalize with the ER compartment. We therefore performed a costaining of caspase 8 and PDI, a typical marker enzyme of the ER (Fig. 2B) (35). This analysis demonstrated that caspase 8 in MCF7 cells is localized only at mitochondria and not in the ER.
FIG. 2.
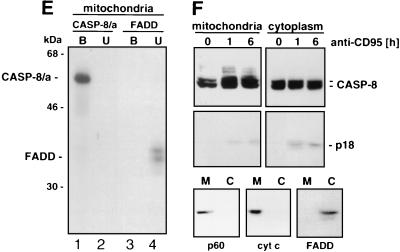
Caspase 8 colocalizes with and binds to mitochondria. (A and B) The breast carcinoma cell line MCF7-Fas was double stained for caspase 8 (anti-caspase 8 C5, FITC labeled; left column) and mitochondria (antimitochondrial antigen, second antibody, PE labeled; center column) (A) or for caspase 8 and ER (anti-PDI antibody, second antibody, PE labeled) (B). The right column presents the overlay of the two stainings. (C and D) HaCat keratinocytes (C) and HepG2 hepatocarcinoma cells (D) were double stained for caspase 8 and mitochondria as for panel A. Bar, 10 μm. (E and F) Biochemical analysis of the subcellular localization of caspase 8. (E) In vitro-translated [35S]caspase-8/a (CASP-8/a) and [35S]FADD were incubated with purified mitochondria, and the amount of bound (B) and unbound (U) in vitro-translated material was determined by SDS-PAGE (12% gel) and subsequent autoradiography. The migration positions of caspase 8 and FADD are indicated. (F) Subcellular fractionation of MCF7-Fas cells. MCF7-Fas cells (5 × 107) were subjected to subcellular fractionation into mitochondria and cytoplasm after treatment with anti-CD95. The Western blot was developed with anti-caspase 8 MAb C15. The migration positions of caspase 8/a and 8/b and the caspase 8 active subunit p18 are indicated. To assess the purity of the mitochondrial (M) and cytoplasmic (C) fractions, a Western blot was developed with antibodies directed against mitochondrial marker proteins p60 (recognized by the antimitochondrial antibody also used for immunofluorescence), cytochrome c (cyt c), and as a cytoplasmic marker FADD.
The mitochondrial localization of caspase 8 was also found in MCF7 cells that did not overexpress CD95, indicating that the mitochondrial localization of caspase 8 was not caused by overexpression of CD95 (data not shown). MCF7 cells have been shown to be caspase 3 deficient due to a deletion in the caspase 3 gene (23). To exclude that the mitochondrial localization of caspase 8 was caused by the absence of caspase 3, a putative substrate of caspase 8, we tested MCF7 cells reconstituted with caspase 3 (23). Again caspase 8 was found to colocalize with mitochondria, excluding any effect due to the absence of caspase 3 (data not shown).
To determine whether procaspase 8 was also associated with mitochondria in cells from other tissues, a human keratinocyte cell line, HaCat, and a hepatocellular carcinoma cell line, HepG2, were double stained for caspase 8 and mitochondria. In both cell lines, a significant part of the mitochondria was positive for procaspase 8 (Fig. 2C and D). However, cytoplasmic staining for caspase 8 could also be observed. Thus, also in cell lines derived from other tissues, significant amounts of procaspase 8 were bound to mitochondria.
To confirm these staining data biochemically, we tested mitochondria for in vitro binding of 35S-labeled caspase 8/a (Fig. 2E). Caspase 8 had a significant affinity for isolated mitochondria (Fig. 2E, lane 1). In contrast, no binding of 35S-labeled FADD (which was taken as a DED-containing control) to mitochondria was found (Fig. 2E, lane 3). Furthermore, we tested whether endogenous caspase 8 was found attached to mitochondria of MCF7-Fas cells during CD95-mediated apoptosis (Fig. 2F). In MCF7 cells, about half of the cellular procaspase 8 was found to be associated with mitochondria after isolation. That did also not change during CD95-mediated apoptosis. Consistent with the staining data, most of active caspase 8 formed in this assay was found in the cytoplasmic fraction. We have previously shown that prolonged treatment of MCF7-Fas cells with anti-CD95 results in complete activation of all cellular caspase 8 (40). The purity of the mitochondrial preparation was confirmed by Western blot analysis identifying the p60 mitochondrial protein recognized by the antimitochondrial MAb (used for immunofluorescence; see Materials and Methods) and cytochrome c, both in the mitochondrial fraction, whereas FADD was exclusively detected in the cytoplasmic fraction (Fig. 2F). To determine whether association of caspase 8 with mitochondria is a general phenomenon, other cells were tested in the same way. We found various amounts of procaspase 8 associated with mitochondria in different cell lines (data not shown). Taken together, our data indicate that in MCF7 cells a significant amount of procaspase 8 is localized at the mitochondria and that this phenomenon is also found in other cells.
Active caspase 8 translocates from mitochondria to plectin.
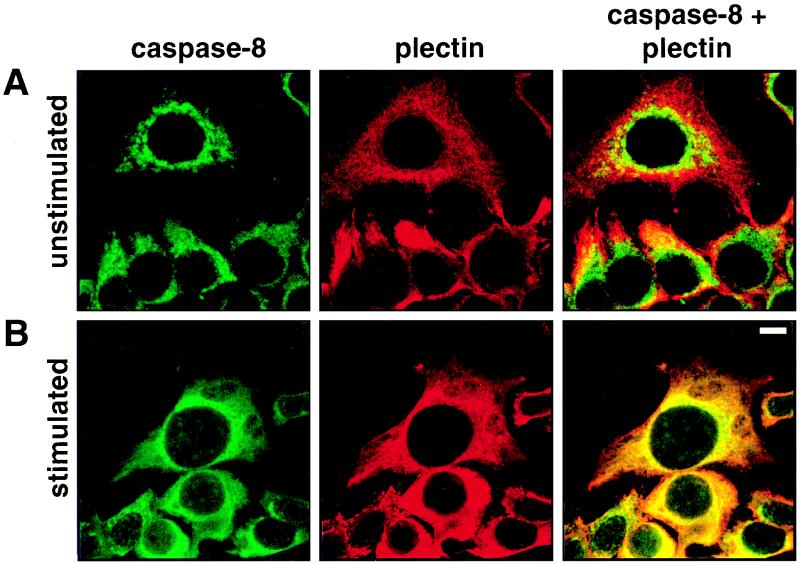
After triggering of CD95, active caspase 8 subunits redistributed into a cytoplasmic meshwork (Fig. 1B-b) similar to that reported for the cytoskeletal protein plectin (71). This staining pattern was significantly different from those of the three major cytoplasmic filament systems of MCF7 cells, i.e., microfilaments, microtubules, and intermediate-sized filaments consisting of CK8 and CK18 (reference 42 and data not shown). To investigate whether active subunits of caspase 8 and plectin indeed colocalized, we performed a double staining with the anti-caspase 8 MAb C5 and a guinea pig anti-plectin-C antiserum (Fig. 3). In unstimulated cells, the mitochondrial staining of caspase 8 was clearly distinct from the antiplectin staining (Fig. 3A). Hardly any overlap was observed, as indicated by the green and red fluorescence signals in the merged images. The staining changed dramatically when CD95 was triggered by treating the cells with the agonistic antibody anti-CD95 and the cells were double stained for both proteins (Fig. 3B). Active caspase 8 subunits had redistributed, and the staining patterns of caspase 8 and plectin were essentially superimposed, although some perinuclear staining of rods and granules corresponding to caspase 8 associated with mitochondria was still seen. Thus, our immunofluorescence microscopy data show for the first time translocation of endogenous active caspase subunits from mitochondria to a putative substrate. These data suggest that the cytolinker plectin (11, 63, 71) may associate with and be a substrate for activated caspase 8.
FIG. 3.
Colocalization of caspase 8 with plectin during CD95-mediated apoptosis. Untreated (A) and anti-CD95-treated (2 μg/ml anti-CD95, 16 h) (B) were double stained for caspase 8 (left column) and plectin (center column). The right column presents the overlay of the two stainings. Panels show representative midsections through the cells. Bar, 10 μm.
Plectin is specifically cleaved by caspases in vivo.
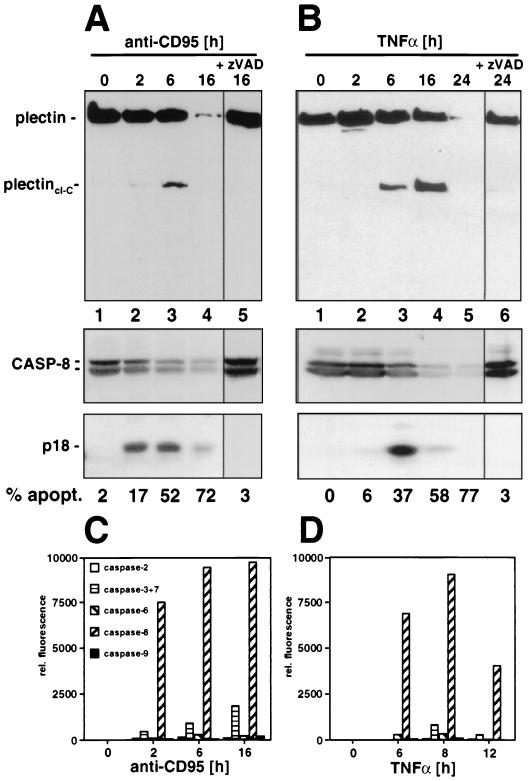
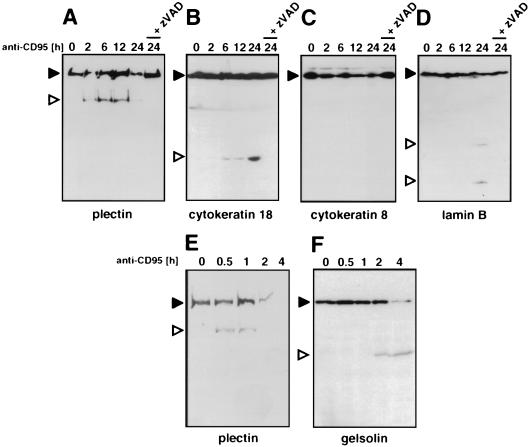
To test whether plectin was an in vivo target for cleavage by caspases during death receptor-mediated apoptosis, cytoskeletal preparations of CD95-stimulated MCF7-Fas cells were subjected to a Western blot analysis using the anti-plectin-C serum (Fig. 4A). After 6 h, we observed a prominent cleavage fragment of about 200 kDa corresponding to the C-terminal half of plectin (plectincl-C) (Fig. 4A, lane 3) which was first detectable after 2 h (lane 2). This cleavage was preceded by appearance of active caspase 8 subunits (lane 2). After 16 h of incubation with anti-CD95, most of plectin was cleaved and the 200-kDa subfragment was further degraded, indicating that it was unstable in that state in these cells (lane 4). Cleavage of both caspase 8 and plectin was blocked by addition of the broad-spectrum caspase inhibitor zVAD-fmk (Fig. 5A, lane 5) or the caspase 8 peptide inhibitor zIETD-fmk (data not shown). Similar results were obtained when cells were treated with TNF-α (Fig. 4B), suggesting that cleavage of plectin is a general phenomenon in death receptor-induced apoptosis. To determine the role of caspase 8 in apoptosis in these cells, we determined the kinetics of activation of the effector caspases 2, 3, 6, 7, 8, and 9 after stimulation with anti-CD95 or TNF-α (Fig. 4C and D), using fluorogenic peptide substrates. In both forms of apoptosis, the most prominent caspase activated was caspase 8, consistent with its cleavage kinetics indicating that caspase 8 was cleaving plectin during death receptor-mediated apoptosis.
FIG. 4.
Cleavage of plectin during death receptor-mediated apoptosis. (A) MCF7-Fas cells were treated with anti-CD95 (2 μg/ml) for the indicated time periods. Plectin was enriched as described in Materials and Methods. For caspase 8 detection, Triton X-100 cell lysates were prepared (see Materials and Methods). Plectin and caspase 8 cleavage was followed by Western blotting using MAb C15 and the anti-plectin-C antibody. For inhibition of caspase 8 activation, cells were incubated for 30 min with 20 μM zVAD-fmk prior to addition of anti-CD95 (2 μg/ml). Migration positions of plectin, caspase 8 (CASP-8), and the caspase 8 active subunit p18 are shown. Apoptosis was quantified using CELLocate coverslips (see Materials and Methods). (B) As for panel A except that cells were treated with TNF-α (20 ng/ml) and CHX (1 μg/ml). (C and D) Caspase activities in cellular lysates of cells treated with anti-CD95 (2 μg/ml) or TNF-α (20 ng/ml) and CHX (1 μg/ml) as in panel B were determined as described in Materials and Methods. Note that in addition to early activation of caspase 8 during CD95-mediated apoptosis (C), a late activation of caspase 7 was detectable, consistent with an earlier report (58).
FIG. 5.
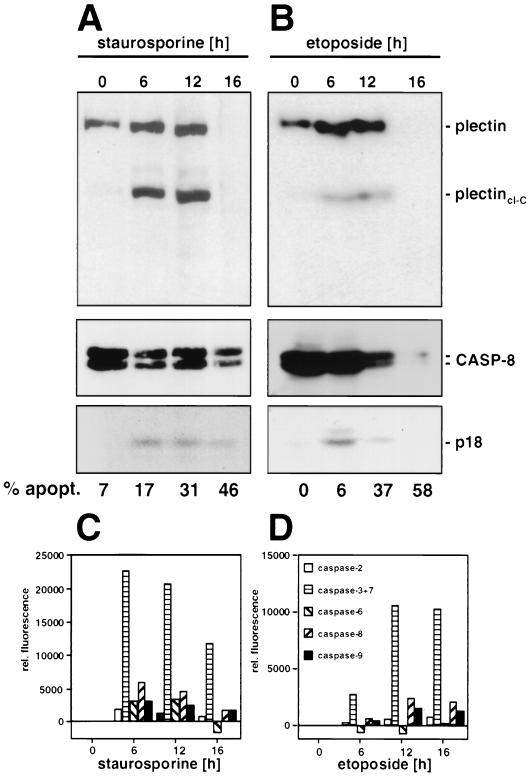
Cleavage of plectin during drug-induced apoptosis. Jurkat cells were incubated with staurosporine (1 μM) (A) or etoposide (20 μg/ml) (B) for the indicated time periods. Analysis of plectin and caspase 8 cleavage fragments was done as described for Fig. 4A and B; quantification of the apoptotic cells was done as described in Materials and Methods. (C and D) Caspase activities during drug-induced apoptosis, determined as described in the legend for Fig. 4C and D.
To determine whether cleavage of plectin also occurs during other forms of apoptosis, we treated Jurkat cells with the broad-spectrum protein serine/threonine kinase inhibitor staurosporine and the topoisomerase II inhibitor etoposide. Both reagents have been shown to induce apoptosis in many cells lines (for reviews, see references 6 and 26). Both staurosporine and etoposide induced apoptosis in Jurkat cells (Fig. 5A and B). Again plectin cleavage fragments of about the same size (200 kDa) were detected, and after 16 h of treatment plectin was completely degraded. However, in this case apoptosis was accompanied by only weak processing of caspase 8, suggesting that caspase 8 may not be the main plectin-cleaving activity during drug-induced apoptosis but instead its activation represents a secondary event. This finding was confirmed when the activities of other effector caspases were determined in this experiment (Fig. 5C and D). Our data are consistent with a recent report which demonstrated that although activation of caspase 8 is essential for death receptor-mediated apoptosis, activation of caspase 8 during drug-induced apoptosis is a secondary event (3). However, it is interesting that also in this experiment only one plectin cleavage fragment was found when the anti-plectin-C antiserum was used for Western blotting (Fig. 5A and B). The fragment was indistinguishable in size from the one generated during death receptor-mediated apoptosis. Taken together, all data suggested that cleavage of plectin is a general feature of many forms of apoptosis and that during death receptor-mediated apoptosis, caspase 8 is the most likely candidate for being the plectin-cleaving caspase.
Caspase 8 is the proteolytical activity that cleaves plectin in the center of the molecule during CD95-mediated apoptosis.
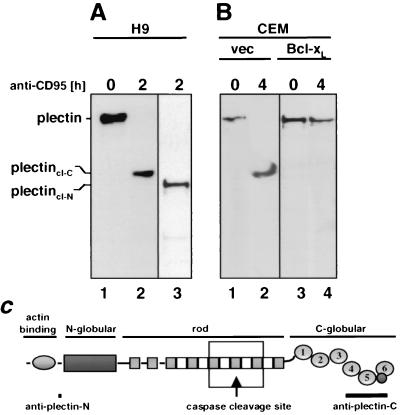
Cleavage of plectin by caspases during CD95-mediated apoptosis was not restricted to MCF7-Fas cells, as revealed by analysis of a number of cell lines after induction of apoptosis. In the T-lymphoma cell line H9, plectin was completely cleaved, also generating a C-terminal fragment of about 200 kDa (Fig. 6A, lane 2). To determine whether plectin was cleaved only at one site, an antiserum specific for the N terminus of plectin was generated. Using this antibody, again only one plectin cleavage fragment (plectin-cl-N) was detected migrating slightly faster than the C-terminal fragment (Fig. 6A, lane 3). These data suggest that during CD95-mediated apoptosis plectin was cleaved by a caspase approximately in the center of the molecule, generating two large fragments similar in size. Activation of caspase 8 in H9 cells occurs early after CD95 triggering, since these cells activate caspase 8 at the DISC (39). Complete cleavage of plectin in these cells coincided with activation of caspase 8 (data not shown). In CEM cells, however, most of caspase 8 activation requires the apoptogenic activity of mitochondria, resulting in a significantly delayed activation of caspase 8 (55). Therefore, complete cleavage of plectin in CEM cells occurred about 2 h later than in H9 cells (Fig. 6B, lane 2), and both activation of caspase 8 and cleavage of plectin were inhibited by overexpression of Bcl-xL (Fig. 6B, lane 4, and reference 56). Complete plectin cleavage was also detected in other cell lines, i.e., the T-lymphoma cell line Jurkat, the B-lymphoblastoid cell line SKW6.4, and the Burkitt's lymphoma cell line BJAB (data not shown), indicating that plectin cleavage is a general feature of CD95-mediated apoptosis in cells of both lymphoid and nonlymphoid origin.
FIG. 6.
Plectin is cleaved by caspase 8 in lymphoid cells. (A) The T-lymphoma cell line H9 was treated with anti-CD95 (2 μg/ml) for the indicated periods of time. The Western blot was developed with anti-plectin-C (lanes 1 and 2) and anti-plectin-N (lane 3) antisera. Migration positions of plectin, of plectincl-C, and of plectincl-N are indicated. (B) The T-lymphoma cell line CEM transfected with vector (vec) or Bcl-xL was treated with anti-CD95 (2 μg/ml) for the indicated times, and the Western blotting for plectin was performed as for panel A. (C) Locations of the putative caspase cleavage site and the antiplectin antiserum recognition sites within the plectin molecule. The box indicates the region of the recombinant plectin fragment used in experiments shown in Fig. 7C to E.
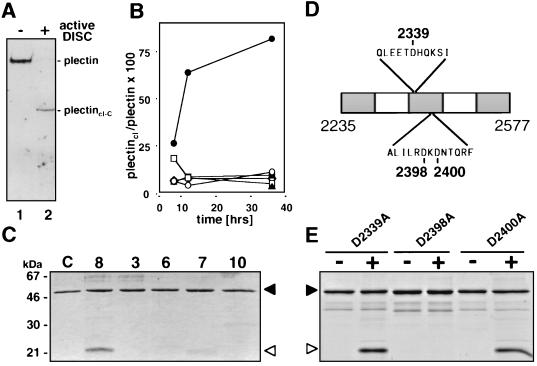
To test whether caspase 8 was the caspase that cleaves plectin, an enriched plectin preparation was incubated with active caspase 8 generated by the DISC (Fig. 7A). This resulted in the formation of a C-terminal plectin fragment of the same size as the one generated in vivo in all tested cells after CD95 triggering (e.g., plectincl-C in Fig. 6A, lane 2) suggesting that caspase 8 was the caspase that cleaved plectin at one single site in the center of the molecule.
FIG. 7.
Plectin is directly cleaved by caspase 8 in the center of the molecule. (A) Active DISC prepared from SKW6.4 cells as described in Materials and Methods was added to an IF preparation obtained from untreated MCF7-Fas cells, incubated overnight at 4°C, and immunoblotted with the anti-plectin-C antiserum. Migration positions of full-length plectin and plectincl-C are indicated. (B) In vitro cleavage of plectin by recombinant caspases. IF preparations obtained from untreated MCF7-Fas cells were incubated with recombinant caspases (active concentration, 10 μM each) for 8 h, 12 h, and 36 h. The Western blot was developed with the anti-plectin C antiserum. Migration positions of plectin and plectincl-C were identical to those in Fig. 5A and B, lanes 2. The densitometric analysis of this Western blot is shown as ratio plectincl/plectin. Data points represent control (▴), caspase 3 (○), caspase 6 (□), caspase 7 (■), caspase 8 (●), and caspase 10 (◊). (C) In vitro cleavage of a recombinant plectin fragment encompassing the putative cleavage site for caspase 8 with recombinant caspases 3, 6, 7, 8, and 10 (active concentration, 40 μM each) or with cleavage buffer (lane C). Proteins were analyzed by SDS-PAGE and Coomassie brilliant blue staining. Edman degradation showed that the 22-kDa fragment generated by caspase 8 represents the N-terminal half of the plectin fragment. The C-terminal half was not found likely due to secondary proteolytic degradation. (D) Schematic representation of the rat plectin fragment covering the part of the plectin rod containing potential caspase cleavage sites. Amino acid positions of the aspartic acid residues that were replaced by alanine are indicated. (E) In vitro cleavage of recombinant plectin fragment mutants. Mutant proteins were incubated in the absence (−) or presence (+) of active caspase 8 for 24 h and analyzed as for panel C. Migration positions of the full-length protein (filled arrowheads) and of the cleavage fragment (open arrowheads) are indicated in panels C and E.
In an attempt to determine the caspase cleavage site by Edman degradation, plectin was enriched from stimulated MCF7-Fas cells to amounts stainable with Coomassie blue (data not shown). Consistent with the Western blot data in this assay, after CD95 triggering we detected two plectin fragments of about 200 kDa, the larger of which corresponded to the C-terminal half (plectincl-C in Fig. 6A, lane 2). However, repeated N-terminal sequencing of this Coomassie blue-stained fragment did not result in sequence information. Low-molecular-weight contaminating protein bands of the same IF preparation could be readily sequenced, suggesting that a modification at the P0 position of the cleavage site had occurred.
Most known apoptosis death substrates are cleaved by caspases 3, 6, and 7 (for a review see reference 64). Many of the death substrates require active caspase 3 for cleavage. Since plectin was cleaved in both caspase 3-expressing and caspase 3-negative MCF7 cells, this effector caspase appeared not to be essential for its degradation. To determine which of the known effector caspases had the highest activity in cleaving plectin in vitro, a plectin-enriched IF preparation was treated with various recombinant active caspases (Fig. 7B). Cleavage kinetics revealed that caspase 8 was the only caspase cleaving plectin efficiently, again generating a C-terminal fragment of about 200 kDa (data not shown).
So far all in vitro plectin cleavage experiments had been performed with plectin-enriched IF preparations. Hence, we could not exclude that caspase 8 did not directly cleave plectin but rather activated a proteolytic activity copurified with the IF. To test this, we recombinantly generated a fragment of rat plectin of about 43 kDa covering the last third of the rod domain containing the putative caspase 8 cleavage site. The primary structures of rat and human plectin in these regions are 96.2% identical, with all potential caspase cleavage sites in the cleavage region conserved. After purification of this His6-tagged plectin fragment from bacteria, the protein was treated with the same recombinant active caspases as used before (Fig. 7B). Again recombinant active caspase 8 was by far the most active caspase to cleave this plectin fragment, indicating that caspase 8 can cleave plectin directly (Fig. 7C). This cleavage was inhibited by addition of zVAD-fmk (data not shown). The observed cleavage was incomplete, likely due to the intrinsic aggregating activity of the plectin rod fragment. Therefore, neither increasing the amount nor repeated addition of active caspase 8 resulted in higher cleavage efficiency (data not shown).
To finally identify the caspase 8 cleavage site within plectin, we individually replaced all three aspartic acid residues in the region of the recombinant plectin fragment corresponding to the cleavage area of full-length plectin by alanine (Fig. 7D). All mutants were subjected to cleavage by active caspase 8 (Fig. 7E). Mutants D2339A and D2400A were readily cleaved, whereas mutant D2398A was completely resistant to cleavage by caspase 8. The data identify Asp 2398 within an ILRD sequence as the caspase 8 cleavage site in the plectin molecule. This position corresponds to position Asp 2395 in human plectin (GenBank accession number Z54367) (32). An ILRD motif represents a very unusual cleavage site for caspase 8. The optimal caspase 8 cleavage site was recently determined to be P4(ADEILPV) P3(E) P2(ITV) P1(D) (66). Plectin contains one such site, EETD, with the P1 position located at amino acid 2336 of the plectin primary structure. However, replacement of aspartic acid at this cleavage site (D2339A in Fig. 7E) did not reduce cleavage of the plectin fragment by caspase 8. We therefore conclude that caspase 8 specifically cleaves plectin at the ILRD motif in the center of the molecule.
Degradation of plectin precedes degradation of CK18, lamin, and gelsolin.
The morphological changes during apoptosis require reorganization of various components of the cytoskeleton such as IF. The major cytokeratins expressed in MCF7 cells are CK8 and CK18 (42). CK18 belongs to the type I keratins and is cleaved by caspases during various forms of apoptosis (7, 29). This cleavage is required for gross reorganization of the cellular structure (7). CK8, like all type II keratins, does not contain a caspase cleavage site and is resistant to degradation during apoptosis (7). We tested whether CK18 is also cleaved by caspases during CD95-mediated apoptosis in MCF7-Fas cells. Again CK18 but not CK8 was degraded (Fig. 8B and C). In line with previous reports, cleavage of CK18 yielded an N-terminal fragment of 29 kDa. The cleavage was caused by caspases, since it could be inhibited by the broad-spectrum caspase inhibitor zVAD-fmk (Fig. 8B). Comparison between the cleavage of CK18 and plectin showed that initial cleavage of plectin occurs about 4 h earlier than that of CK18 (Fig. 8A and B), consistent with caspase 8 being the cleaving activity. Immunoblotting revealed that the nuclear IF protein lamin B was also cleaved, although much later, with major cleavage products of 21 and 28 kDa accumulating by 24 h (Fig. 8D). Another major cytoskeletal caspase substrate is gelsolin, a microfilament-associated protein that regulates actin polymerization. It was shown to be cleaved by caspase 3 very early after CD95 stimulation (28). Since MCF7 cells are caspase 3 deficient, we compared the kinetics of gelsolin and plectin proteolytical degradation in Jurkat T cells after triggering cells with anti-CD95 (Fig. 8E and F). In this system cleavage of plectin significantly preceded degradation of gelsolin, again supporting the view that cleavage of plectin is one of the earliest cleavage events during CD95-mediated apoptosis.
FIG. 8.
Cleavage of plectin precedes cleavage of CK18, lamin B, and gelsolin during CD95-mediated apoptosis. (A) MCF7-Fas cells were stimulated with anti-CD95 (2 μg/ml) for the indicated time periods or treated with 20 μM zVAD-fmk prior to addition of 2 μg of anti-CD95 per ml. The corresponding IF preparations were analyzed by Western blotting using the anti-plectin-C antiserum. (B to D) Cells were treated as for panel A, but Triton X-100 cell lysates were prepared (see Materials and Methods). Western blots were developed with anti-CK 8 (B), anti-CK 18 (C), or anti-lamin B (D) antibody. (E to F) Jurkat T cells were stimulated with anti-CD95 (2 μg/ml) for the indicated time periods. Plectin IF preparations (E) or Triton X-100 cell lysates (F) were analyzed for plectin or gelsolin. Migration positions of the full-length proteins are indicated by filled arrowheads; migration positions of the cleavage products are marked by open arrowheads. Molecular masses of the cleavage products: plectin, 200 kDa; CK18, 28 kDa; lamin B, 28 and 21 kDa; gelsolin, 48 kDa.
Plectin is required for actin reorganization.
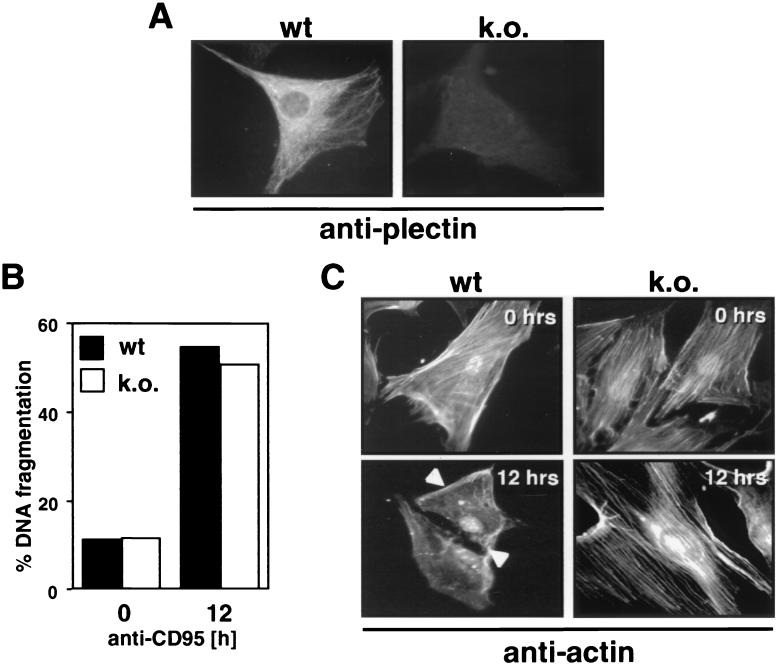
Establishing cell lines overexpressing noncleavable plectin has turned out to be extraordinarily difficult due to the abundance and enormous size of the protein (data not shown). To determine whether plectin plays a role in the execution of apoptosis in general, we therefore tested primary fibroblasts from plectin-deficient mice (Fig. 9A) (2). To induce expression of CD95, these cells (data not shown) were treated with IFN-γ for 36 h, rendering them sensitive to apoptosis induction through CD95. Wild-type and plectin-deficient fibroblasts were equally active in fragmenting their DNA (as determined in cleavage kinetics, of which one representative time point is shown), indicating that plectin did not play a role in the pathway that leads to activation of endonucleases (Fig. 9B). However, since plectin binds actin and reorganization of actin stress fibers has been implicated in apoptosis, we stained CD95-treated cells for actin (Fig. 9C). In fibroblasts from wild-type mice, actin stress fibers were visible. After induction of apoptosis for 12 h, however, all of the actin fibers had disappeared, with only a few membrane ruffles left (Fig. 9C, lower left). The extent of actin stress fibers in fibroblasts from plectin-deficient mice was similar to that in wild-type fibroblasts (Fig. 9C, upper right). However, no reorganization of the actin cytoskeleton was observed after triggering CD95 (Fig. 9C, lower right). These data indicate that during CD95-mediated apoptosis, plectin plays an active role in actin depolymerization.
FIG. 9.
Plectin is required for actin reorganization during CD95-mediated apoptosis. (A) Staining of primary fibroblasts from wild-type (wt) and plectin-deficient (knockout [k.o.]) mice with the anti-plectin-C antiserum using immunofluorescence microscopy. (B) Sensitivity of IFN-γ-treated primary fibroblasts to CD95-mediated DNA fragmentation. Cells were treated with IFN-γ for 36 h, and apoptosis was induced by using anti-CD95 antibody Jo2 (10 μg/ml; Pharmingen), protein A (10 ng/ml), and CHX (1 μg/ml). (C) Staining of actin in primary fibroblasts after incubation with anti-CD95 for 0 or 12 h. Arrowheads point to membrane ruffles.
DISCUSSION
The list of caspase targets that are specifically cleaved during apoptosis is growing rapidly (for a review, see reference 64). A number of approaches have been used to identify death substrates and their corresponding cleaving caspase.
(i) Specific antibodies were used in Western blotting experiments to monitor the cleavage of putative caspase substrates. However, this method does not allow one to identify specific caspases due to the lack of caspase selective inhibitors. None of the available peptide-based caspase inhibitors are specific enough to block the activity of one caspase only at the concentrations usually applied (15).
(ii) Most caspases that cleave specific substrates were identified in vitro using recombinant caspases. However, in such assays caspases and/or substrates are used at concentrations much higher than those found intracellularly. Furthermore, the generation of recombinant active caspases requires removal of the prodomains, which possibly have a regulating function.
(iii) Recently, MCF7 cells were identified to be deficient for caspase 3, allowing one to determine whether caspase 3 was essential for cleavage of several death substrates (22). Death substrates that were shown to require caspase 3 for cleavage include cytoskeletal proteins such as α-fodrin (22), topoisomerase II (54), or the inhibitor of the endonuclease CAD (DFF40) I-CAD (DFF45) (65). However, these data are not proof that a substrate is cleaved by caspase 3 directly.
The availability of a panel of subunit-specific MAbs against caspase 8 allowed us for the first time to monitor endogenous active caspase subunits from one location inside a cell to one of its putative substrates. Therefore, plectin is the first target of a caspase that has been identified in situ by monitoring the translocation of the active caspase subunits within a cell. These findings were substantiated by biochemical in vivo and in vitro data demonstrating that plectin is a major target for caspases during death receptor-mediated apoptosis and that it can most efficiently be cleaved by caspase 8. Furthermore, the substantial cleavage of plectin occurs very early during death receptor- and drug-mediated apoptosis in all cells tested.
In nonactivated MCF7 cells, most of caspase 8 was found to be associated with mitochondria (Fig. 10), as determined by immunofluorescence microscopy. Our data are therefore in contrast to a previous report demonstrating that caspase 8 has a diffuse cytosolic and nuclear staining pattern (65). However, these data were obtained with overexpressed hemagglutinin epitope-tagged caspase 8, whereas we studied authentic endogenous caspase 8. Our observation was additionally confirmed by two biochemical approaches: (i) caspase 8 bound to isolated mitochondria in an in vitro binding assay; (ii) in a fractionation experiment, the majority of cellular caspase 8 was associated with mitochondria in vivo. In contrast to other caspases (2, 3, and 9) that are present in the intermembrane space of mitochondria in certain tissues (36, 62), caspase 8 is loosely attached with the mitochondrial outer membrane (A. H. Stegh and M. E. Peter, submitted for publication), providing an explanation for the difference in the almost quantitative association of caspase 8 with mitochondria detected by immunofluorescence and the much smaller amount of caspase 8 found on isolated mitochondria in the fractionation experiments, likely caused by a loss of caspase 8 during the isolation procedure. Recent data suggest that caspase 8 binds to a novel mitochondrial DED-containing protein, BAR, through a DED-DED interaction (74). BAR is highly expressed on the surface of mitochondria of MCF7 cells (unpublished data).
FIG. 10.
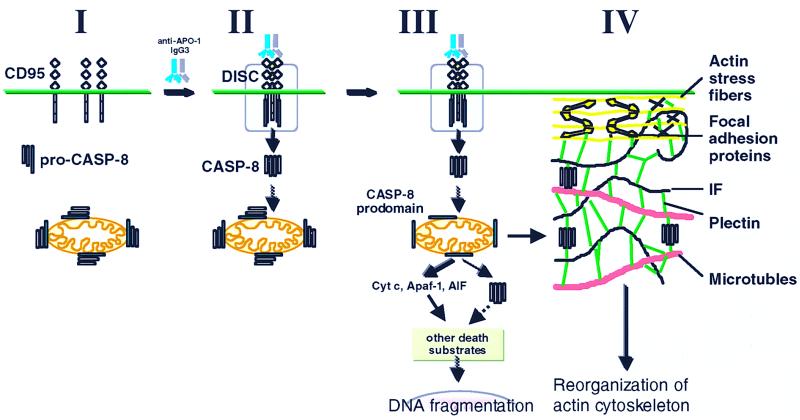
Model of apoptosis signaling in MCF7-Fas cells. MCF7-Fas cells express CD95 on the cell surface. Most procaspase 8 in these cells is localized to mitochondria (I). Upon CD95 triggering, procaspase 8 (pro-CASP-8) is recruited to the DISC and subsequently activated by proteolytical cleavage, resulting in release of active subunits (orange and purple boxes) into the cytoplasm (II) (39). Recent data indicate that active caspase 8 directly and specifically activates procaspase 8 on mitochondria (III) (unpublished data). In contrast to the prodomain (green box) that remains bound at the mitochondria, the active caspase 8 subunits translocate to plectin and cleave this cytoskeleton-associated regulatory protein (IV). This cleavage may be required for the reorganization of the actin cytoskeleton typical for apoptosis by an unknown mechanism. The DISC is shown in a simplified form without FADD. Cyt c, cytochrome c.
Testing a number of different cell lines, we did not find a correlation between the varying caspase 8 association with mitochondria and the apoptosis cell type (type I or II [56]). However, binding of caspase 8 was much stronger when mitochondria were isolated from MCF7-Fas cells overexpressing Bcl-xL, suggesting that Bcl-xL affects the binding activity of mitochondria for caspase 8 (unpublished data). This again is consistent with a caspase 8-BAR association since BAR's activity to bind procaspase 8 is regulated by Bcl-xL (74). Future studies are needed to determine how active caspase 8 subunits, generated at the DISC, activate caspase 8 bound at the mitochondria (Fig. 10) and whether this process involves the new adapter protein.
We have previously shown that overexpression of Bcl-xL in MCF7-Fas cells can protect these cells from CD95-mediated apoptosis without inhibiting activation of caspase 8 (39). In the light of our present data, the question arises whether in these cells plectin is still cleaved and whether cytoskeletal changes can be observed. We recently found that overexpression of Bcl-xL in these cells gives their mitochondria the activity to not only bind procaspase 8 but also efficiently sequester active caspase 8 generated at the DISC, preventing it from reaching and cleaving its targets such as plectin or BID (Stegh and Peter, submitted).
Plectin belongs to a multigene family termed plakins (13, 51), for which the name “cytolinkers” has recently been suggested since these proteins have a common structural principle and are involved in cross-linking various cytoskeletal components (71). Four other members of this family are known: desmoplakin, bullous pemphigoid antigen 1, envoplakin, and periplakin (51, 52). Except for periplakin, which is expressed only in epithelial cells, none of these proteins contains a potential caspase 8 consensus site or the caspase 8 cleavage site ILRD used in plectin, suggesting that cleavage of plectin by caspase 8 is unique for this cytolinker.
The fact that cleavage of plectin was first detected in MCF7 cells, which are deficient of caspase 3, demonstrated that this cleavage was independent of caspase 3. In addition, caspase 8 active subunits also translocated to plectin in TNF-α-treated apoptosing MCF7 cells, as confirmed by colocalization experiments using confocal immunofluorescence microscopy (data not shown). In addition, during TNF-α-induced apoptosis, plectin was cleaved by caspases in vivo. It has recently been shown that in MCF7 cells treated with TNF-α, only caspase 8, not caspase 1, 2, 5, 7, 9, or 10, becomes activated (22), supporting the unique role for caspase 8 in the cleavage of plectin. Our data confirm these findings and show that caspase 8, which so far has been shown to function only as an initiator caspase cleaving other caspases such as caspase 3 (61), can also act as an effector caspase directly cleaving a structural protein.
Is plectin a specific caspase 8 substrate? During death receptor-mediated apoptosis of MCF7 cells, the only significant caspase activity that we detected was caspase 8. However, these cells are deficient for caspase 3 (23). During CD95-mediated apoptosis of caspase 3-expressing Jurkat cells, we found that prior to massive late activation of caspase 3 at the time of first detection of the plectin cleavage fragments, the only caspase activity detected was caspase 8 (data not shown and reference 55). Together, the finding that plectin could very efficiently be cleaved by the DISC (at 4°C) that contains only caspase 8 and the fact that no effector caspase other than caspase 8 could cleave a plectin fragment containing the cleavage site suggest that during death receptor-mediated apoptosis, plectin is a specific caspase 8 substrate. The very early cleavage of plectin during apoptosis is consistent with that conclusion.
Plectin is one of the largest polypeptides known (4,684 amino acids [32]). It is ubiquitously expressed in many cell types from skin to heart muscle (for a review, see reference 71). Through its terminal protein interaction domains, it has the unique ability to cross-link with one another important constituents of the cytoskeleton such as myosin II, actin, IF, microtubules, and focal contact proteins (for reviews, see references 13, 14, and 71). In accordance with its central role in maintaining the structural integrity of the cell, plectin is very abundant, corresponding to up to 1% of the total cellular protein (21). A number of cytoskeletal components have been shown to be cleaved by caspases during apoptosis (for reviews, see references 50 and 64). Plectin binds to a number of these components including cytokeratins (12), which are also substrates for caspases (7, 29). MCF7 cells mainly express CK8 and CK18. Whereas CK8 was resistant to degradation, CK18 was cleaved by caspases during apoptosis induced by etoposide and daunomycin (7) or anisomycin (29). Caspase 6 was identified as the caspase cleaving CK18 at the cleavage site VEVD (7), a site frequently found also in other type I cytokeratins. However, cleavage of plectin occurred about 4 h earlier than that of CK18 and about 20 h earlier than that of lamin B, consistent with the progressive and ordered activation of different caspases during the course of apoptosis and supporting a model of a hierarchical cleavage of cytoskeletal proteins.
Due to plectin's abundance, size, and multiple splice variants, each with potentially different binding activities (71), we were unable to generate cells harboring mutated full-length plectin in order to study the effect of its cleavage on apoptosis directly. In addition, overexpression of full-length plectin in plectin-deficient fibroblasts was shown to result in collapse of the intermediate and the microfilament systems (reference 1 and data not shown). However, naturally occurring mutations in the plectin gene are the cause for epidermolysis bullosa simplex with muscular dystrophy, characterized by skin blistering and muscle degeneration (37, 57). This indicates that intact plectin is needed to support cellular stability against mechanical stress. In addition, recent results from gene inactivation studies in mice confirm that in the absence of plectin various cells and tissues are extremely fragile, probably being the cause the early postnatal death of plectin-null mice (2). These data underscore the central role of plectin in the establishment of a functional cytoarchitecture. Its ability to integrate the stress-resistant and dynamic filament systems, i.e., IF with microtubules and the actomyosin system (63), is seemingly vital for cellular integrity.
Apoptosis is characterized by dramatic structural changes. It has been shown that IF proteins such as keratins undergo major structural reorganization during apoptosis, and these changes are very likely involved in the profound morphological alterations observed during apoptosis (7). Thus, cleavage of plectin, a key factor integrating all of these structural elements (71), prior to degradation of other cytoskeletal proteins may allow this process to be initiated. Moreover, it has recently been reported that plectin does not function just as a scaffolding protein but also as an active regulator of actin cytoskeleton dynamics (1). We now provide evidence that also structural changes during apoptosis, e.g., reorganization of the actin cytoskeleton, depend on the presence of functional plectin. Apoptosis-dependent cleavage of plectin by far precedes degradation of gelsolin, cleavage of which has recently been reported to occur early during CD95-mediated apoptosis and to be important for the actin cytoskeletal collapse (16). It is tempting to hypothesize that cleavage of plectin is important for the dramatic changes in the actin cytoskeleton during CD95-mediated apoptosis.
Our data show that the apoptosis pathway leading to DNA degradation is distinct from the pathway leading to depolymerization of actin since DNA fragmentation occurred normally in plectin-deficient cells. Overexpression of the N-terminal actin binding domain (ABD) of plectin alone in plectin-deficient fibroblasts significantly reduced the number of actin stress fibers. In fact, the intracellular distribution of depolymerized actin in the ABD-transfected cells (1) looked very similar to the actin staining pattern seen in apoptosing wild-type fibroblasts (Fig. 9C), suggesting that plectin fragments such as the ones generated by caspase 8 cleavage might play an active role in this process (1). However, overexpression of full-length plectin had similar effects (data not shown), precluding an interpretation of our data in this way. We hypothesize that the early cleavage of plectin by caspase 8 in the center of the molecule may be the first trigger to disintegrate the stable extended scaffold of the cells, subsequently initiating the dynamic structural reorganization typical for apoptosis.
Recently, is was shown that caspase 8 not only functions in apoptosis but also is activated during and required for T-cell activation (27), demonstrating the versatility of this enzyme. Since cytoskeletal rearrangements are also found in other processes such as cell migration or mitosis, it is conceivable that caspase 8 cleavage may be required not only for apoptosis but also to regulate other cellular processes. In fact, a recent study demonstrated that caspase activity is required for spreading of NIH 3T3 cells on collagen-coated plates (69) that showed no signs of apoptosis. The authors of this report did not identify the caspase responsible but could exclude caspase 3. Our initial identification of plectin as a major early caspase 8 substrate may provide the basis for explaining the observed effects since plectin is a major component of hemidesmosomes, submembrane structures that have been shown to transduce signals required for cell spreading (69). Future studies will be aimed at determining the in vivo role of the cleavage of plectin in apoptosis and apoptosis-independent cellular processes.
ACKNOWLEDGMENTS
We thank U. Matiba and D. Süss for excellent technical assistance, H. Heid for microsequencing fragments of recombinant plectin, and Branislav Nikolic for generating plectin cDNA expression plasmids and isolation of recombinant proteins. The MCF7-Fas and MCF7-Fas-Bcl-xL cells and the MCF7-caspase 3 cells were generous gifts from M. Jäättelä and A. Porter, respectively. We are grateful to G. Salvesen for providing active recombinant caspases, to H. Söling for providing the anti-PDI antibody, and to P. Lichter for critically reading the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Li 406/3-1 and PE 653/1-2), the Bundesministerium für Forschung und Technologie, the Tumor Center Heidelberg/Mannheim, the Deutsche Leukämieforschungshilfe, and the Austrian Science Foundation (PI2389 and SFB006/661). A.H.S. was supported by a stipend from the Boehringer Ingelheim Fonds.
REFERENCES
- 1.Andrä K, Nikolic B, Stöcher M, Drenckhahn D, Wiche G. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Genes Dev. 1998;12:3442–3451. doi: 10.1101/gad.12.21.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrä K, Lassmann H, Bittner R, Shorny S, Fassler R, Propst F, Wiche G. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev. 1997;23:3143–3156. doi: 10.1101/gad.11.23.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boesen-de Cock J G, Tepper A D, de Vries E, van Blitterswijk W J, Borst J. Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and gamma-radiation downstream from caspase-8 activation. J Biol Chem. 1999;274:14255–14261. doi: 10.1074/jbc.274.20.14255. [DOI] [PubMed] [Google Scholar]
- 4.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 5.Boldin M P, Varfolomeev E E, Pancer Z, Mett I L, Camonis J H, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 6.Caponigro F, French R C, Kaye S B. Protein kinase C: a worthwhile target for anticancer drugs? Anticancer Drugs. 1997;8:26–33. doi: 10.1097/00001813-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Caulín C, Salvesen G S, Oshima R G. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinnaiyan A M, O'Rourke K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes-Alnemri T, Armstrong R C, Krebs J, Srinivasula S M, Wang L, Bullrich F, Fritz L C, Trapani J A, Tomaselli K J, Litwack G, Alnemri E S. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogel M, Lifschitz-Mercer B, Moll R, Kushnir H, Jacob N, Waldherr R, Livoff A, Franke W W, Czernobislky B. Heterogeneity of intermediate filament expression in human testicular seminomas. Differentiation. 1991;46:143–145. doi: 10.1111/j.1432-0436.1990.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 11.Foisner, R., W. Bohn, K. Mannweiler, and G. Wiche. Distribution and ultrastructure of plectin arrays on subclones of rat glioma C6 cells deferring in intermediate filament protein (vimentin) expression. J. Struct. Biol. 115:304–317. [DOI] [PubMed]
- 12.Foisner R, Leichtfried R E, Herrmann H, Small J V, Laurson D, Wiche G. Cytoskeleton-associated plectin in situ localization, in vitro reconstitution, and binding to immobilized intermediate filament proteins. J Cell Biol. 1988;106:723–733. doi: 10.1083/jcb.106.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs E, Yang Y. Crossroads on cytoskeletal highways. Cell. 1999;98:547–550. doi: 10.1016/s0092-8674(00)80041-0. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs E, Cleveland D W. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Calvo M, Peterson E P, Leiting B, Ruel R, Nicholson D W, Thornberry N A. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 16.Geng Y J, Azuma T, Tang J X, Hartwig J H, Muszynski M, Wu Q, Libby P, Kwiatkowski D J. Caspase-3-induced gelsolin fragmentation contributes to actin cytoskeletal collapse, nucleolysis, and apoptosis of vascular smooth muscle cells exposed to proinflammatory cytokines. Eur J Cell Biol. 1998;77:294–302. doi: 10.1016/S0171-9335(98)80088-5. [DOI] [PubMed] [Google Scholar]
- 17.Greidinger E L, Miller D K, Yamin T T, Casciola-Rosen L, Rosen A. Sequential activation of three distinct ICE-like activities in Fas-ligated Jurkat cells. FEBS Lett. 1996;390:299–303. doi: 10.1016/0014-5793(96)00678-3. [DOI] [PubMed] [Google Scholar]
- 18.Gross A, Yin X-M, Eang K, Wei M C, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann H, Hofmann I, Franke W W. Identification of a nonapeptide motif in the vimentin head domain involved in intermediate filament assembly. J Mol Biol. 1992;223:637–650. doi: 10.1016/0022-2836(92)90980-x. [DOI] [PubMed] [Google Scholar]
- 20.Herrmann H, Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J Biol Chem. 1987;262:1320–1325. [PubMed] [Google Scholar]
- 21.Herrmann H, Wiche G. Specific in situ phosphorylation of plectin in detergent-resistant cytoskeletons from cultured Chinese hamster ovary cells. J Biol Chem. 1983;23:14610–14618. [PubMed] [Google Scholar]
- 22.Jänicke R U, Ng P, Sprengart M L, Porter A G. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem. 1998;273:15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- 23.Jänicke R U, Sprengart M L, Wati M R, Porter A G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 24.Jonkman M F. Hereditary skin diseases of hemidesmosomes. J Dermatol Sci. 1999;20:103–121. doi: 10.1016/s0923-1811(99)00017-1. [DOI] [PubMed] [Google Scholar]
- 25.Juo P, Kuo C J, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol. 1998;8:1001–1008. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann S H. Cell death induced by topoisomerase-targeted drugs: more questions than answers. Biochim Biophys Acta. 1998;1400:195–211. doi: 10.1016/s0167-4781(98)00136-5. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy N J, Kataoka T, Tschopp J, Budd R C. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry T J, Kirschner M W, Koths K, Kwiatkowski D J, Williams L T. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 29.Ku N-O, Liao J, Omary M B. Apoptosis generates stable fragments of human type I keratins. J Biol Chem. 1997;272:33197–33203. doi: 10.1074/jbc.272.52.33197. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Zhu H, Xu C J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y, Devin A, Rodriguez Y, Liu Z. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C-G, Maercker C, Castanon M J, Hauptmann R, Wiche G. Human plectin: organization of the gene, sequence analysis, and chromosome localization (8q24) Proc Natl Acad Sci USA. 1996;93:4278–4283. doi: 10.1073/pnas.93.9.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Li P, Widlak P, Zou H, Luo X, Garrard W T, Wang X. The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc Natl Acad Sci USA. 1998;95:8461–8466. doi: 10.1073/pnas.95.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 35.Macer D R, Koch G L. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. J Cell Sci. 1988;91:61–70. doi: 10.1242/jcs.91.1.61. [DOI] [PubMed] [Google Scholar]
- 36.Mancini M, Nicholson D W, Roy S, Thornberry N A, Peterson E P, Casciola-Rosen L A, Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLean W H, Pulkkinen L, Smith F J, Rugg E L, Lane E B, Bullrich F, Burgeson R E, Amano S, Hudson D L, Owaribe K, McGrath J A, McMillan J R, Eady R A, Leigh I M, Christiano A M, Uitto J. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev. 1997;10:1724–1735. doi: 10.1101/gad.10.14.1724. [DOI] [PubMed] [Google Scholar]
- 38.McKinney R M, Spillane J T, Pearce G W. A simple method for determining the labeling efficiency of fluorescein isothiocyanate products. Anal Biochem. 1966;14:421–428. doi: 10.1016/0003-2697(66)90284-3. [DOI] [PubMed] [Google Scholar]
- 39.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medema J P, Scaffidi C, Krammer P H, Peter M E. Bcl-xL acts downstream of caspase-8 activation by the CD95 death-inducing signaling complex. J Biol Chem. 1998;273:3388–3393. doi: 10.1074/jbc.273.6.3388. [DOI] [PubMed] [Google Scholar]
- 41.Mertens C, Kuhn C, Franke W W. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moll R, Francke W, Schiller D L. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 43.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 44.Ng F W, Nguyen M, Kwan T, Branton P E, Nicholson D W, Cromlish J A, Shore G C. p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol. 1997;139:327–338. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;22:229–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 46.Nikolic B, MacNulty E, Mir B, Wiche G. Basic amino acid residue cluster within nuclear targeting sequence motif is essential for cytoplasmic plectin-vimentin network junctions. J Cell Biol. 1996;134:1455–1467. doi: 10.1083/jcb.134.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez D, White E. E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE. J Cell Biol. 1998;141:1255–1266. doi: 10.1083/jcb.141.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peter M E, Scaffidi C, Medema J P, Kischkel F C, Krammer P H. The death receptors. In: Kumar S, editor. Apoptosis: biology and mechanisms. 23. Results and problems in cell differentiation. Heidelberg, Germany: Springer-Verlag; 1998. pp. 25–63. [DOI] [PubMed] [Google Scholar]
- 49.Peter M E, Hellbardt S, Schwartz-Albiez R, Westendorp M O, Moldenhauer G, Grell M, Krammer P H. Cell surface sialylation plays a role in modulating sensitivity towards APO-1-mediated apoptotic cell death. Cell Death Differ. 1995;2:163–171. [PubMed] [Google Scholar]
- 50.Prasad S C, Thraves P J, Kuettel M R, Srinivasarao G Y, Dritschilo A, Soldatenkov V A. Apoptosis-associated proteolysis of vimentin in human prostate epithelial tumor cells. Biochem Biophys Res Commun. 1998;249:332–338. doi: 10.1006/bbrc.1998.9137. [DOI] [PubMed] [Google Scholar]
- 51.Ruhrberg C, Watt F M. The plakin family: versatile organizers of cytoskeletal architecture. Curr Opin Genet. 1997;3:392–397. doi: 10.1016/s0959-437x(97)80154-2. [DOI] [PubMed] [Google Scholar]
- 52.Ruhrberg C, Hajibagheri M A N, Parry D A D, Watt F M. Periplakin, a novel component of cornified envelopes and desmosomes that belongs to the plakin family and forms complexes with envoplakin. J Cell Biol. 1997;139:1835–1849. doi: 10.1083/jcb.139.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 54.Samejima K, Svingen P A, Basi G S, Kottke T, Mesner P W Jr, Stewart L, Durrieu F, Poirier G G, Alnemri E S, Champoux J J, Kaufmann S H, Earnshaw W C. Caspase-mediated cleavage of DNA topoisomerase I at unconventional sites during apoptosis. J Biol Chem. 1999;274:4335–4340. doi: 10.1074/jbc.274.7.4335. [DOI] [PubMed] [Google Scholar]
- 55.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scaffidi C, Medema J P, Krammer P H, Peter M E. FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J Biol Chem. 1997;272:26953–26958. doi: 10.1074/jbc.272.43.26953. [DOI] [PubMed] [Google Scholar]
- 57.Smith F J, Eady R A, Leigh I M, McMillan J R, Rugg E L, Kelsell D P, Bryant S P, Spurr N K, Geddes J F, Kirtschig G, Milana G, de Bono A G, Owaribe K, Wiche G, Pulkkinen L, Uitto J, McLean W H, Lane E B. Plectin deficiency results in muscular dystrophy with epidermolysis bullosa. Nat Genet. 1996;13:450–457. doi: 10.1038/ng0896-450. [DOI] [PubMed] [Google Scholar]
- 58.Srinivasan A, Li F, Wong A, Kodandapani L, Smidt R, Jr, Krebs J F, Fritz L C, Wu J C, Tomaselli K J. Bcl-xL functions downstream of caspase-8 to inhibit Fas- and tumor necrosis factor receptor 1-induced apoptosis of MCF7 breast carcinoma cells. J Biol Chem. 1998;273:4523–4529. doi: 10.1074/jbc.273.8.4523. [DOI] [PubMed] [Google Scholar]
- 59.Stegh A H, Schickling O, Ehret A, Scaffidi C, Peterhänsel C, Hoffmann T G, Grummt I, Krammer P H, Peter M E. DEDD, a novel death effector containing apoptosis-inducing protein targeted to nucleoli. EMBO J. 1998;17:5974–5986. doi: 10.1093/emboj/17.20.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steller H. Mechanisms and genes of cellular suicide. Science. 1985;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 61.Stennicke H R, Jürgensmeier J M, Shin H, Deveraux Q, Wolf B B, Yang X, Zhou Q, Ellerby H M, Ellerby L M, Bredesen D, Green D R, Reed J C, Froelich C J, Salvesen G S. Procaspase-3 is a major physiologic target of caspase-8. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 62.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost M C, Alzari P M, Kroemer G. Mitochondrial release of caspase-2 and -9 during the apoptotic process. J Exp Med. 1999;189:381–393. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Svitkina T M, Verkhovsky A, Borisy G G. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biol. 1996;4:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan X, Wang J Y J. The caspase-Rb connection in cell death. Trends Cell Biol. 1998;8:116–120. doi: 10.1016/s0962-8924(97)01208-7. [DOI] [PubMed] [Google Scholar]
- 65.Tang D, Kidd V J. Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis. J Biol Chem. 1998;273:28549–28552. doi: 10.1074/jbc.273.44.28549. [DOI] [PubMed] [Google Scholar]
- 66.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 67.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1998;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 68.Varfolomeev E E, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann J S, Mett I L, Rebrikov D, Brodianski V M, Kemper O C, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham K B, Goncharov T, Holtmann H, Lonai P, Wallach D. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe Y, Akaike T. Possible involvement of caspase-like family in maintenance of cytoskeleton integrity. J Cell Physiol. 1999;179:45–51. doi: 10.1002/(SICI)1097-4652(199904)179:1<45::AID-JCP6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 70.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 71.Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci. 1998;111:2477–2486. doi: 10.1242/jcs.111.17.2477. [DOI] [PubMed] [Google Scholar]
- 72.Wiche G, Baker M A. Cytoplasmic network arrays demonstrated by immunolocalization using antibodies to high mol wt protein present in cytoskeletal preparations from cultured cells. Exp Cell Res. 1982;138:15–29. doi: 10.1016/0014-4827(82)90086-6. [DOI] [PubMed] [Google Scholar]
- 73.Wiche G, Becker B, Luber K, Weitzer G, Castanon M J, Hauptmann R, Stratowa C, Stewart M. Cloning and sequencing of rat plectin indicates a 466-kD polypeptide chain with a three-domain structure based on a central alpha-helical coiled coil. J Cell Biol. 1991;114:83–99. doi: 10.1083/jcb.114.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H, Xu Q, Krajewski S, Krajewska M, Xie Z, Fuess S, Kitada S, Godzik A, Pawlowski K, Reed J. BAR: a novel apoptosis regulator at the intersection of caspases and Bcl-2 family proteins. Proc Natl Acad Sci USA. 2000;97:2597–2602. doi: 10.1073/pnas.97.6.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]