Summary
We report that decreased expression of miR-30c in tumor compared to adjacent tissue is sex-dependent in colorectal cancer (CRC) patients. High expression of miR-30c was associated with better survival in the whole cohort. When the cohort was split into male and female subcohorts, decreased miR-30c expression in tumor compared to adjacent tissue was observed only in males. Expression of miR-30c was decreased in CRC tumor tissue in male patients with nodes involvement compared to those without metastases in nodes and this difference was not observe in females. Next dependency of miR-30c expression on oestrogen receptor β (ERβ) mRNA levels in tumor was tested. In males with low expression of ERβ, we observed a significant decrease in miR-30c levels in patients with nodes involvement compared to those without nodes involvement. This difference was not observed in males with high ERβ mRNA levels and in females. Accordingly, males with low expression of ERβ and high expression of miR-30c showed a better survival that those with low expression ERβ and low expression of miR-30c. It is possible to conclude that whole cohort survival dependence on miR-30c is mostly generated by a subcohort of males with low expression of ERβ mRNA in tumor tissue.
Keywords: Oestrogen receptor β, Males, Females, Metastases, Survival
Introduction
Colorectal cancer (CRC) is recently the fourth leading cause of cancer-related death worldwide (Bray et al. 2018). According SEER (2010–2016) a proportion of patients diagnosed with CRC surviving at least 5-years is 65.6 % in the USA. With this survival CRC takes 10th position in the list of 24 malignant cancers after mesothelioma, pancreas, liver, oesophagus, lung, stomach, brain, and ovary cancers, and myeloma. Based on information provided by cancer registries of Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the UK 5-years survival of CRC patients shows continual improvement from 60 % during the period 1995–1999 up to 70 % in years 2010–2014 (Arnold et al. 2019). In spite of obvious advances in CRC management, there is still acute demand for tools useful in determination of patient exact diagnosis and prognosis. In this respect miRNAs are frequently studied to assess cancer progression (Dave et al. 2019).
miR-30c-5p (miR-30) belongs to a large family of small noncoding miRNAs. Today approximately 1900 miRNAs were identified in the human genome and their number probably will be growing (Griffiths-Jones 2004, Li and Kowdley 2012). miRNAs are extensively studied because of their role in gene silencing. Based on the complementarity of miRNA seed sequence and 3′-untranslated regions of mRNA miRNA in cooperation with argonaute protein, endonuclease dicer and other components create RNA-induced silencing complex (RISC) that targets mRNA. Complete RISC assures degradation and/or translation inhibition of complementary mRNA (Slack and Chinnaiyan 2019).
miR-30c usually exerts an oncostatic role, although some exceptions have also been shown (Yang et al. 2017). In the gastrointestinal tract, the tumor suppressive influence of miR-30c was demonstrated in several experimental models (Strubberg and Madison 2017). In human colon carcinoma cell line Caco-2 miR-30c regulates expression of KRAS that is involved in cancer development via epidermal growth factor (Nakayama et al. 2017). In LOVO and WE480 CRC cell lines, miR-30c downregulation of BCL9 (B cell lymphoma 9) expression has been shown. BCL9 exerts proliferative influence and over-expression in several malignancies (Zhao et al. 2019). In the stable gastric cancer cell line MGC-803 miR-30c downregulated metastasis-associated protein 1 (MTA1) that is strongly associated with tumorigenesis (Cao et al. 2017). miR-30c also inhibited expression of ADAM19 in HTC116 human colon cancer cells. ADAM19 is involved in a variety of pathologies including promotion of colon cancer cells invasiveness (Zhang et al. 2015).
The downregulation of miR-30c in cancer tissue compared to adjacent tissue is documented in breast, ovarian, endometrial, lung, gastric, and bladder cancers; neuroblastomas, mesotheliomas, and renal cell carcinomas (Irani and Hussain 2015, Kong et al. 2014, Han et al. 2020). Moreover, a decreased expression of miR-30c in malignant tissue has also been observed in patients suffering from colorectal cancer (Zhao et al. 2019, Zhang et al. 2015). On the basis of this knowledge, miR-30c has been suggested as a potential biomarker (Han et al. 2020).
Even though miR-30c is usually downregulated in tumor tissue compared to adjacent tissue (Irani and Hussain 2015), there are two studies that have implicated increased levels of miR-30c in the circulation of CRC patients compared to healthy control (Ostenfeld et al. 2016, Jacob et al. 2017). This observation can be explained by the secretion of oncostatic miRNAs from cancer cells as a disposal route of tumor suppressive elements (Ostenfeld et al. 2016).
In our previous studies (Hasakova et al. 2017, Hasakova et al. 2019), we investigated sex-dependent differences in miRNAs levels in CRC tumor tissue compared to adjacent tissues dependent on clinicopathological features. In this respect our attention turned to possible influence of oestrogen on CRC progression (Herichova et al. 2019).
Oestrogen exerts most of its effect via nuclear oestrogen receptors α and β (ERα and ERβ, respectively) than influence gene transcription via oestrogen-response element (ERE). ERβ is predominant form of oestrogen receptor in the bowel. Except of genomic action, oestrogen receptors can also influence gene expression by indirect genomic signaling via protein-protein interactions with other transcriptional factors and regulatory elements or by oestrogen independent mechanism (Fuentes and Silveyra 2019). We observed a significant decrease in ERβ expression dependent on staging in females that was not observed in male patients diagnosed with CRC. In males a pronounced decrease in ERβ expression compared to adjacent tissues was present already at the very early stage of disease (Herichova et al. 2019). ERβ is known for its inverse relationship with CRC staging and grading, presence of colorectal polyps and capacity to mediate a beneficial response for patients. Therefore, ERβ selective agonists are investigated in preclinical and clinical studies with promising results (Williams et al. 2016).
Since effect of oestrogen on miRNA expression in CRC experimental model was reported recently (He et al. 2012) we decided to elucidate if there is a sex-dependent difference in miR-30c expression and correlate it with expression of ERβ that mediates oestrogen regulation in colorectum.
Material and Methods
The patient cohort consisting of 64 patients (averaged age 69 years, males 69±1.7 years, females 69±2.8 years) was described in Table 1. Samples of tumor tissue (C18–C20), proximal (≥10 cm from tumor) and distal tissues (≥2 cm from tumor) were collected during the surgery. Tissue samples were frozen into liquid nitrogen and stored under −70 °C until mRNA and miRNA extraction. Surgery and histological examinations of samples were performed at the First Surgery Department of the Faculty of Medicine of Comenius University and the University Hospital Bratislava in Slovakia. An experimental protocol corresponding with Helsinki declaration was approved by the Ethics Committee of Comenius University and explained to each patient. All participants provided written informed consent.
Table 1.
Patient’s gender, tumor location and clinicopathological characteristics.
| Females | Males | Sum | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Cohort | 26 | 41 | 38 | 59 | 64 | 100.0 | |
|
| |||||||
| Tumor location | Right-side | 14 | 22 | 11 | 17 | 25 | 39.1 |
| Left-side | 12 | 19 | 27 | 42 | 39 | 60.9 | |
|
| |||||||
| Grading stage | G 1–1.5 | 5 | 8 | 7 | 11 | 12 | 18.8 |
| G 2–2.5 | 20 | 31 | 29 | 45 | 49 | 76.6 | |
| G3 | 1 | 2 | 2 | 3 | 3 | 4.7 | |
|
| |||||||
| Clinical stage | I | 1 | 2 | 3 | 5 | 4 | 6.3 |
| IIA, IIB | 8 | 13 | 21 | 33 | 29 | 45.3 | |
| IIIA, IIIB | 10 | 16 | 5 | 8 | 15 | 23.4 | |
| IVA, IVB | 7 | 11 | 9 | 14 | 16 | 25.0 | |
|
| |||||||
| TNM classification | |||||||
|
| |||||||
| Primary tumor invasion | T1–T2 | 1 | 2 | 3 | 5 | 4 | 6.3 |
| T3 | 20 | 31 | 28 | 44 | 48 | 75.0 | |
| T4 | 5 | 8 | 7 | 11 | 12 | 18.8 | |
|
| |||||||
| Regional lymph node | N0 | 10 | 16 | 25 | 39 | 35 | 54.7 |
| N1 | 6 | 9 | 7 | 11 | 13 | 20.3 | |
| N2 | 10 | 16 | 6 | 9 | 16 | 25.0 | |
|
| |||||||
| Distant metastasis | M0 | 19 | 30 | 29 | 45 | 48 | 75.0 |
| M1 | 7 | 11 | 9 | 14 | 16 | 25.0 | |
n=number, T=tumor invasion, N=nodal status, M=distant metastasis; right-sided colon cancers include C18.0–C18.4, left-sided CRCs include C18.5–C20.
miRNA was isolated from 70 mg of tissue using RNAzol according to the vendor’s instructions (MRC, USA; protocol for isolation of large RNA and small RNA fractions). Before reverse transcription poly(A) tailing kit (Life Technologies, USA) was employed to polyadenylate 1 μg of miRNA. Polyadenylated template (100 ng) was transcribed using ImProm-II™ reverse transcription kit (Promega, USA) and a primer attached a universal tag with sequence 5′-CAGGTCCAGTTTTTTTTTTTTTTTVN-3′to miRNAs to extend their sequence (Busk 2014). hsa-miR-30c-5p expression was measured by real-time polymerase chain reaction (PCR) using miScript SYBR green PCR kit (Qiagen, Germany) and the StepOnePlus™ real-time PCR system (Applied Biosystems, USA). Primers for miR-30c (MI0000254) measurement: sense, 5′-GCAGTGTAAACATCCTACACTCT-3′; antisense, 5′-TCCAGTTTTTTTTTTTTTTTGCTGA-3′ were designed by software miRprimer (Busk 2014). Splitting of the cohort according to low and high mRNA oestrogen receptor β (ERβ) levels was based on median. ERβ mRNA expression was published in the previous study Herichova et al. (2019).
Real-time PCR conditions for miR-30c-5p measurement were: activation of hot-start polymerase at 95 °C for 15 min followed by 35 cycles at 94 °C for 15 s, 55 °C for 30 s and 72 °C for 30 s. Melting curve analysis was used for validation of PCR product specificity. Nuclear RNA U6 was used for gene normalization. Measurement of U6 is described elsewhere (Herichova et al. 2019).
Statistical analysis
Evaluation of overall survival was performed by Kaplan-Meier survival curves followed by a log-rank testing related to the median miR-30c expression. The starting point for the log-rank test was the day when surgery was performed. Expression of miR-30c in cancer and adjacent tissues was compared by paired t-test. Expression of miR-30c between two clusters of patients was compared by unpaired t-test. Association between miR-30c expression and clinicopathological stages of patients was analysed by regression analysis. Data in histograms were provided as a mean ± standard error of the mean (SEM). The level of significance was set at p<0.05.
Results
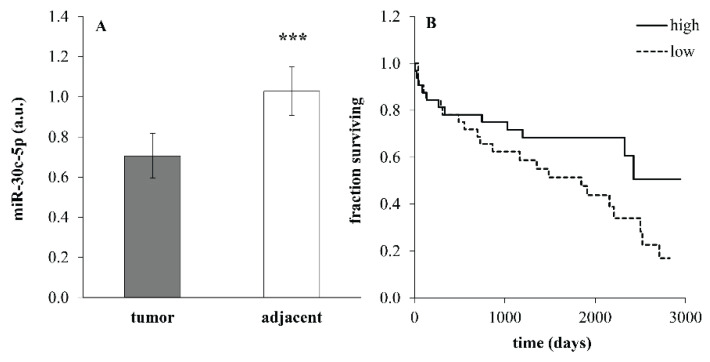
Expression of miR-30c in the whole cohort was significantly decreased in tumor compared to adjacent tissues (Fig. 1A; p=1.2*10−4, paired t-test). High expression of miR-30c was associated with better survival and low miR-30c levels corresponded with worse survival in the whole cohort of patients (Fig. 1B; p=0.045, log-rank test).
Fig. 1.
Expression of miR-30c (A) in tumor and adjacent tissues in the whole cohort of patients (n=64) undergoing surgery because of colorectal carcinoma. Data were provided as mean ± SEM. *** p<0.001 (paired t-test). Expression in adjacent tissue was calculated as an average of expression in the healthy tissues sampled proximally and distally from the tumor (a.u.=arbitrary units). (B) Kaplan-Meier survival curves for the entire cohort of patients with low miR-30a expression (≤median, dotted line) and high miR-30c expression (>median, solid line) in tumor tissue. p=0.045 (log-rank test).
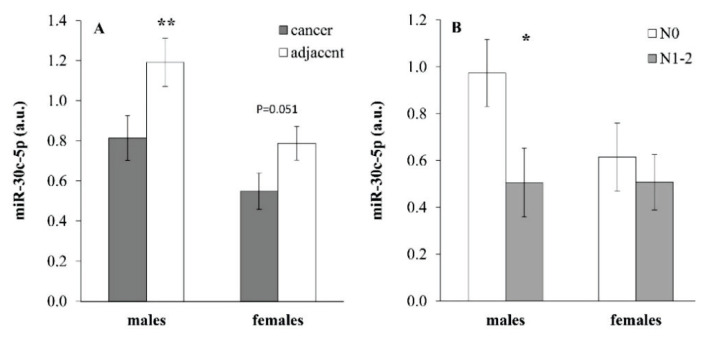
miR-30c expression in cancer and adjacent tissues exerted sex-dependent differences. While miR-30c expression in males showed a pronounced decrease in tumor tissue compared to adjacent tissue (Fig. 2A; p=0.001, paired t-test), in females this difference achieved a borderline only level of significance (Fig. 2A; p=0.051, paired t-test). Expression of miR-30c in adjacent tissue of males was significantly higher compared to females (p=0.015, unpaired t-test). The difference in miR-30c expression in the tumor tissue between the two genders did not achieve a level of significance (p=0.09, unpaired t-test). When subcohorts of males and females were split according to presence of metastases in nodes, there was a significant difference in miR-30c expression in the tumor tissue depending on nodes metastases in males (Fig. 2B; p=0.044, unpaired t-test) while this difference was not observed in females.
Fig. 2.
Expression of miR-30c (A) in tumor and adjacent tissues in male (n=38) and female (n=26) patients with colorectal cancer. Data are provided as mean ± SEM. ** p<0.01 (paired t-test). (B) miR-30c expression in tumor tissue of males and females with or without metastases in nodes (n=10–25). N0=without metastases in nodes, N1–2=with metastases in nodes. * p<0.05 (unpaired t-test).
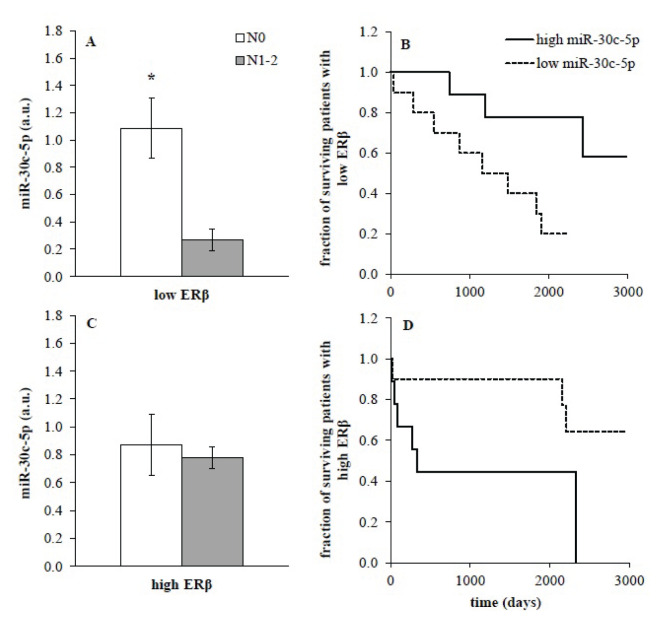
Next, we evaluated the dependence of miR-30c on ERβ mRNA expression. In male subcohort with low ERβ expression in tumor miR-30c levels showed a significant difference between samples from patients without metastases in nodes compared to those with nodes involvement (Fig. 3A; p=0.014, unpaired t-test). This difference was not observed in males with high levels of ERβ mRNA in tumor (Fig. 3C) and in females (data not shown). Accordingly, males with low expression of ERβ mRNA exerted significant association between miR-30c expression and survival. High miR-30c expression in males with low ERβ was associated with better survival and low miR-30c expression was linked to worse survival (Fig. 3B; p=0.017, log-rank test). This dependence was not observed in males with high ERβ mRNA expression (Fig. 3D) and females.
Fig. 3.
miR-30c expression (A, C) in male subcohort split according to low (n=9, A) or high (n=19, C) expression of oestrogen receptor β (ERβ). Data were provided as mean ± SEM. Low ERβ – expression of ERβ≤median, high ERβ – expression>median; N0=without metastases in nodes, N1–2=with metastases in nodes; * p<0.05 (unpaired t-test). (B, D) Kaplan-Meier survival curves for the male subcohort of patients with low (B) and high (D) ERβ expression were calculated in relation to low miR-30c expression (≤median, dotted line, p=0.017, log-rank test) and high miR-30c expression (>median, solid line) in tumor tissue.
Gender and ERβ dependent associations between miR-30c expression and clinicopathological parameters of patients are provided in Table 2. We observed a significant regression between miR-30c expression and nodes involvement, presence of distant metastases, grading and clinical stage in male patients with low ERβ expression. These correlations were not observed in female patients and males with high ERβ expression. Therefore, it is possible to conclude that survival dependence on miR-30c expression (Fig. 1B) is mostly generated by the subcohort of males with low expression of ERβ mRNA in tumor tissue (Table 2).
Table 2.
The association between miR-30c expression, ERβ expression and clinicopathological stage of patients.
| miR-30c-5p expression (a.u.) | Regression analysis | Log-rank test | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| T | N | M | Grading | Clinical stage | Survival | |||
| Females | low ERβ |
beta | 0.128 | 0.054 | 0.212 | −0.129 | 0.163 | n/a |
| R | 0.125 | 0.069 | 0.212 | −0.129 | 0.205 | n/a | ||
| p | ns | ns | ns | ns | ns | ns | ||
|
| ||||||||
| high ERβ |
beta | 0.263 | −0.140 | −0.050 | −0.215 | −0.052 | n/a | |
| R | 0.345 | −0.290 | −0.033 | −0.244 | −0.095 | n/a | ||
| p | ns | ns | ns | ns | ns | ns | ||
|
| ||||||||
| Males | low ERβ |
beta | −0.185 | −0.479 | −0.802 | −0.775 | −0.337 | n/a |
| R | −0.253 | −0.547 | −0.522 | −0.493 | −0.465 | n/a | ||
| p | ns | 0.015 | 0.022 | 0.032 | 0.045 | 0.006 | ||
|
| ||||||||
| high ERβ |
beta | 0.377 | −0.088 | 0.583 | 0.212 | 0.172 | n/a | |
| R | 0.367 | −0.093 | 0.333 | 0.135 | 0.237 | n/a | ||
| p | ns | ns | ns | ns | ns | ns | ||
T=tumor invasion, N=nodal status, M=distant metastasis, ERβ=oestrogen receptor β, beta=slope of the regression line, R=regression coefficient, ns=not significant, p=probability value, a.u.=arbitrary units, n/a=not applicable.
Discussion
Our study showed that expression of miR-30c exerts sex-dependent expression in patients with CRC. We observed a significant downregulation of miR-30c expression in tumor tissue compared to adjacent tissue, and better survival associated with high miR-30c expression. This observation is in good agreement with previous findings (Zhang et al. 2015, Zhao et al. 2019). When the cohort was split according to gender and ERβ expression, it became obvious that difference observed at the level of the whole cohort was generated mostly by the male subcohort exerting low expression of ERβ (Table 2).
In our previous studies, we demonstrated sex-dependent differences in miRNA-21, miR-16 and miR-34a expression in the colorectal tumor tissue (Hasakova et al. 2017, Hasakova et al. 2019). When we directly compared miR-30c expression between male and female patients, there was only a nonsignificant trend to increased levels in males compared to females in the tumor tissue. However, when the cohort was split according to nodes involvement, a difference between the two genders became obvious. Correlation analysis also indicates that miR-30c expression depends on the TNM stage much more in males compared to females. Sex-dependent differences in miRNAs expression have also been observed by other authors (Guo et al. 2017, Cui et al. 2018); however, an explanation for this finding is not readily available.
Since plasma concentration of oestrogen is similar in males and females at this age (Ober et al. 2008) we focused on the regulation of ERβ in CRC patients. Expression of ERβ is much more abundant in the gastrointestinal tract compared to ERα and the most of oestrogen effects in this tissue are expected to be mediated via ERβ (Fuentes and Silveyra 2019). ERβ executes its function mainly through ERE influencing transcription of oestrogen target genes (Deroo et al. 2010). There are two miR-30c encoding genes and both are located within intron of other gene. In the case of the intronic position of miRNA, miRNA is frequently transcribed together with the host gene (Baskerville and Bartel 2005, Pidikova et al. 2020).
miR-30c-1 gene is located in the intron of abundantly expressed nuclear transcription factor Y subunit C (NFYC) (Gurtner et al. 2017). However, full transcriptome analysis of ERα and ERβ mediated gene regulation did not reveal presence of ERE binding site in its sequence (Wiliams et al. 2008). Moreover, NFYC was shown to be up-regulated in CRC and its high expression was associated with worse survival of patients (Kottorou et al. 2012). Therefore, NFYC does not seem to be a key factor in determination of miR-30c expression.
miR-30c-2 is found in the intron of long non-coding RNA LINC00472 (C6orf155, P53RRA) that does not produce a protein (Irani and Hussain 2015). Unlikely NFYC, LINC00472 possess functional ERE site that via ERα induces LINC00472 expression (Wang et al. 2019). Activation of ERE located in LINC00472 by ERβ was not tested in CRC tissue yet. LINC00472 is, similarly like miR-30c, considered to be tumor-suppressor in CRC and shows decreased expression in CRC tumor tissue compared to adjacent tissue (Ye et al. 2018).
In our previous study (Herichova et al. 2019), we observed sex-dependent differences in ERβ expression in tumor tissue of CRC patients. While the expression of ERβ mRNA showed a decrease dependent on the TNM stage in females, in males, a decrease in ERβ expression was observed already at the very early stage of disease without nodes involvement. It is very likely that a decrease in ERβ expression in males occurs even before or during cancer onset. Therefore, it is possible that the ERβ and gender dependent miR-30c expression can be attributed to changed oestrogen signaling via ERβ receptors in early stages of CRC.
Although this assumption needs to be tested, it is in according with experiments implicating that miRNA expression is responsive to oestradiol (E2). E2 induced miR-30c expression in MCF-7 (Bhat-Nakshatri et al. 2009). On the other hand, it was reported, that E2 down-regulated miR-30c expression in Ishikawa and HEC-1-B cells (Kong et al. 2014) and transcription of the whole set of miRNAs was inhibited in MCF-7 cell line after E2 treatment (Mailot et al. 2009). In colon adenocarcinoma COLO205 cell line E2 induced expression of ERβ and inhibited expression of several miRNAs. There results were partially confirmed in human cohort (He et al. 2012).
Taken together, miR-30c exerts decreased levels in tumor tissue compared to adjacent tissue in patients diagnosed with CRC, and low levels of miR-30c are associated with worse survival. Expression of miR-30c is more related to the TNM status in males, especially in those with low expression of ERβ compared to females, which is also reflected by the survival curves. Males with low expression of ERβ and high expression of miR-30c show better survival than other clusters of the cohort. This finding is in accordance with previously observed sex-dependent differences in ERβ expression in CRC between males and females, and the inhibitory effect of oestrogen on miRNAs expression. We propose that subclustering of the clinical cohort can significantly contribute to personalized medicine and can help to more precisely depict which group of patients generates differences observed at the level of the whole cohort.
Acknowledgements
Research was supported by grants APVV-16-0209, APVV-14-0318 and VEGA 1/0679/19.
Footnotes
Conflict of interest
There is no conflict of interest.
References
- ARNOLD M, RUTHERFORD MJ, BARDOT A, FERLAY J, ANDERSSON TM, MYKLEBUST TÅ, TERVONEN H, THURSFIELD V, RANSOM D, SHACK L, WOODS RR, TURNER D, LEONFELLNER S, RYAN S, SAINT-JACQUES N, DE P, McCLURE C, RAMANAKUMAR AV, STUART-PANKO H, ENGHOLM G, ET AL. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASKERVILLE S, BARTEL DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–247. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHAT-NAKSHATRI P, WANG G, COLLINS NR, THOMSON MJ, GEISTLINGER TR, CARROLL JS, BROWN M, HAMMOND S, SROUR EF, LIU Y, NAKSHATRI H. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009;37:4850–4861. doi: 10.1093/nar/gkp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY F, FERLAY J, SOERJOMATARAM I, SIEGEL RL, TORRE LA, JEMAL A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- BUSK PK. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics. 2014;15:29. doi: 10.1186/1471-2105-15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAO JM, LI GZ, HAN M, XU HL, HUANG KM. MiR-30c-5p suppresses migration, invasion and epithelial to mesenchymal transition of gastric cancer via targeting MTA1. Biomed Pharmacother. 2017;93:554–560. doi: 10.1016/j.biopha.2017.06.084. [DOI] [PubMed] [Google Scholar]
- CUI C, YANG W, SHI J, ZHOU Y, YANG J, CUI Q, ZHOU Y. Identification and analysis of human sex-biased microRNAs. Genomics Proteomics Bioinformatics. 2018;6:200–211. doi: 10.1016/j.gpb.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVE VP, NGO TA, PERNESTIG AK, TILEVIK D, KANT K, NGUYEN T, WOLFF A, BANG DD. MicroRNA amplification and detection technologies: opportunities and challenges for point of care diagnostics. Lab Invest. 2019;99:452–469. doi: 10.1038/s41374-018-0143-3. [DOI] [PubMed] [Google Scholar]
- DEROO BJ, BUENSUCESO AV. Minireview: Estrogen receptor-beta: mechanistic insights from recent studies. Mol Endocrinol. 2010;24:1703–1714. doi: 10.1210/me.2009-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUENTES N, SILVEYRA P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135–170. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITHS-JONES S. The microRNA registry. Nucleic Acids Res. 2004;J32:D109–D111. doi: 10.1093/nar/gkh023. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GURTNER A, MANNI I, PIAGGIO G. NF-Y in cancer: Impact on cell transformation of a gene essential for proliferation. Biochim Biophys Acta Gene Regul Mech. 2017;1860:604–616. doi: 10.1016/j.bbagrm.2016.12.005. [DOI] [PubMed] [Google Scholar]
- GUO L, ZHANG Q, MA X, WANG J, LIANG T. miRNA and mRNA expression analysis reveals potential sex-biased miRNA expression. Sci Rep. 2017;7:39812. doi: 10.1038/srep39812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN W, CUI H, LIANG J, SU X. Role of MicroRNA-30c in cancer progression. J Cancer. 2020;11:2593–2601. doi: 10.7150/jca.38449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASÁKOVÁ K, BEZAKOVA J, VICIAN M, REIS R, ZEMAN M, HERICHOVA I. Gender-dependent expression of leading and passenger strand of miR-21 and miR-16 inhuman colorectal cancer and adjacent colonic tissues. Physiol Res. 2017;66(Suppl 4):S575–S582. doi: 10.33549/physiolres.933808. [DOI] [PubMed] [Google Scholar]
- HASAKOVA K, REIS R, VICIAN M, ZEMAN M, HERICHOVA I. Expression of miR-34a-5pis up-regulated in human colorectal cancer and correlates with survival and clock gene PER2 expression. PLoS One. 2019;14:e0224396. doi: 10.1371/journal.pone.0224396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE YQ, SHENG JQ, LING XL, FU L, JIN P, YEN L, RAO J. Estradiol regulates miR-135b and mismatch repair gene expressions via estrogen receptor-β in colorectal cells. Exp Mol Med. 2012;44:723–732. doi: 10.3858/emm.2012.44.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERICHOVA I, REIS R, HASAKOVA K, VICIAN M, ZEMAN M. Sex-dependent regulation of estrogen receptor beta in human colorectal cancer tissue and its relationship with clock genes and VEGF-A expression. Physiol Res. 2019;68(Suppl 3):S297–S305. doi: 10.33549/physiolres.934352. [DOI] [PubMed] [Google Scholar]
- IRANI S, HUSSAIN MM. Role of microRNA-30c in lipid metabolism, adipogenesis, cardiac remodeling and cancer. Curr Opin Lipidol. 2015;26:139–146. doi: 10.1097/MOL.0000000000000162. [DOI] [PubMed] [Google Scholar]
- JACOB H, STANISAVLJEVIC L, STORLI KE, HESTETUN KE, DAHL O, MYKLEBUST MP. Identification of a sixteen-microRNA signature as prognostic biomarker for stageII and III colon cancer. Oncotarget. 2017;8:87837–87847. doi: 10.18632/oncotarget.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG X, XU X, YAN Y, GUO F, LI J, HU Y, ZHOU H, XUN Q. Estrogen regulates the tumour suppressor MiRNA-30c and its target gene, MTA-1, in endometrial cancer. PLoS One. 2014;9:e90810. doi: 10.1371/journal.pone.0090810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTTOROU AE, ANTONACOPOULOU AG, DIMITRAKOPOULOS FI, TSAMANDAS AC, SCOPA CD, PETSAS T, KALOFONOS HP. Altered expression of NFY-C and RORA in colorectal adenocarcinomas. Acta Histochem. 2012;114:553–561. doi: 10.1016/j.acthis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- LI Y, KOWDLEY KV. MicroRNAs in common human diseases. Genomics Proteomics Bioinformatics. 2012;10:246–253. doi: 10.1016/j.gpb.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAILLOT G, LACROIX-TRIKI M, PIERREDON S, GRATADOU L, SCHMIDT S, BÉNÈS V, ROCHÉ H, DALENC F, AUBOEUF D, MILLEVOI S, VAGNER S. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009;69:8332–8340. doi: 10.1158/0008-5472.CAN-09-2206. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA T, FUNAKOSHI-TAGO M, TAMURA H. Coffee reduces KRAS expression in Caco-2 human colon carcinoma cells via regulation of miRNAs. Oncol Lett. 2017;14:1109–1114. doi: 10.3892/ol.2017.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBER C, LOISEL DA, GILAD Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSTENFELD MS, JENSEN SG, JEPPESEN DK, CHRISTENSEN LL, THORSEN SB, STENVANG J, HVAM ML, THOMSEN A, MOURITZEN P, RASMUSSEN MH, NIELSEN HJ, ØRNTOFT TF, ANDERSENCL miRNA profiling of circulating EpCAM(+) extracellular vesicles: promising biomarkers of colorectal cancer. J Extracell Vesicles. 2016;5:31488. doi: 10.3402/jev.v5.31488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIDÍKOVA P, REIS R, HERICHOVA I. miRNA clusters with down-regulated expression in human colorectal cancer and their regulation. Int J Mol Sci. 2020;21:4633. doi: 10.3390/ijms21134633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLACK FJ, CHINNAIYAN AM. The role of non-coding RNAs in oncology. Cell. 2019;179:1033–1055. doi: 10.1016/j.cell.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRUBBERG AM, MADISON BB. MicroRNAs in the etiology of colorectal cancer: pathways and clinical implications. Dis Model Mech. 2017;10:197–214. doi: 10.1242/dmm.027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Z, KATSAROS D, BIGLIA N, SHEN Y, LOO L, YU X, LIN H, FU Y, CHU WM, FEI P, NI Y, JIA W, DENG X, QIAN B, YU H. ERα upregulates the expression of long non-coding RNA LINC00472 which suppresses the phosphorylation of NF-κB in breast cancer. Breast Cancer Res Treat. 2019;175:353–368. doi: 10.1007/s10549-018-05108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS C, EDVARDSSON K, LEWANDOWSKI SA, STRÖM A, GUSTAFSSON JA. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27:1019–1032. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C, DILEO A, NIV Y, GUSTAFSSON JÅ. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016;372:48–56. doi: 10.1016/j.canlet.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG SJ, YANG SY, WANG DD, CHEN X, SHEN HY, ZHANG XH, ZHONG SL, TANG JH, ZHAO JH. The miR-30 family: Versatile players in breast cancer. Tumour Biol. 2017;39:1010428317692204. doi: 10.1177/1010428317692204. [DOI] [PubMed] [Google Scholar]
- YE Y, YANG S, HAN Y, SUN J, XV L, WU L, WANG Y, MING L. Linc00472 suppresses proliferation and promotes apoptosis through elevating PDCD4 expression by sponging miR-196a in colorectal cancer. Aging. 2018;10:1523–1533. doi: 10.18632/aging.101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO DW, LI MM, HAN JP, WANG Y, JIANG LX, CHANG HL. MiR-30c exerts tumor suppressive functions in colorectal carcinoma by directly targeting BCL9. Eur Rev Med Pharmacol Sci. 2019;23:3335–3343. doi: 10.26355/eurrev_201904_17696. [DOI] [PubMed] [Google Scholar]
- ZHANG Q, YU L, QIN D, HUANG R, JIANG X, ZOU C, TANG Q, CHEN Y, WANG G, WANG X, GAO X. Role of microRNA-30c targeting ADAM19 in colorectal cancer. PLoS One. 2015;10:e0120698. doi: 10.1371/journal.pone.0120698. [DOI] [PMC free article] [PubMed] [Google Scholar]