Abstract
OCT4 is a core transcription factor involved in pluripotency maintenance in the early mammalian embryo. The POU5F1 gene that encodes the OCT4 protein is highly conserved across species, suggesting conserved function. However, studies in several species including mice, cattle, and pigs, suggest that there are differences in where and when OCT4 is expressed. Specifically, in the horse, several studies have shown that exposure to the uterine environment may be necessary to induce OCT4 expression restriction to the inner cell mass (ICM) of the developing embryo, suggesting that there may be equine-specific extrinsic regulators of OCT4 expression that have not yet been investigated. However, an alternative hypothesis is that this restriction may not be evident in equine embryos because of our inability to culture them to the epiblast stage, preventing the observation of this restriction. In vitro studies have identified that OCT4 is expressed in the immature equine oocyte and in the early equine embryo, but OCT4 expression has not been studied after the formation of the ICM in the equine embryo. Despite the gaps in knowledge about equine-specific functions of OCT4, this factor has been used in studies assessing equine embryonic stem cells and to induce pluripotency in equine somatic cells. This review describes the role of OCT4 in the equine embryo and its applications in equine stem cell research.
Keywords: OCT4, POU5F1, Equine, Embryo, Regulation, Induced pluripotent stem cells
1. Introduction
Oct4, or octamer binding transcription factor 4, is a transcription factor that is highly expressed in early embryos. The octamer binding motif is a cis-acting regulatory element that is present in many different promoters and enhancers in the mammalian genome [1]. Members of the octamer binding transcription factor family bind to the octamer motif (5′-ATTTGCAT-3′) to modulate transcription [2]. When initially discovered, Oct3 and 4 were identified as distinct proteins because they were expressed at different developmental stages and in different murine tissues [1], but they have since been found to be isomers of the same gene, Pou5f1.
The Pou5f1 gene is in the Pou family of transcription factors. This family consists of three classes of transcription factors: pituitary-specific (Pit1), octamer binding, and Unc86 [3]. There are six identified classes of Pou transcription factors. Four of these classes evolved before the last common ancestor of animals, and thus, these genes are expressed throughout the animal kingdom [4]. However, the Pou5 class is evolutionarily unique to vertebrates [4].
In addition to being unique in vertebrates, this gene is also well conserved across species. In an investigation of protein sequence homology, it was reported that the cattle OCT4 protein has a 90.6% sequence homology to its human ortholog and 81.7% homology to the mouse ortholog [5]. Through an NCBI protein BLAST alignment comparison, we found that the equine OCT4 protein has a 95.0% sequence similarity to the human protein, 94.4% similarity to the cattle protein, and 83.1% similarity to the murine protein. These findings suggest that the POU5F1 gene and OCT4 protein are well conserved across mammalian species, indicating that they likely play an important role in the developing equine embryo.
Functional studies of OCT4 have been performed using loss-of-function knockout approaches. This work was done in mice [6], humans [7] and cattle [8,9], but not equine embryos. Thus, most studies on OCT4 in equine cells have extrapolated its function from studies in other species. OCT4 has been used as a pluripotency marker and as a method to induce pluripotency in equine embryos, but not much is known about its specific function in horses.
1.1. OCT4 in the Immature Oocyte
In the mouse, Oct4 is expressed in the primordial germ cell (PGC) lineage of males and females and continues to be produced in the female germline, appearing in the unfertilized egg, but not in sperm or testis tissue [1]. Oct4’s presence in male PGCs and continued presence in oocytes suggests an important role for Oct4 in the germ cell lineage, helping to maintain the pluripotency of those cells [10]. In this regard, loss of Oct4 expression in PGCs leads to apoptosis, further indicating that Oct4 is essential for maintaining mammalian germ cells [11]. The OCT4 protein is present in the cytoplasm and nucleus of the immature equine oocyte (Figure 1), similar to its expression in the mouse [12]. Furthermore, OCT4 expression has been identified in the PGCs of all species assessed to date, highlighting its vital role in maintaining pluripotency in that lineage.
Fig. 1.
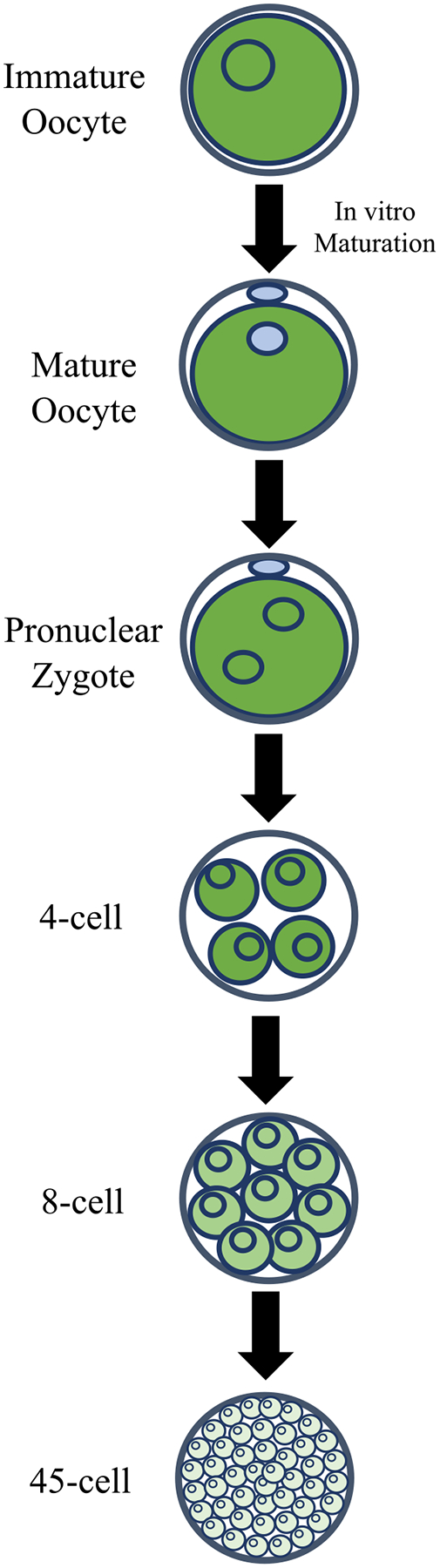
OCT4 expression during in vitro equine embryo development. OCT4 expression is designated in green with shade demonstrating the amount of protein expressed. Blue coloring represents weak or no OCT4 staining. OCT4 is expressed in the nucleus and cytoplasm of the immature oocyte, but after metaphase II, OCT4 expression restricts to the oocyte cytoplasm [12]. After fertilization, OCT4 continues to be expressed in the nucleus and the cytoplasm of the blastomeres until approximately the 6-cell stage. Expression of OCT4 then decreases until it reaches its lowest point at approximately the 40-cell stage [12].
1.2. OCT4 Expression in the Developing Embryo
Residual Oct4 expression from the oocyte is present in the early embryo until embryonic genome activation occurs. Initially, Oct4 transcripts are present in equal amounts in all blastomeres, but, as the embryo grows and differentiates, Oct4 expression decreases in the outer layer of cells, which will become the trophectoderm (TE), while Oct4 expression is maintained in the inner cell mass (ICM). It has been proposed that Oct4 is maintained in the ICM lineage to help retain the pluripotency of that lineage by regulating other pluripotency-associated genes [6,13]. In mice, this decrease in expression in the TE occurs at around four days post conception [14].
Choi and coworkers [12] investigated the presence of OCT4 protein through immunofluorescence staining at difference stages of in vitro–produced equine embryos and found OCT4 protein abundance dropped from fertilization until about day five post fertilization, or about the 40-cell stage, presenting both nuclear and cytoplasmic localization (Figure 1). After day five, OCT4 protein levels increased again and were restricted to the nuclei of all blastomeres. Thus, these in vitro produced blastocysts presented similar levels of OCT4 protein in both ICM and TE cells. In contrast, in vivo–produced embryos showed higher intensity for OCT4 immunostaining in ICM cells than TE cells on day seven post fertilization, with complete restriction to the ICM in day ten blastocysts [12]. Additionally, RNA-seq expression profiling of in vivo–produced day eight equine embryos showed OCT4 mRNA present predominantly in the ICM with reduced levels in the TE [15]. Interestingly, in vitro–produced embryos transferred into a mare for 2–3 days showed differential expression of OCT4 like in vivo–produced embryos (Figure 2) [12]. The authors interpreted this finding as indicating a need for embryo uterine exposure to achieve ICM-specific OCT4 expression. However, it is also likely that such restriction is only achieved after ten days of culture, which in vitro systems cannot effectively support.
Fig. 2.
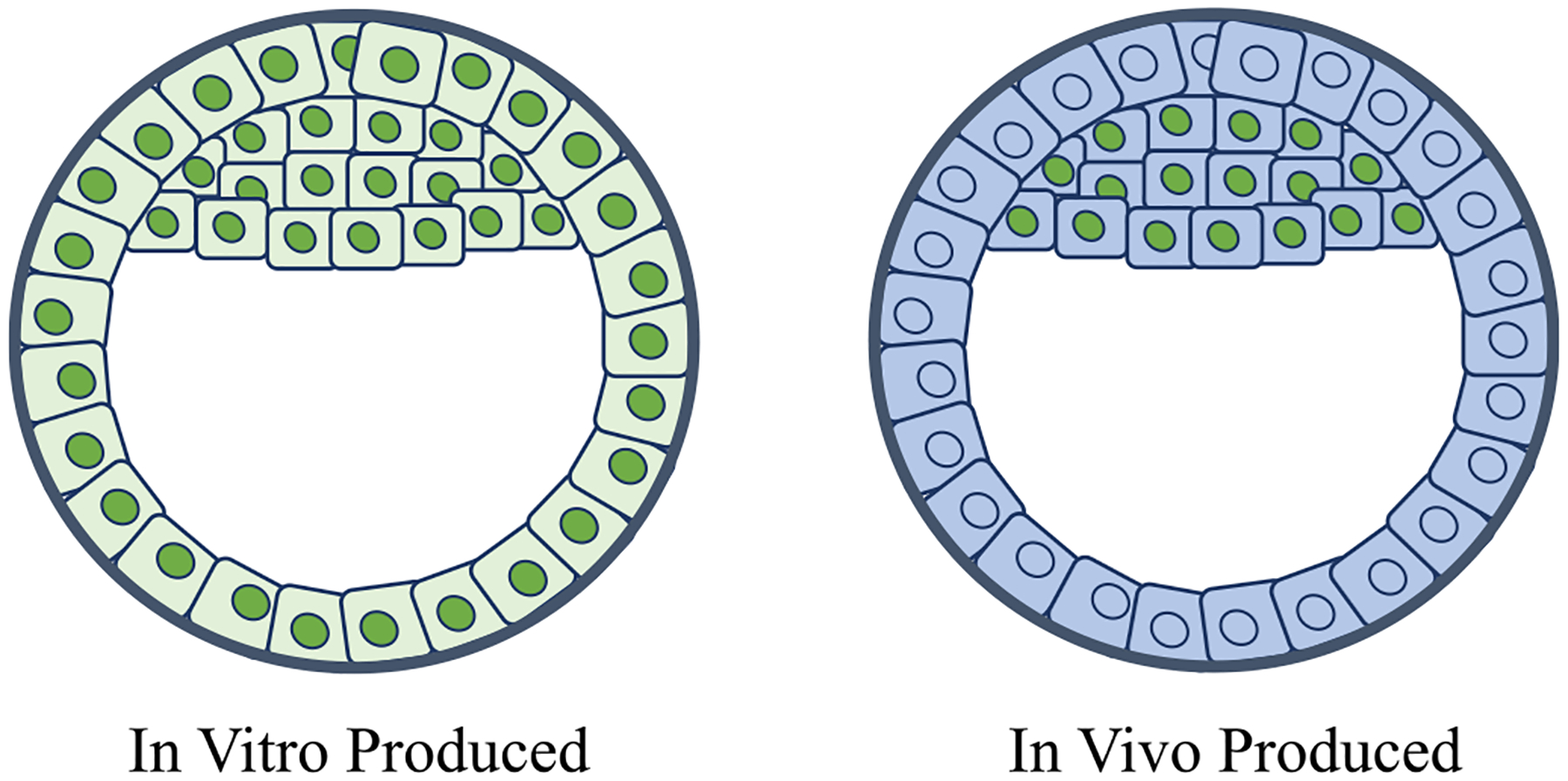
Comparison of OCT4 expression in in vitro produced and in vivo produced equine blastocysts. As the embryo develops into a blastocyst in vitro, OCT4 expression increases in the nuclei of all cells with no discernible expressional differences between the inner cell mass (ICM) and the trophectoderm (TE) [12]. On the other hand, in vivo conceived or in vitro produced embryos that are transferred into a mare and are subsequently recovered show OCT4 expression only in the nuclei of the ICM [12].
While in the mouse, Oct4 is strictly confined to the ICM lineage from early stages of blastocyst formation [6], in other species, OCT4 has been detected in both the TE and ICM, with restriction to the ICM occurring at different times in different species. In horses, this happens at around day ten of development with the embryo still presenting blastocyst morphology [12]. In pigs, OCT4 only becomes restricted in the epiblast cells in posthatching embryos [16]. In cattle embryos, OCT4 is present in the TE and this residual presence in the TE lineage had been attributed to higher protein stability and inheritance from the morula stage, as in situ hybridization detected OCT4 mRNA restriction to the ICM. On the other hand, more sensitive qPCR methods have detected OCT4 mRNA in both the ICM and TE of bovine embryos, with significantly higher expression levels in the ICM. Regardless, it appears that across species OCT4 is restricted to the pluripotent lineage with reduced expression in the TE cells, suggesting that OCT4 presence is not sufficient for pluripotency but is required to maintain it.
1.2.1. OCT4 Expression and the Timing of Placentation
Based on their findings in murine, bovine, and porcine embryos, Kirchhof and colleagues [17] suggested that the decrease in OCT4 expression in the TE corresponds with the timing of placentation. In their study, they found that murine embryos had no Oct4 expression in the TE at day five, whereas bovine and porcine embryos still showed OCT4 expression in both the ICM and TE. However, this study only assessed bovine and porcine embryos at days five and eight post conception. The authors suggested that investigating porcine and bovine embryos closer to the time of their implantation could show that these embryos have the same pattern of expression as murine embryos [17]. But this hypothesis does not hold true for equine embryos. In horses, the decrease in OCT4 expression of in vivo produced blastocysts appears to occur around day ten [12] when placentation is not until day 40 [18]. As approximately 10% of equine pregnancies are lost by day 35 [19], more work into the specific functions of OCT4 in equine embryos could help better our understanding of early pregnancy loss.
1.3. OCT4 in Late Embryonic Development
As the murine embryo continues to develop, Oct4 expression further restricts until it is only expressed in the epiblast [10]. Oct4 is initially expressed throughout the epiblast, but as gastrulation ensues, Oct4 expression is downregulated until it is only expressed in the posterior aspect of the murine epiblast [14]. Oct4 expression continues to decrease until expression is only detectable in the PGC lineage. In mice, this restriction occurs approximately 8.5 days post coitum [14]. Then, Oct4 expression is maintained in the PGCs until sexual differentiation occurs [10]. It is thought that Oct4 is expressed in this lineage to maintain the pluripotency of the germ cells. In contrast to all that is known about Oct4 restriction in late murine embryonic development, the temporal and spatial expression of OCT4 after the formation of the ICM in equine embryos is unknown, so further studies are needed to provide clarity into the role of OCT4 in late equine embryonic development.
1.4. OCT4 as a Marker of Embryonic Development
As OCT4 is expressed in the early equine embryo, it has been used as a marker of embryonic development in several studies. For example, in a study on the effects of medium glucose concentration on equine embryo development, OCT4 staining was used to investigate the quality of the embryos [20]. OCT4 expression was also used as a marker to assess the developmental effects of aggregation, or co-culturing of multiple embryos, on cloned equine embryos [21]. This study identified that zona-free cloned embryos expressed OCT4 throughout the embryo in both the ICM and TE [21]. This finding could support the hypothesis that restriction of OCT4 to the ICM in the equine embryo is dependent on the uterine environment [12] or the hypothesis that the equine embryos require more time to reach the epiblast stage, which cannot be achieved in vitro. In mice, the formation of aggregates improved Oct4 expression in cloned embryos [22], but in the equine clonal aggregates, there was a decreased expression of OCT4 at day 16 in the trial with three zona-free reconstructed embryos in one culture [21]. This finding further suggests that there are regulatory differences in OCT4 expression between equine and murine embryos.
1.5. Functional Investigation of OCT4 Through Gene Knockout Experiments
To further study the regulatory functions of OCT4, OCT4 knockout (KO) embryos have been made for several species. This was first performed by Nichols and colleagues [6] with the creation of Oct4 null mice from homologous recombination in embryonic stem cells (ESCs). More recently, CRISPR-induced OCT4 knockouts have been made in human [7] and cattle [8,9] embryos, but no equine knockouts have been reported yet. KO studies highlight that OCT4 is vital for the formation of the ICM and the blastocyst, and for embryo survival. Additionally, knockouts are useful to identify the genes that are regulated by OCT4. However, these knockouts are limited as these embryos fail after the formation of the ICM, likely due to a lack of OCT4 and the genes it regulates. But, there are methods that can be used to bypass this embryonic lethality, such as the use of conditional knockouts, in which the gene is inactivated in a certain tissue, or the use of inducible knockouts, in which the gene is inactivated after a certain developmental point. For example, Kehler and coworkers [11] created a strain of conditional Oct4 knockout mice through the Cre/IoxP gene targeting approach to study the role of Oct4 in PGCs, a cell lineage that undergoes apoptosis in the absence of Oct4. Thus, the creation of an equine OCT4 knockout would be a valuable way to assess the role of OCT4 in the early embryo and better understand the genes that it regulates and the creation of a conditional or inducible knockout would allow researchers to further study the role of OCT4 after formation of the blastocyst.
2. Regulation
2.1. Regulation of POU5F1 Expression
OCT4 is regulated by three upstream cis-acting elements. These include a proximal promoter, a proximal enhancer, and a distal enhancer. A comparative alignment analysis of these regulatory regions was performed in mice, humans, and cattle that identified four conserved regions (CR1 to 4; one in the proximal promoter, one in the proximal enhancer, and two in the distal enhancer) with 66–94% conservation between these species [23]. The four CRs were identified in the equine genome through a nucleotide BLAST search using the CR sequences identified by Nordhoff and colleagues [23]. This investigation found that the equine sequences share a high sequence similarity with the human and cattle CRs (Table 1). Similar to the results of the study by Nordhoff and colleagues [23], the murine CRs showed less similarity to the equine sequence, but they did still show regions of homology with the equine sequence.
Table 1.
Comparison of horse sequence with cattle, mouse, and human conserved regions.
| Conserved Regions (CR) | Cattle | Mouse | Human |
|---|---|---|---|
| CR1 | 92.31% (120/130) | 85.38% (111/130) | 94.62% (123/130) |
| CR2 | 96.43% (189/197) | 93.50% (187/200) | 95.41% (187/196) |
| CR3 | 85.85% (91/106) | 80.00% (84/105) | 85.71% (90/105) |
| CR4 | 93.28% (125/134) | 70.15% (94/134) | 91.79% (123/134) |
The percent of sequence identity is presented along with the numbers of equal base pairs out of the total base pairs for each conserved region.
Subsequent studies have identified numerous transcription factors that bind to these four conserved regions. The most important are Sox2 (sex determining region Y box 2), Nanog, and Lrh1 (liver receptor homolog 1) [24]. Sox2 binds to CR4 to control transcription of Oct4 [24]. Additionally, Sox2 acts to maintain the levels of Oct4 expression through upregulation of positive regulators of Oct4 expression and by downregulating negative regulators [25]. Nanog binds at CR2 to open the chromatin, organize the formation of a transcription factor complex, and respond to activation signals [24]. Lrh1 acts at CR1 to initiate transcription by preventing nucleosome formation in the region and to organize the formation of a transcription factor complex at this locus [24].
In addition to local mediators, DNA methylation and histone modifications play an important role in regulating gene expression. The role of DNA methylation in regulating Oct4 expression was assessed in murine cells, which demonstrated that the Pou5f1 gene is unmethylated during the blastocyst stage but becomes methylated at 6.5 days post coitum and remains that way in adult tissues [26]. This methylation event leads to a severe decrease in gene expression due to the inability of RNA polymerase to bind to the region. There have not yet been investigations into how histone modifications effect POU5F1 expression nor have there been any investigations on the regulation of OCT4 to date in equine embryos.
There are also several reported repressors of Oct4 expression. Caudal related homeobox 2 (Cdx2) was found to repress Oct4 when it is overexpressed in mouse embryos [27]. It was also found that Coup-tf1 (chicken ovalbumin upstream promoter-transcription factor 1) binds to an Oct4-specific retinoic acid responsive element, modulating Oct4 expression [28]. Additionally, germ cell nuclear factor (Gcnf) was found to repress Oct4 expression directly through binding to the proximal promoter [29]. Again, the effects of these factors on OCT4 expression have not been assessed in equine embryos.
These studies show that there are several factors that play a role in regulating Oct4 expression, but none of these studies have identified the trigger(s) for the decreased expression of Oct4 when cells differentiate. Additionally, the specific factors that trigger its initial expression in the developing embryo are not known. The identification of these triggers would better enable scientists to maintain the pluripotency of cells in culture and would increase our understanding of the embryonic regulators of differentiation.
2.2. Regulation by OCT4
Chromatin immunoprecipitation followed by sequencing (ChIP-Seq) analysis of human ESCs identified that OCT4 associates with the promoter regions of 3% of the known coding genes in the human genome [30]. Interestingly, OCT4 binds to its own promoter and to the promoters for SOX2, NANOG, CDX2, and many others. Additionally, about half of the promoters that OCT4 bound to were also bound by SOX2 [30], where they act synergistically to activate Oct-Sox enhancers [25]. These enhancers act to positively self-regulate Oct4 and Sox2 expression along with regulating the expression of other pluripotency-associated genes, like Nanog. Interestingly, this study found that Sox2 is not essential for Oct-Sox enhancer activity as Sox2 knockout cells still had some enhancer activity, suggesting that Oct4 is the essential factor in activating these enhancers [25]. However, the binding affinity of Oct4 to the Oct-Sox enhancers is increased in the presence of Sox2 as Sox2 binds to the DNA first and assists in Oct4 binding [31].
Interestingly, over 90% of the shared Oct4 and Sox2 binding sites were also bound by Nanog [30]. This finding was further supported by Loh and colleagues [32] who found a substantial overlap of Oct4 and Nanog binding targets. These studies have opened the door to investigating the regulatory role of Oct4 in the early embryo, but there are still many functions of Oct4 that are not well understood, such as if the targets for Oct4 regulation change over time as embryo development occurs.
2.3. Equine Regulation of and by OCT4
Overall, there is a lack of studies investigating how OCT4 is regulated or how OCT4 regulates development in the equine embryo. A DNA methylation investigation in early equine embryos was performed, but the methylation patterns for the promoter region of OCT4 were not included in this investigation [33], which is unfortunate as this is the only study to date that has investigated the epigenetic regulation of the early equine embryo. As mentioned in section 2.2, Choi and coworkers [12] identified a difference in OCT4 expression between in vitro– and in vivo– produced equine embryos that they attributed to the uterine environment, but no further work has been done to investigate the specific factor(s) in the equine uterine environment that would cause this difference. The regulation of this gene in the equine embryo has been assumed from experiments performed in mice, but the differences seen in embryo development and placentation suggest that there could be different mechanisms for the regulation of OCT4 and/or a different role for OCT4 in the equine embryo compared to the murine embryo. Thus, further work into the regulation and function of OCT4 in equine embryos is necessary to gain a better understanding of the equine-specific developmental process.
3. Stem Cell Applications of OCT4
Murine Oct4 knockout embryos fail to establish ESC lines [6], highlighting how important this transcription factor is in maintaining pluripotency, both in vivo and in culture. As OCT4 appeared to play a role in the formation of ESCs, this gene was also investigated as a potential stem cell marker and indicator of pluripotency in equine cell lineages. Successful use of OCT4 as a marker led to its use to reprogram somatic cells into induced pluripotent stem cells (iPSCs).
3.1. OCT4 as an Embryonic Stem Cell Marker
To ascertain the pluripotency of cells in culture, markers needed to be established to distinguish pluripotent cells from differentiated cells. Pluripotent cells derived from mouse embryos express Oct4 in culture, suggesting that this gene could be a viable marker for pluripotency in mouse ESCs [6]. Additionally, mouse-derived ESCs present alkaline phosphatase activity and express stage-specific embryonic antigen 1 (Ssea1) [34,35]. Human ESCs were found to express SSEA3, SSEA4, tumor rejection antigen-1-60 (TRA-1-60), TRA-1-81, OCT4, and alkaline phosphatase, but not SSEA1 [36–38]. Based on these results, OCT4 and alkaline phosphatase had the potential to be universal markers for ESCs and could have importance in future studies of equine embryo biology.
Oct4 is required for the establishment of ESCs [6] and its expression level is critical for the maintenance of ESC characteristics in culture. A specific concentration of Oct4 was determined in mouse ESCs as vital to maintaining the self-renewal and pluripotent characteristics of those cell lines [13]. It was further shown that a less than two-fold increase in Oct4 expression led the cells to differentiate into endoderm and mesoderm, whereas a decrease in Oct4 expression led to a loss of pluripotency and differentiation into TE. Thus, Niwa and coworkers [13] concluded that there is a specific concentration of Oct4 that is required to sustain the cells in a pluripotent state and prevent cellular differentiation. These findings indicate that strict control of Oct4 concentration is essential to maintain the pluripotency of stem cells in culture.
Saito and colleagues [39] described the first attempt to isolate equine ESCs. These cells, derived from frozen blastocysts, expressed some of the same ESC markers as human and mouse cells, such as alkaline phosphatase and OCT4. But they found that the equine ES-like cells had an expression profile that was more similar to that of the mouse in that the embryos expressed SSEA1, but not SSEA3 or 4 [39]. A different study showed that equine ES-like cells also express TRA-1-60 and TRA-1-81 [40], which are present in human ESCs but not mouse ESCs. These findings suggest that early equine embryos have a unique expression profile, leading to potentially different phenotypic profiles from human and mouse embryos. Finally, Guest and Allen [41] found that OCT4 is the only marker shared across the ESCs of mice, humans, and horses. This finding suggests that OCT4 plays an essential role in maintaining pluripotency and the ability to self-renew across different species. However, none of these equine ES-like cells were capable of producing teratomas in immunodeficient mice [39,40], suggesting that they are not truly ESCs. This finding severely limits the conclusions that can be drawn regarding all the previously described putative ESC markers in the horse as they were described in lineages with questionable pluripotency.
OCT4 is frequently used as a marker of pluripotency in other species and it has been used as a marker in equine studies as well. For example, the presence of OCT4 expression was investigated via RT-PCR and immunofluorescence in ES-like cells derived from embryos created from somatic nuclear transfer and parthenogenesis (embryos produced without fertilization with sperm) [42]. OCT4 was identified to be expressed in the ICM of these embryos, but it was also found to be expressed in the TE, which could be attributed to the lack of exposure to a uterine environment [12] or inability to culture embryos to the epiblast stage. This study highlights the potential problem of using OCT4 as a marker for pluripotency in equine embryos in culture as cells of the TE lineage, which are not pluripotent, express OCT4 as well.
3.2. Role of OCT4 in iPSCs
Takahashi and Yamanaka [43] were the first to implement the idea of induced pluripotency in mouse cells. In that study, the authors identified 24 candidate genes that could potentially be used to induce pluripotency when culturing mouse somatic cells. In initial cultures with single gene additions, pluripotency was not induced in any of the cells, but in a culture with all 24 gene products, pluripotency was induced. From there, the authors iteratively removed genes to determine which were essential for inducing pluripotency. Thus, they identified that four genes: Oct4, Sox2, Klf4, and c-Myc (referred to as OSKM or “Yamanaka Factors”) were essential to induce pluripotency [43].
After its use in numerous mouse and human stem cell studies, the Yamanaka method was tested in equine somatic cells to induce pluripotency. In 2011, Nagy and colleagues [44] induced pluripotency in equine fibroblasts using the piggybac transposon method to insert the OSKM genes. In addition to expressing the anticipated pluripotency markers, these cells also had the ability to form teratomas (the gold standard for pluripotency) with all three embryonic tissue layers being expressed in immunocompromised mice [44], suggesting that these cells were truly pluripotent in contrast to the earlier studies done to produce equine ES-like cells [39,40].
3.2.1. Therapeutic Applications of Equine iPSCs
Khodadadi and coworkers [45] investigated the potential to create equine iPSCs without the use of c-MYC. In their study, they induced pluripotency in equine fibroblasts through retroviral transduction with only OCT4, SOX2 and KLF4 with similar expression of pluripotency markers and ability to form teratomas as the iPSCs formed with the use of c-MYC. This gene was excluded because it has been identified as a proto-oncogene that is frequently expressed in immortalized tumor cell lines [46]. The authors hypothesized that the exclusion of this gene could lead to the formation of iPSCs that are less risky for use in autologous transplantation for equine cartilage and tendon injury repair [45].
In addition to tendon and cartilage repair, equine iPSCs have been studied for their therapeutic application for several other tissue types. For example, iPSCs were created from equine adipose-derived stem cells that, when transplanted into mice, were incorporated into injured muscle tissue and increased muscle regeneration [47]. Further research showed that these adipose-derived iPSCs, when induced to differentiate into mesenchymal stem cells (MSC), could be used in horses to help treat various musculoskeletal diseases including fractures, arthritis, tendonitis, and osteochondritis [48]. In a recent study, pluripotency was induced in equine keratinocytes through a retroviral vector [49]. Through modification of growth conditions, the authors were able to generate cholinergic motor neurons, representing the first characterization of fully functional cells derived from equine iPSCs [49]. As these studies show, there is a variety of equine clinical conditions that could be treated using OCT4-stimulated iPSCs.
These equine studies all relied on transposon, retroviral, or lentiviral introduction of transgenes to induce pluripotency. Transgene integration into the genome poses a significant risk for iPSCs intended for clinical use as they lead to permanent genetic modifications that could affect endogenous gene expression or introduce mutations, potentially leading to the formation of tumors when implanted into an individual [50]. A way to decrease these risks and increase the therapeutic potential of equine iPSCs is through the creation of transgene-free iPSCs. This could be done using nonintegrating viral vectors or nonviral approaches, such as minicircle vectors or episomal vectors [50], but to date, no there have been no reports of the use of these alternative methods in equine cells.
3.3. The Use of OCT4 in Investigations of Adult Stem Cells
One unique investigation into the expression of equine OCT4 was performed on primary progenitor cells in the hoof wall [51]. This study aimed to culture these primary progenitor cells to further investigate their role in producing and maintaining deeper hoof tissues, thus investigating their ability to act as adult stem cells with regenerative capacity [51]. The authors subsequently identified the presence of OCT4 using RT-PCR [51], but this method does not assess the presence of the OCT4 protein. Detection of OCT4 mRNA does not necessitate the production of a functional protein, so, without clear nuclear presence of the OCT4 protein, it cannot be concluded that these cells are pluripotent.
A similar investigation was performed in which bone marrow–derived mesenchymal progenitor cells (MPCs) were assessed for the markers of pluripotency [33]. This study found that OCT4 was only detectable in iPSC lines and that, in MPCs, OCT4 expression was not statistically different from the expression in chondrocytes, a differentiated cell type, suggesting that the MPCs were not a pluripotent lineage in adult tissues [33]. Unlike the previous study, the absence of OCT4 mRNA does suggest that the OCT4 protein would be absent in these cells.
4. Conclusion and Future Directions
As shown here, there are many applications for which OCT4 can be used, and yet, there have been few studies on the specific function of OCT4 in equine embryos or cells. The few studies that have investigated gene expression in the early equine embryo have determined that the expression profile is different than the embryos of other species, but no additional work into those differences has been performed. The creation of an equine OCT4 knockout would allow for further investigation into the specific function of OCT4 in the early equine embryo. Additionally, the creation of an inducible or conditional knockout would allow for further study on the restriction of and the effects of OCT4 after the formation of the ICM. However, there are few equine oocytes available for research, thus increasing the difficulty in performing knockout studies in equine embryos compared to other species, such as mice and cattle. Similarly, isolation of equine ESCs with in vivo pluripotency potential and/or additional work in creating transgene-free iPSCs could significantly advance our understanding of OCT4 in equine pluripotency.
Acknowledgments
The authors thank the Students Training in Academic Research (STAR) Program for providing EAH with financial support through the UC Davis School of Veterinary Medicine Endowment Fund.
Financial disclosure:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Animal welfare/ethical statement: As this article is a review, the authors did not perform direct experimentation on animals and, therefore, do not have any ethical considerations to declare.
Conflict of interest statement: The authors declare no conflicts of interest.
References
- [1].Schöler HR, Hatzopoulos AK, Balling R, Suzuki N, Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J 1989;8:2543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Singh H, Sen R, Baltimore D, Sharp PA. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature 1986;319:154–8. [DOI] [PubMed] [Google Scholar]
- [3].Herr W, Sturm RA, Clerc RG, Corcoran LM, Baltimore D, Sharp PA, et al. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev 1988;2:1513–6. [DOI] [PubMed] [Google Scholar]
- [4].Gold DA, Gates RD, Jacobs DK. The early expansion and evolutionary dynamics of POU class genes. Mol Biol Evol 2014;31:3136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Eijk MJ, van Rooijen MA, Modina S, Scesi L, Folkers G, van Tol HT, et al. Molecular cloning, genetic mapping, and developmental expression of bovine POU5F1. Biol Reprod 1999;60:1093–103. [DOI] [PubMed] [Google Scholar]
- [6].Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998;95:379–91. [DOI] [PubMed] [Google Scholar]
- [7].Fogarty NME, McCarthy A, Snijders KE, Powell BE, Kubikova N, Blakeley P, et al. Genome editing reveals a role for OCT4 in human embryogenesis. Nature 2017;550:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Daigneault BW, Rajput S, Smith GW, Ross PJ. Embryonic POU5F1 is required for expanded bovine blastocyst formation. Sci Rep 2018;8:7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Simmet K, Zakhartchenko V, Philippou-Massier J, Blum H, Klymiuk N, Wolf E. OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proc Natl Acad Sci U S A 2018;115:2770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pesce M, Gross MK, Schöler HR. In line with our ancestors: oct-4 and the mammalian germ. Bioessays 1998;20:722–32. [DOI] [PubMed] [Google Scholar]
- [11].Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, et al. Oct4 is required for primordial germ cell survival. EMBO Rep 2004;5:1078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Choi YH, Harding HD, Hartman DL, Obermiller AD, Kurosaka S, McLaughlin KJ, et al. The uterine environment modulates trophectodermal POU5F1 levels in equine blastocysts. Reproduction 2009;138:589–99. [DOI] [PubMed] [Google Scholar]
- [13].Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000;24:372–6. [DOI] [PubMed] [Google Scholar]
- [14].Schöler HR, Dressler GR, Balling R, Rohdewohld H, Gruss P. Oct-4: a germline-specific transcription factor mapping to the mouse t-complex. EMBO J 1990;9: 2185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iqbal K, Chitwood JL, Meyers-Brown GA, Roser JF, Ross PJ. RNA-seq transcriptome profiling of equine inner cell mass and trophectoderm. Biol Reprod 2014;90:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vejlsted M, Offenberg H, Thorup F, Maddox-Hyttel P. Confinement and clearance of OCT4 in the porcine embryo at stereomicroscopically defined stages around gastrulation. Mol Reprod Dev 2006;73:709–18. [DOI] [PubMed] [Google Scholar]
- [17].Kirchhof N, Carnwath JW, Lemme E, Anastassiadis K, Schöler H, Niemann H. Expression pattern of Oct-4 in preimplantation embryos of different species. Biol Reprod 2000;63:1698–705. [DOI] [PubMed] [Google Scholar]
- [18].van Niekerk CH, Allen WR. Early embryonic development in the horse. J Reprod Fertil Suppl 1975;23:495–8. [PubMed] [Google Scholar]
- [19].Morris LH, Allen WR. Reproductive efficiency of intensively managed Thor-oughbred mares in Newmarket. Equine Vet J 2002;34:51–60. [DOI] [PubMed] [Google Scholar]
- [20].Choi YH, Ross P, Velez IC, Macías-García B, Riera FL, Hinrichs K. Cell lineage allocation in equine blastocysts produced in vitro under varying glucose concentrations. Reproduction 2015;150:31–41. [DOI] [PubMed] [Google Scholar]
- [21].Gambini A, Jarazo J, Olivera R, Salamone DF. Equine cloning: in vitro and in vivo development of aggregated embryos. Biol Reprod 2012;87:1–9. [DOI] [PubMed] [Google Scholar]
- [22].Boiani M, Eckardt S, Leu NA, Schöler HR, McLaughlin KJ. Pluripotency deficit in clones overcome by clone-clone aggregation: epigenetic complementation? EMBO J 2003;22:5304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nordhoff V, Hübner K, Bauer A, Orlova I, Malapetsa A, Schöler HR. Comparative analysis of human, bovine, and murine Oct-4 upstream promoter sequences. Mamm Genome 2001;12:309–17. [DOI] [PubMed] [Google Scholar]
- [24].Li YQ. Networks of transcription factors for Oct4 expression in mice. DNA Cell Biol 2017;36:725–36. [DOI] [PubMed] [Google Scholar]
- [25].Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol 2007;9:625–35. [DOI] [PubMed] [Google Scholar]
- [26].Gidekel S, Bergman Y. A unique developmental pattern of Oct-3/4 DNA methylation is controlled by a cis-demodification element. J Biol Chem 2002;277:34521–30. [DOI] [PubMed] [Google Scholar]
- [27].Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 2005;123:917–29. [DOI] [PubMed] [Google Scholar]
- [28].Ben-Shushan E, Sharir H, Pikarsky E, Bergman Y. A dynamic balance between ARP-1/COUP-TFII, EAR-3/COUP-TFI, and retinoic acid receptor:retinoid X receptor heterodimers regulates Oct-3/4 expression in embryonal carcinoma cells. Mol Cell Biol 1995;15:1034–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fuhrmann G, Chung AC, Jackson KJ, Hummelke G, Baniahmad A, Sutter J, et al. Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev Cell 2001;1:377–87. [DOI] [PubMed] [Google Scholar]
- [30].Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005;122:947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen J, Zhang Z, Li L, Chen BC, Revyakin A, Hajj B, et al. Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell 2014;156: 1274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet 2006;38:431–40. [DOI] [PubMed] [Google Scholar]
- [33].Hackett CH, Greve L, Novakofski KD, Fortier LA. Comparison of gene-specific DNA methylation patterns in equine induced pluripotent stem cell lines with cells derived from equine adult and fetal tissues. Stem Cells Dev 2012;21:1803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wobus AM, Holzhausen H, Jäkel P, Schöneich J. Characterization of a pluripotent stem cell line derived from a mouse embryo. Exp Cell Res 1984;152:212–9. [DOI] [PubMed] [Google Scholar]
- [35].Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A 1978;75:5565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–7. [DOI] [PubMed] [Google Scholar]
- [37].Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H, et al. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells 2002;20:329–37. [DOI] [PubMed] [Google Scholar]
- [38].Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 2000;18:399–404. [DOI] [PubMed] [Google Scholar]
- [39].Saito S, Ugai H, Sawai K, Yamamoto Y, Minamihashi A, Kurosaka K, et al. Isolation of embryonic stem-like cells from equine blastocysts and their differentiation in vitro. FEBS Lett 2002;531:389–96. [DOI] [PubMed] [Google Scholar]
- [40].Li X, Zhou SG, Imreh MP, Ahrlund-Richter L, Allen WR. Horse embryonic stem cell lines from the proliferation of inner cell mass cells. Stem Cells Dev 2006;15:523–31. [DOI] [PubMed] [Google Scholar]
- [41].Guest DJ, Allen WR. Expression of cell-surface antigens and embryonic stem cell pluripotency genes in equine blastocysts. Stem Cells Dev 2007;16:789–96. [DOI] [PubMed] [Google Scholar]
- [42].Desmarais JA, Demers SP, Suzuki J Jr, Laflamme S, Vincent P, Laverty S, et al. Trophoblast stem cell marker gene expression in inner cell mass-derived cells from parthenogenetic equine embryos. Reproduction 2011;141:321–32. [DOI] [PubMed] [Google Scholar]
- [43].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126: 663–76. [DOI] [PubMed] [Google Scholar]
- [44].Nagy K, Sung HK, Zhang P, Laflamme S, Vincent P, Agha-Mohammadi S, et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev Rep 2011;7:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Khodadadi K, Sumer H, Pashaiasl M, Lim S, Williamson M, Verma PJ. Induction of pluripotency in adult equine fibroblasts without c-MYC. Stem Cells Int 2012;2012:429160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 2005;132:885–96. [DOI] [PubMed] [Google Scholar]
- [47].Lee EM, Kim AY, Lee EJ, Park JK, Park SI, Cho SG, et al. Generation of equine-induced pluripotent stem cells and analysis of their therapeutic potential for muscle injuries. Cell Transplant 2016;25:2003–16. [DOI] [PubMed] [Google Scholar]
- [48].Chung MJ, Park S, Son JY, Lee JY, Yun HH, Lee EJ, et al. Differentiation of equine induced pluripotent stem cells into mesenchymal lineage for therapeutic use. Cell Cycle 2019;18:2954–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sharma R, Livesey MR, Wyllie DJ, Proudfoot C, Whitelaw CB, Hay DC, et al. Generation of functional neurons from feeder-free, keratinocyte-derived equine induced pluripotent stem cells. Stem Cells Dev 2014;23:1524–34. [DOI] [PubMed] [Google Scholar]
- [50].Haridhasapavalan KK, Borgohain MP, Dey C, Saha B, Narayan G, Kumar S, et al. An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene 2019;686:146–59. [DOI] [PubMed] [Google Scholar]
- [51].Yang Q, Pinto VMR, Duan W, Paxton EE, Dessauer JH, Ryan W, et al. In vitro characteristics of heterogeneous equine hoof progenitor cell isolates. Front Bioeng Biotechnol 2019;7:155. [DOI] [PMC free article] [PubMed] [Google Scholar]


