Abstract
Introduction:
Cardiac amyloidosis (CA) is an infiltrative cardiomyopathy and a common cause of heart failure with preserved and mid-range ejection fraction (HFpEF and HFmrEF). Left ventricular (LV) systolic assessment is pivotal in differential diagnostic and prognostic stratification in CA. However, nondeformation and deformation-based parameters classically implied had many limitations. Myocardial work (MW) has been recently introduced for the evaluation of myocardial performance, in a load-independent fashion, in patients with cardiomyopathies.
Aims:
This study aimed to evaluate MW parameters in LV performance assessment in CA and their possible role in differential diagnosis between AL and ATTR forms, compared with other echocardiographic parameters, also exploring the possible association between MW parameters and blood biomarkers.
Materials and Methods:
The study population consisted of 25 patients with CA (10 with AL amyloidosis and 15 with wild-type ATTR [ATTRwt] form) and HFpEF or HFmrHF, enrolled between March 2018 and December 2019, undergoing a comprehensive clinical, biochemical, and imaging evaluation. Ten healthy individuals were studied as controls. ATTR patients had a noninvasive diagnosis of wtATTR-CA (positive 99mTc-hydroxy methylene-diphosphonate scintigraphy with a negative hematological screening), while AL patients underwent endomyocardial biopsy. All patients underwent standard transthoracic echocardiography. MW and related indices were estimated using a vendor-specific module.
Results:
Compared to the ATTRwt group, patients in the AL group showed a more pronounced myocardial performance impairment assessed by Global Word Efficiency (GWE: 83.5% ± 6.3% vs. 88.2% ± 3.6%; P = 0.026). In multiple linear regression analysis, cardiac troponin I (Β = −0.55; P < 0.0001), global longitudinal strain (Β =0.35; P < 0.008), and regional relative strain ratio (Β = −0.30; P < 0.016) were significant predictors of GWE reduction in CA patients. At receiver operating characteristics curve analysis, among all other deformation-based and nondeformation-based echocardiographic parameters, GWE showed the highest area under the curve (AUC) (AUC 0.74; 95% CI: 0.55–0.96; P < 0.04). The optimal cutoff was determined by sensitivity/specificity analysis: a GWE < 86.5% identified patients with AL amyloidosis with a sensitivity and specificity, respectively, of 80.0% and 66.7%.
Conclusions:
The results of our pivotal study seem to highlight the importance of new deformation parameters to study myocardial performance in patients with CA, and to differentiate between AL CA and ATTR CA.
Keywords: Cardiac amyloidosis, heart failure, myocardial work
INTRODUCTION
Cardiac amyloidosis (CA) is infiltrative cardiomyopathy characterized by extracellular deposition of misfolded proteins: [1] immunoglobulin light chains in AL amyloidosis[2] and transthyretin in ATTR amyloidosis.[3] Left ventricular (LV) myocardial infiltration in CA typically starts from the subendocardial layers of the basal and middle regions, sparing the ventricular apical segments until the latest stages of the disease. Then, the diseases progress toward the more superficial layers in a nonuniform way.[4,5] The increased myocardial wall thickness and stiffness only partially account of the ventricular dysfunction that is also due to the effects exerted by the amyloid deposition in terms of abnormal cytoarchitecture, subendocardial ischemia, and direct cytotoxic effect; this latter effect mainly caused by the free light chains in the AL form.[6] Those features picture CA as cardiomyopathy with hypertrophic phenotype, restrictive physiology, and early systolic dysfunction.[7] Amyloid infiltration is inhomogeneous, progressive, and clinically important, showing the poorest prognosis in AL CA and a more benign course in ATTR CA, especially in wild-type forms.[8,9,10]
It is well accepted that multimodality imaging represents an essential approach in the clinical setting for differential diagnosis, etiology definition, and prognosis stratification in patients with CA.[11,12] Echocardiography is widely used in clinical practice to characterize morphological and functional consequences of amyloid deposition on myocardial function.[13] Left ventricular ejection fraction (LVEF), which represents the most common method to evaluate LV function, often remains preserved until advanced stages of disease.[14,15,16] Therefore, LV global longitudinal strain (GLS) emerged in the latest years as an accurate tool to evaluate global myocardial function and to detect subclinical myocardial dysfunction.[17] Moreover, GLS and other deformation-derived parameters (e.g. relative regional strain ratio [RRSR], ejection fraction strain ratio [EFSR]) have shown to be useful both in diagnosis and risk stratification.[18,19,20] However, deformation parameters also suffer from load dependency, and this limits its use in patients with CA due to the wide use of diuretics for symptoms management in this population.[21]
Recently, novel echocardiographic methods have been introduced for the evaluation of myocardial performance in a load-independent fashion. Myocardial work (MW) represents a noninvasive LV pressure-strain loop (LV-PSL) analysis derived from echocardiographic and clinical data, offering a surrogate of the invasively-determined LV-PVL that correlates well with invasive hemodynamic parameters and cardiac metabolism assessed by positron emission tomography.[22] This method allows estimation of global and segmental work, expressed in mmHg% unit, as a surrogate of power over the cardiac cycle.[23]
The better knowledge of MW parameters and their routine evaluation in patients with CA would provide an accurate tool to refine follow-up myocardial function, when systemic blood pressure might be extremely variable, and to assess the effect of different treatments.
Aims
This study aimed to: (i) evaluate MW indices in patients with CA and their association with clinical status, blood biomarkers, and conventional and deformation-derived echocardiographic parameters and (ii) evaluate the possible role of MW parameters in the differential diagnosis between AL and ATTR CA.
MATERIALS AND METHODS
Population
The study population consisted of 25 patients with CA (10 with AL amyloidosis and 15 with wild-type ATTR [ATTRwt] form) referred to the Inherited and Rare Cardiovascular Diseases Unit of the Monaldi Hospital, Naples, Italy, and 10 healthy subjects. All patients were enrolled between March 2018 and December 2019 and the data were collected prospectively. All patients in the AL group had endomyocardial biopsy-proven CA and positive immune histology for AL amyloidosis. In contrast, patients in the ATTR group had a noninvasive diagnosis of wtATTR-CA with positive 99mTc-hydroxy methylene-diphosphonate scintigraphy, negative hematological screening, and negative genetic testing for TTR gene mutations.[24] All patients have been evaluated after an adequate period of optimal medical therapy to avoid excessive fluctuations of blood pressure and volemic state. Patients with LV dysfunction due to other causes (e.g. coronary artery disease, LV dyssynchrony), left ventricular outflow obstruction, and significant valvulopathy (e.g. aortic stenosis of a more than moderate degree) were excluded from the study.
Laboratory analysis
Serum levels of high sensitivity cardiac troponin I (HS-c-TnI) and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) were measured from peripheral venous blood samples in all patients using standard commercially available assays. Reference values were as follows: NT-proBNP <125 ng/L and HS-cTnI <0.04 μg/L.
Echocardiography
All patients underwent standard transthoracic echocardiography using a Vivid E9 ultrasound system (GE Healthcare, Horten, Norway) equipped with an M5S 3.5-MHz transducer. Two-dimensional (2D), color Doppler, pulsed wave, and continuous wave Doppler data were acquired and then stored on a dedicated workstation for the offline analysis (EchoPAC Version 202, GE Vingmed Ultrasound, Norway). Appropriate echocardiographic windows were obtained in all the patients. Chamber quantification was carried out according to current recommendations.[25] Diastolic parameters were collected in apical four-chamber view by wave and tissue Doppler.[26] Myocardial contraction fraction (MCF), a volumetric measure of myocardial shortening independent of chamber size and geometry, was calculated as the ratio of stroke volume (SV) on myocardial volume.[27] SV was obtained by the Simpson biplane method, while myocardial volume was calculated as the ratio of LV mass (LVM) over 1.05 (myocardial density). Peak systolic longitudinal strain (LS) and 2D Speckle-Tracking (2D-ST) strain measurements were performed in the three standard apical views, and an automatically generated bulls' eye of segmental peak systolic LS values was obtained. GLS was calculated by the average of regional LS values, as recommended.[28] Regional LS for six basal, six mid, and five apical views of the LV was averaged to obtain three mean regional LS values (basal, mid, and apical). RRSR was calculated by dividing the average apical LS by the sum of the average basal and mid-LS values.[29]
Myocardial work analysis
MW and related indices were estimated using a vendor-specific module (EchoPAC 202.0.0; see above). MW was estimated, as a function of time, throughout the cardiac cycle combining LV strain data by 2D-ST analysis and a noninvasively estimated LV pressure (LVP) curve.[30] A 17-segment model was used for the estimation of segmental MW. 2D grayscale images were acquired in the standard apical views at a frame rate of ≥60 frames/s to estimate LV GLS. The instantaneous systolic pressure was estimated by a brachial artery cuff and assumed to be equal to peak systolic LVP and to be uniform throughout the ventricle. The reliability of this noninvasively estimated LVP curve has been previously validated;[31] the software constructs a noninvasive LVP curve adjusted according to the duration of isovolumic and ejection phases defined by valvular timing events through the cardiac cycle. Strain and pressure data were synchronized using the R wave on the ECG as a standard time reference. MW was then quantified multiplying the rate of segmental shortening of the strain curve by the instantaneous LVP. This result is integrated over time to obtain MW as a function of time, from mitral valve closure (MVC) to mitral valve opening (MVO), comprising the isovolumic contraction and LV ejection period. During this phase, segmental shortening contributes to LV ejection and represents constructive work, whereas segmental stretch or lengthening in the same phase represents wasted work. The opposite occurs during LV relaxation. The following parameters were then calculated: (i) Global Work Index (GWI), as the total work within the area of the LV-PSL calculated from MVC to MVO; (ii) Global constructive work (GCW), as the work that contributes to LV ejection in systole (myocytes shortening in systole and myocytes lengthening during isovolumic relaxation phase); (iii) Global Wasted Work (GWW), as the work which does not contribute to LV ejection (myocytes lengthening rather than shortening in systole and shortening rather than lengthening during isovolumic relaxation phase); (iv) Global Work Efficiency (GWE), as the estimation of the proportion of constructive work in systole by the formula: (GCW)/(GCW + GWW) ×100. In healthy hearts, all myocardial segments shorten in systole and relax in diastole simultaneously, contributing together efficaciously to MW.[32]
Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test and compared accordingly using Student's t or Mann–Whitney U-test; for comparisons among multiple groups, a one-way ANOVA with post hoc Kruskal–Wallis analysis was used. Categorical data were expressed as frequencies and percentages and compared by the Chi-square test. Univariate linear regression analysis with a bias-corrected bootstrap method with 10.000 resamplings was used to find predictors for the amount of GWE reduction in CA patients. Multivariate analysis was performed by entering into the model a set of variables that were considered significant on univariate analysis (P < 0.05) to identify the parameters that best predicted the reduction in GWE. The limited number of patients in the study group allows us to test only three variables at a time. Receiver operating characteristics (ROCs) curve (area under the curve [AUC]) was determined to evaluate the diagnostic performance of different parameters for the diagnosis of AL amyloidosis in CA patients (discriminating AL from ATTR CA). An optimal cutoff value for the diagnosis of AL amyloidosis was chosen to maximize the Youden's index (sensitivity + specificity ‒ 1). All statistical analysis was performed using a standard statistical software program (SPSS Version 23.0, IBM, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Study population
Baseline characteristics are presented in Table 1.
Table 1.
Demographic data and biochemistry results in AL, ATTR, and control group
| Variable | AL group (n=10), n (%) | ATTRwt group (n=15), n (%) | Control (n=10), n (%) |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 66.0±12.1# | 80.0±6.1† | 58.0±13.5 |
| Gender male | 4 (40)# | 12 (80) | 5 (50) |
| BSA (m2) | 1.68±0.17 | 1.81±0.14 | 1.80±0.10 |
| SBP (mmHg) | 102.5±19.6 | 112.0±17.1 | 115.5±10.9 |
| DBP (mmHg) | 63.5±11.3 | 68.0±11.3 | 68.5±11.5 |
| Peripheral neuropathy | 3 (30) | 8 (53) | 0 |
| Cardiovascular dysautonomia | 4 (40) | 7 (47) | 0 |
| NYHA functional class | |||
| I | 0 | 1 (6) | 10 (100) |
| II | 4 (40) | 9 (60) | 0 |
| III | 4 (40) | 5 (33) | 0 |
| IV | 2 (20) | 0 | 0 |
| Cardiac biomarkers | |||
| cTnI (μg/L) | 0.46±0.43#,* | 0.09±0.07 | 0.01±0.01 |
| NT-proBNP (pg/L) | 10817±15158* | 6272±6407 | 48±24 |
*AL groups versus all other groups; P<0.05, #AL group versus ATTR group; P<0.05, †ATTR group versus control group; P<0.05. ATTRwt=ATTR wild type, BSA=Body surface area, cTnI=Cardiac troponin I, DBP=Diastolic blood pressure, NT-pro-BNP=N-terminal prohormone of brain natriuretic peptide, NYHA=New York Heart Association, SBP=Systolic blood pressure
As expected, patients with CA showed significantly higher values of cardiac biomarkers compared to the control group. Moreover, patients in the ATTR group were older and prevalently male, while those in the AL group showed significantly higher c-Tn levels compared to ATTR group.
Echocardiography
Conventional echocardiographic characteristics are described in Table 2.
Table 2.
Global echocardiographic morphological and functional parameters in AL, ATTR wild type, and control group
| Variable | AL group (n=10) | ATTRwt group (n=15) | Control group (n=10) |
|---|---|---|---|
| Morphology | |||
| LVEDV (ml) | 63.3±44.7 | 80.1±28.3 | 99.2±23.9* |
| LVESV (ml) | 36.5±33.9 | 42.9±15.6 | 38.9±10.9 |
| LVEDd (mm) | 39.8±7.9 | 43.5±6.8 | 45.3±4.8 |
| LVESd (mm) | 27.8±9.4 | 31.3±4.8 | 28.2±5.4 |
| IVSd (mm) | 17.0±3.7 | 18.2±4.6 | 8.8±1.4*,# |
| PWd (mm) | 15.4±3.8 | 16.3±4.2 | 8.3±1.3*,# |
| RWT | 0.80±0.25 | 0.77±0.29 | 0.37±0.06*,# |
| ECC index | 1.11±1.10 | 1.13±0.22 | 1.06±0.06 |
| LVM index (g/m2) | 158.1±57.8 | 185.5±72.4 | 70.1±19.3*,# |
| LAD (mm) | 43.6±10.0† | 52.6±8.5 | 36.3±3.1*,# |
| LAV index (ml/m2) | 38.7±11.4† | 61.3±16.5 | 26.3±6.2*,# |
| Diastolic function | |||
| E wave (cm/s) | 95.2±29.1 | 90.3±23.6 | 79.1±14.9 |
| A wave (cm/s) | 53.6±24.7 | 54.0±31.3 | 56.2±9.6 |
| E/A ratio | 1.92±1.08 | 2.19±1.14 | 1.44±0.39 |
| DecT (ms) | 144.6±38.4 | 145.6±42.4 | 217.0±56.2*,# |
| E/E’ | 25.7±12.6 | 18.9±4.1 | 6.5±0.8*,# |
| Myocardial function | |||
| LVEF (%) | 46.2±8.9 | 46.9±8.8 | 61.1±2.3*,# |
| SV index (ml/m2) | 15.6±6.1 | 20.4±7.6 | 33.3±6.4*,# |
| MCF (%) | 11.0±4.7 | 12.6±5.0 | 51.5±9.2*,# |
*Control group versus AL group; P<0.05, #Control group versus ATTR group; P<0.05, †AL group versus ATTR group; P<0.05. DecT=Deceleration time, ECC=Eccentricity, IVSd=Interventricular septum diameter, LAD=Left atrium diameter, LAV=Left atrium volume, LVEDd=Left ventricle end-diastolic diameter, LVEDV=Left ventricle end-diastolic volume, LVEF=Left ventricle ejection fraction, LVESd=Left ventricle end-systolic diameter, LVESV=Left ventricle end-systolic volume, LVM=Left ventricle mass, MCF=Myocardial contraction fraction, PWd=Posterior wall diameter, RWT=Relative wall thickness, SV=Stroke volume, ATTRwt=ATTR wild type
Compared to healthy subjects, patients with CA were characterized by the symmetric increase of LV wall thickness and mass (concentric hypertrophy) with nondilated ventricles, a significant reduction in LV systolic function assessed by nondeformation parameters (LVEF, MCF, SV index), and higher LV filling pressures (E/E').
The comparison between groups with CA showed no differences in LV geometry (RWT), wall thickness (IVSd, PWd), mass (LVM), size (LVEDd, LVESd, ECC index), systolic function (LVEF, MCF, SV index), and filling pressures (E/E'). The only conventional echocardiographic parameters statistically different between AL and ATTR group were LAD and LAVI: indeed, patients with ATTRwt form showed a more significant LA enlargement when compared to AL patients (P < 0.001).
Deformation-based and MW-derived features are described in Table 3.
Table 3.
Deformation-based and myocardial work-derived parameters in AL, ATTR wild type, and control group
| Variable | AL group (n=10) | ATTRwt group (n=15) | Control group (n=25) |
|---|---|---|---|
| Deformation-based parameters | |||
| GLS (%) | 8.5±2.1 | 9.5±2.6 | 20.2±1.2*,# |
| EFSR | 5.6±1.3 | 5.1±1.0 | 3.0±0.1*,# |
| RRSR | 1.12±0.44 | 1.06±0.52 | 0.44±0.04*,# |
| Myocardial work parameters | |||
| GWI (mmHg%) | 751.3±425.6 | 795.0±257.6 | 2372.0±226.4*,# |
| GCW (mmHg%) | 845.0±406.6 | 964.9±270.5 | 2522.9±237.7*,# |
| GWW (mmHg%) | 128.1±86.2† | 79.7±34.6 | 92.2±26.9 |
| GWE (%) | 83.5±6.3† | 88.2±3.6 | 96.3±1.0*,# |
*Control group versus AL group; P<0.05, #Control group versus ATTR group; P<0.05, †AL group versus ATTR group; P<0.05. EFSR=Ejection fraction on strain ratio, GCW=Global constructive work, GLS=Global longitudinal strain, GWE=Global work efficiency, GWI=Global work index, GWW=Global wasted word, RRSR=Relative regional strain ratio, ATTRwt=ATTR wild type
All deformation-based (GLS, EFSR, RRSR) and MW-derived parameters (GWI, GCW, GWE) were significantly abnormal in CA compared to group control (GWI, GCW, GWE) [Figure 1]. Longitudinal deformation properties, evaluated with regional quantitative LS assessment (RRSR), were significantly different in CA than in the control group, reflecting a prevalent impairment in basal and midventricular segments.
Figure 1.
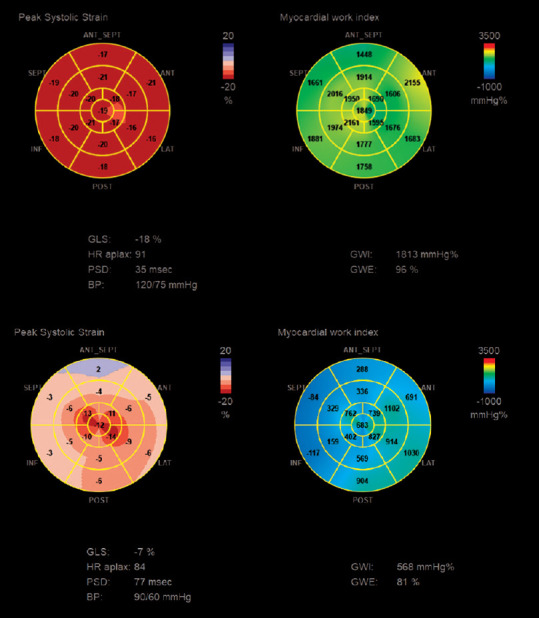
An example of peak systolic strain and myocardial work index bull's eye in a healthy subject (upper panel) and in a patient with advanced AL cardiac amyloidosis (lower panel)
Moreover, among deformation-based and MW-derived parameters, GWW and GWE showed a statistically suggestive difference between ATTRwt and AL patients, suggesting a more pronounced myocardial performance dysfunction in this group (83.5% ±6.3% vs. 88.2% ±3.6%; P = 0.026).
Univariate and multivariate linear regression analysis performed between GWE and demographic and echocardiographic variables in CA patients are displayed in Table 4.
Table 4.
Univariate and multivariate associations of global work efficiency
| GWE (adjusted r2=0.705) | Univariate | Multivariate | |||
|---|---|---|---|---|---|
|
|
|
||||
| Adjusted r2 | B (95% CI) | P | B (95% CI) | P | |
| cTnI | 0.468 | −0.70 (−15.3-−5.90) | <0.0001 | −0.55 (−12.0-−4.7) | <0.0001 |
| ECC index | 0.182 | 0.46 (2.45-25.06) | 0.019 | ||
| SV index | 0.180 | 0.46 (0.06-0.61) | 0.020 | ||
| MCF | 0.137 | 0.41 (0.02-0.88) | 0.038 | ||
| GLS | 0.317 | 0.59 (0.51-2.01) | 0.002 | 0.35 (0.22-1.28) | 0.008 |
| EFSR | 0.151 | −0.43 (−3.75-−0.19) | 0.031 | ||
| RRSR | 0.215 | −0.50 (−9.56-−1.35) | 0.011 | −0.30 (−5.9-−0.7) | 0.016 |
ECC=Eccentricity, cTnI=Cardiac troponin I, EFSR=Ejection fraction on strain ratio, GLS=Global longitudinal strain, GWE=Global work efficiency, MCF=Myocardial contraction fraction, RRSR=Relative regional strain ratio, SV=Stroke volume, CI=Confidence interval
By univariate linear regression analysis, GWE correlated inversely with cTnI serum levels, EFSR and RRSR and directly with ECC index, SV index, MCF, and GLS. In multiple linear regression analysis, cTnI (B = −0.55; P < 0.0001), GLS (B =0.35; P < 0.008), and RRSR (B = −0.30; P < 0.016) were significant predictors of GWE reduction in CA patients. Sequential multivariate model, including these significant variables, has been employed to determine whether deformation-based parameters (GLS, RRSR) and plasma HS-cTnI levels could predict MW efficiency. It showed that reasonable predictive value (adjusted r2 = 0.705) for the GWE reduction in CA and HS-cTnI gave the greatest contribution [Figure 2].
Figure 2.
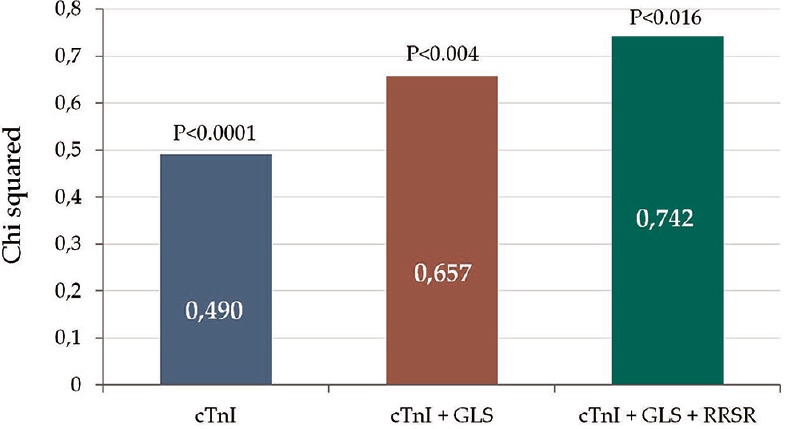
Sequential multivariate model employed to determine whether echocardiography-based and biohumoral-based indices of myocardial injury could predict myocardial work efficiency. Note the improved predictive value of cardiac troponin I in respect to echocardiographic (global longitudinal strain, relative regional strain ratio) data
Diagnostic accuracy of nondeformation-based and deformation-based echo parameters in detecting AL amyloidosis among patients with CA is displayed in Table 5.
Table 5.
Receiver-operating characteristics curve characteristics and cutoff points of nondeformation-based and deformation-based echocardiographic parameters for differential diagnosis of AL versus ATTR wild-type amyloidosis
| Variable | AUC | P | Cutoff | 95% CI | Sens (%) | Spec (%) |
|---|---|---|---|---|---|---|
| LVM index (g/m2) | 0.59 | 0.44 | 0.36-0.82 | |||
| RWT | 0.57 | 0.56 | 0.32-0.82 | |||
| ECC index | 0.50 | 0.98 | 0.27-0.73 | |||
| LVEF (%) | 0.52 | 0.87 | 0.28-0.76 | |||
| SV index (ml/m2) | 0.69 | 0.11 | 0.47-0.91 | |||
| MCF (%) | 0.59 | 0.11 | 0.36-0.82 | |||
| GLS (%) | 0.60 | 0.12 | 0.37-0.83 | |||
| EFSR (%) | 0.59 | 0.12 | 0.36-0.82 | |||
| RRSR | 0.57 | 0.54 | 0.32-0.82 | |||
| GWI (mmHg%) | 0.61 | 0.34 | 0.37-0.86 | |||
| GCW (mmHg%) | 0.67 | 0.15 | 0.44-0.90 | |||
| GWW (mmHg%) | 0.68 | 0.13 | 0.46-0.90 | |||
| GWE (%) | 0.74 | 0.04 | 86.5 | 0.55-0.96 | 80.0 | 66.7 |
AUC=Area under the curve, CI=Confidence interval, ECC=Eccentricity, EFSR=Ejection fraction on strain ratio, GCW=Global constructive work, GLS=Global longitudinal strain, GWE=Global work efficiency, GWI=Global work index, GWW=Global wasted work, LVEF=Left ventricle ejection fraction, LVM=Left ventricle mass, MCF=Myocardial contraction fraction, RRSR=Relative regional strain ratio, RWT=Relative wall thickness, SAB=Septal apical-to-base ratio, Sens=Sensibility, Spec=Specificity, SV=Stroke volume
At ROC curve analysis, among all other deformation-based and nondeformation-based echocardiographic parameters, GWE showed the highest AUC (AUC 0.74; 95% CI: 0.55–0.96; P < 0.04). The optimal cutoff was determined by sensitivity/specificity analysis with calculation of Youden's J index: a GWE < 86.5% identified patients with AL amyloidosis with a sensitivity and specificity, respectively, of 80.0% and 66.7%.
DISCUSSION
This study was performed to explore the role of MW indices of LV myocardial performance in patients with CA in comparison with other conventional and deformation-based echocardiographic parameters; and their possible relationship with known prognosis parameters such as patient's clinical status and cardiac biomarkers.
MW indices, as expected, are significantly lower in CA patients compared to group control, reflecting the significant myocardial performance impairment detectable in CA. Interestingly, except for GWE, the comparison between AL and ATTRwt patients showed no significant differences in LV myocardial performance assessed by conventional, deformation-based, and other MW-derived indices. Indeed, GWE was significantly lower in AL patients that in ATTR group.
To our knowledge, this is the first study reporting a significant difference in terms of GWE between AL and ATTRwt patients. Previous studies reported the value of MW indices in the clinical evaluation of patients with CA but showed no significant differences in terms of GWE between patients with AL and ATTR CA.[33]
These discrepancies may be attributable to the differences in terms of myocardial performance status between the two populations: patients in the French study were presumably enrolled in earlier stages of the diseases, as suggested by preserved LVEF values, while our population showed a significant reduction in LV systolic function parameters.
The significant reduction in GWE CA could be attributable to extensive myocardial infiltration which reduces myocardial deformation properties. A more pronounced reduction on GWE values in AL group compared to ATTRwt group could be probably attributable to the direct cytotoxic effect exerted by circulating free light chain in this setting, and the higher values of c-Tn detected in AL could be a marker of such toxic damage.[34] Indeed, the variables that best predicted GWE reduction at multiple linear regression analysis were c-Tn and deformation-based parameters such as GLS and RRSR. On the other hand, at multivariate sequential analysis, c-Tn gave the best contribution to GWE reduction. However, a larger study including also patients in earlier stages of disease is necessary to confirm our hypothesis.
Furthermore, MW-derived indices have been compared with other conventional and deformation-based parameter in AL amyloidosis detection among patients with CA. At ROC curve analysis, GWE showed the best ability in predicting AL amyloidosis, suggesting the importance of myocardial performance dysfunction differences between groups in the differential diagnosis.
Clinical implications
Our results seem to suggest that MW-derived indices are more accurate than other echocardiography-based parameters in myocardial performance evaluation, being theoretically free from many of their limitations, including load dependency. As known, cardiac involvement in systemic amyloidosis negatively influences prognosis, and its early recognition is crucial for starting appropriate treatment timely to modify the natural history of the disease. Moreover, as known, AL CA form carries the worst prognosis compared to other CA forms. Our results seem promising since they facilitate noninvasive differential diagnosis allowing the recognition of AL patients with sufficient sensitivity and specificity and accelerating the diagnostic process, with all that this entails.
GWE seems to be the most promising parameter in this setting: as showed, myocardial efficiency, which reflects the myocardial ability to conduct work, is significantly impaired in CA.[30] Moreover, it is useful to remember that also the MW, determined noninvasively with LV-PSL, is correlated with regional glucose-based cardiac metabolism which is known to be abnormal in CA.
However, this pivotal study has been conducted on a small sample size, and the results need to be confirmed on a larger study population.
Study limitations
This study is a single-center experience conducted on a limited number of patients. There were significant differences in age and sex between AL and ATTR groups that potentially may influence myocardial oxidative metabolism and myocardial deformation parameters. As describe, the predictive performance of the GWE cutoff obtained is modest and could be more limited in the early stages of disease. Moreover, SV was calculated with the biplane Simpson method, which may have potentially underestimated its value.
Finally, most patients enrolled in the study were in advanced stages of the disease, as suggested by the reduced LVEF values and the increased cardiac biomarkers levels, representing a possible bias in translating our results to patients at early stages of the disease.
CONCLUSIONS
The results of our pivotal study seem to highlight the importance of new deformation parameters to study myocardial performance in patients with CA and to differentiate between AL and ATTR CA.
Ethical clearance
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Financial support and sponsorship
Nil.
Conflicts of interest
GL had travel grant from AMICUS and speaker fee from Pfizer.
REFERENCES
- 1.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–96. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 2.Falk RH, Alexander KM, Liao R, Dorbala S. AL (light-chain) cardiac amyloidosis: A review of diagnosis and therapy. J Am Coll Cardiol. 2016;68:1323–41. doi: 10.1016/j.jacc.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 3.Gertz MA, Benson MD, Dyck PJ, Grogan M, Coelho T, Cruz M, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol. 2015;66:2451–66. doi: 10.1016/j.jacc.2015.09.075. [DOI] [PubMed] [Google Scholar]
- 4.Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–8. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 5.Sperry BW, Vranian MN, Tower-Rader A, Hachamovitch R, Hanna M, Brunken R, et al. Regional variation in technetium pyrophosphate uptake in transthyretin cardiac amyloidosis and impact on mortality. JACC Cardiovasc Imaging. 2018;11:234–42. doi: 10.1016/j.jcmg.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Jordan TL, Maar K, Redhage KR, Misra P, Blancas-Mejia LM, Dick CJ, et al. Light chain amyloidosis induced inflammatory changes in cardiomyocytes and adipose-derived mesenchymal stromal cells. Leukemia. 2020;34:1383–93. doi: 10.1038/s41375-019-0640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–9. doi: 10.1161/CIRCULATIONAHA.113.006242. [DOI] [PubMed] [Google Scholar]
- 8.Falk RH. Pondering the prognosis and pathology of cardiac amyloidosis: Answers breed questions. JACC Cardiovasc Imaging. 2016;9:139–41. doi: 10.1016/j.jcmg.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–12. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 10.Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121:3420–7. doi: 10.1182/blood-2012-12-473066. [DOI] [PubMed] [Google Scholar]
- 11.Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2-evidence base and standardized methods of imaging. J Card Fail. 2019;25:e1–39. doi: 10.1016/j.cardfail.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 2 of 2-diagnostic criteria and appropriate utilization. J Card Fail. 2019;25:854–65. doi: 10.1016/j.cardfail.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Falk RH, Quarta CC. Echocardiography in cardiac amyloidosis. Heart Fail Rev. 2015;20:125–31. doi: 10.1007/s10741-014-9466-3. [DOI] [PubMed] [Google Scholar]
- 14.Mele D, Nardozza M, Ferrari R. Left ventricular ejection fraction and heart failure: An indissoluble marriage? Eur J Heart Fail. 2018;20:427–30. doi: 10.1002/ejhf.1071. [DOI] [PubMed] [Google Scholar]
- 15.Konstam MA, Abboud FM. Ejection fraction: Misunderstood and overrated (changing the paradigm in categorizing heart failure) Circulation. 2017;135:717–9. doi: 10.1161/CIRCULATIONAHA.116.025795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triposkiadis F, Giamouzis G, Boudoulas KD, Karagiannis G, Skoularigis J, Boudoulas H, et al. Left ventricular geometry as a major determinant of left ventricular ejection fraction: Physiological considerations and clinical implications. Eur J Heart Fail. 2018;20:436–44. doi: 10.1002/ejhf.1055. [DOI] [PubMed] [Google Scholar]
- 17.Pagourelias ED, Mirea O, Duchenne J, Van Cleemput J, Delforge M, Bogaert J, et al. Echo parameters for differential diagnosis in cardiac amyloidosis: A head-to-head comparison of deformation and nondeformation parameters. Circ Cardiovasc Imaging. 2017;10:e005588. doi: 10.1161/CIRCIMAGING.116.005588. [DOI] [PubMed] [Google Scholar]
- 18.Sun JP, Stewart WJ, Yang XS, Donnell RO, Leon AR, Felner JM, et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103:411–5. doi: 10.1016/j.amjcard.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 19.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54:618–24. doi: 10.1016/j.jacc.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 20.Liu D, Hu K, Niemann M, Herrmann S, Cikes M, Störk S, et al. Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:1066–72. doi: 10.1161/CIRCIMAGING.113.000683. [DOI] [PubMed] [Google Scholar]
- 21.Baxter SC, Kedar S, Parker JW, et al. Limitations of strain estimation techniques from discrete deformation observations. Geophys Res Lett. 2011;38:L01305. [Google Scholar]
- 22.Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur Heart J. 2012;33:724–33. doi: 10.1093/eurheartj/ehs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubert A, Le Rolle V, Leclercq C, Galli E, Samset E, Casset C, et al. Estimation of myocardial work from pressure-strain loops analysis: An experimental evaluation. Eur Heart J Cardiovasc Imaging. 2018;19:1372–9. doi: 10.1093/ehjci/jey024. [DOI] [PubMed] [Google Scholar]
- 24.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–12. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 25.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–60. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 27.Milani P, Dispenzieri A, Gertz MA, et al. In patients with light-chain (AL) amyloidosis myocardial contraction fraction (MCF) is a simple, but powerful prognostic measure that can be calculated from a standard echocardiogram. Blood. 2015;126:1774. [Google Scholar]
- 28.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–93. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Senapati A, Sperry BW, Grodin JL, Kusunose K, Thavendiranathan P, Jaber W, et al. Prognostic implication of relative regional strain ratio in cardiac amyloidosis. Heart. 2016;102:748–54. doi: 10.1136/heartjnl-2015-308657. [DOI] [PubMed] [Google Scholar]
- 30.Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Gjesdal O, et al. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am J Physiol Heart Circ Physiol. 2013;305:H996–1003. doi: 10.1152/ajpheart.00191.2013. [DOI] [PubMed] [Google Scholar]
- 31.Hwang IC, Cho GY, Yoon YE, Park JJ. Association between global longitudinal strain and cardiovascular events in patients with left bundle branch block assessed using two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2018;31:52–63.e6. doi: 10.1016/j.echo.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Manganaro R, Marchetta S, Dulgheru R, Ilardi F, Sugimoto T, Robinet S, et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2019;20:582–90. doi: 10.1093/ehjci/jey188. [DOI] [PubMed] [Google Scholar]
- 33.Roger-Rollé A, Cariou E, Rguez K, Fournier P, Lavie-Badie Y, Blanchard V, et al. Can myocardial work indices contribute to the exploration of patients with cardiac amyloidosis? Open Heart. 2020;7:e001346. doi: 10.1136/openhrt-2020-001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cappelli F, Baldasseroni S, Bergesio F, Perlini S, Salinaro F, Padeletti L, et al. Echocardiographic and biohumoral characteristics in patients with AL and TTR amyloidosis at diagnosis. Clin Cardiol. 2015;38:69–75. doi: 10.1002/clc.22353. [DOI] [PMC free article] [PubMed] [Google Scholar]


