Abstract
Purpose:
The purpose of the study was to evaluate the ability of periostin when impregnated onto varied collagen matrices to influence osteoblast cell adhesion, proliferation, and activity.
Materials and Methods:
Saos-2 osteoblast cells were cultured and seeded onto two different collagen matrices as follows: Group A: absorbable collagen sponge (ACS), Group B: ACS impregnated with recombinant human periostin, Group C: nanocrystalline hydroxyapatite collagen (NcHC), and Group D: NcHC impregnated with recombinanant human periostin. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed to evaluate cell viability as well as adhesion and proliferation on 2nd, 5th, and 7th day. Osteoblast activity was studied using alkaline phosphatase (ALP) assay for the study groups.
Results:
The periostin-treated absorbable collagen matrices showed a statistically significant increase in the osteoblast adhesion compared to periostin-treated NcHC on days 2, 5, and 7 (P < 0.001). The osteoblast activity as evaluated by ALP assay showed that there is increased activity in the periostin-treated ACS compared to the periostin-treated NcHC.
Conclusion:
From the observations of this study, it is evident that Periostin has a significant role in the modulating cellular response of the osteoblast cells. Further, incorporation of periostin into the ACS has been shown to increase the cell viability, proliferation, and adhesion of osteoblast-like Saos-2 cells.
Keywords: Absorbable collagen sponge, nanocrystalline hydroxyapatite collagen, osteoblast cells, periostin
INTRODUCTION
There is a persistent search, for a new biomolecule that would appropriately modulate periodontal wound healing and stimulate bone formation. In that context, periostin, a secreted disulfide-linked 90-kDa protein belonging to Fasciclin family, plays an essential role in bone and tooth formation.[1] It is a matricellular adhesion protein expressed by osteoblasts of the periosteum and by the fibroblasts of the periodontal ligament.[2] Periostin has been demonstrated as vital for periodontal tissue integrity, homeostasis, migration, attachment,[3] proliferation, as well as matrix formation of osteoblasts.[4,5,6] Periostin seems to be a therapeutically promising extracellular matrix molecule that promotes and accelerates osteoblastic proliferation and differentiation in vitro and stimulates bone formation in vivo. This hints at the functional importance of periostin in the development and in the regenerative process, following tissue injury.
Therefore, the current study proposes to evaluate the ability of recombinant human periostin when impregnated onto varied collagen matrices like absorbable collagen sponge (ACS) and nanocrystalline hydroxyapatite collagen (NcHC) matrix to influence osteoblast adhesion proliferation and activity. Based on our understanding of the function of periostin from preclinical study models, this in vitro study hypothesized that the addition of periostin onto the collagen matrices would favorably increase the activity of osteoblasts in these matrices.
MATERIALS AND METHODS
Osteoblast cell line, Saos-2, was procured from the National Centre for Cell Science, Pune, India, and were cultured to achieve 80% confluence [Figure 1a and b]. The cells were then seeded onto two different collagen matrices, namely ACS (Kolspon-Eucare pharmaceuticals, Source: Fish origin) and NcHC matrix (Sybograf-GBR, Eucare Pharmaceuticals), and the four groups of the study were as follows:
Figure 1.
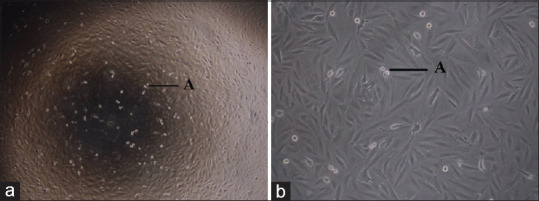
(a) Saos-2 cell line under 4× magnification; (b) Saos-2 cell line under 10× magnification
Group A – ACS without any modification
Group B – ACS impregnated with recombinant human periostin
Group C – NcHC without any modification
Group D – NcHC impregnated with recombinant human periostin.
Cell culture
Cells were maintained in McCoy's 5A medium supplemented with sodium bicarbonate, 10% fetal bovine serum, L-Glutamine, and antibiotic antimycotic solution at 37°C in a humidified atmosphere of 5% CO2. Cells were removed from the growth surface with a trypsin-ethylenediaminetetraacetic acid solution. Subsequently, cells at passages 5–6 were utilized for this experiment and were suspended in the culture media at the density of 106 cells/ml [Figure 1a and b].
Standardization of periostin for adhesion
On a sterile petri dish, varying concentrations (5, 10, 25, 50, and 100 ng/ml) of human recombinant periostin (R and D Systems, Minneapolis, USA; Source: Mouse myeloma cell line) were placed in a circular manner at regular intervals in triplicates. The plates were then allowed to dry overnight and were washed with 1× phosphate-buffered saline twice. The following day, trypsinized Saos-2 cells were mixed in a 15 ml McCoy basal medium. This cell mixture was then flooded onto the Petri dish and incubated with 5% CO2 in an incubator for 24 h. The cells were then stained with crystal violet and observed after drying. At a concentration of 50 ng/ml, an increased number of cells were found to be adhered compared to the other concentrations of periostin. Hence, 50 ng/ml was decided to be optimal to carry out further work.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed in two groups of Saos-2 cells one treated with recombinant human periostin and the other not treated with periostin to assess cell viability. Both the groups were evaluated on 2nd, 5th, and 7th days. Similarly, the MTT assay was performed on the matrices (ACS and NcHC) treated with and without recombinant periostin to assess osteoblast cell viability. The matrices were cut into 5 mm × 5 mm size and were placed onto a 24-well culture plate. Recombinant human periostin (50 ng/ml) was added to the scaffold and incubated for 24 h. Thereafter, Saos-2 cells were seeded on the culture plate at the density of 1 × 104 cells/well per scaffold in triplicates and incubated at 37°C in CO2. The MTT assay was performed on days 2, 5, and 7. On the day of assessment, 20 μl of MTT reagent (thiazolyl blue tetrazolium bromide 5 mg/ml) was added and incubated for 4 h at 37°C in CO2 incubator. The solutions were discarded and 100 μl of acidified isopropanol was added and incubated for 1 h at 37°C. The absorbance readings at 570 nm were recorded in a microplate plate reader. For matrices not treated with periostin, the above-mentioned procedure was performed without the addition of recombinant human periostin.
Alkaline phosphatase activity
The alkaline phosphatase (ALP) assay was performed using a human ALP enzyme-linked immunosorbent assay kit (Bioassay Technology Laboratory, China) following the manufacturer's instructions. The cells on the matrices with and without periostin were subjected to ALP assay at the 7th and 14th days. The absorbance readings measured at 450 nm were recorded in a microplate reader. For matrices not treated with periostin, the above-mentioned procedure was performed without the addition of recombinant human periostin Flow chart 1.
Flow chart 1.
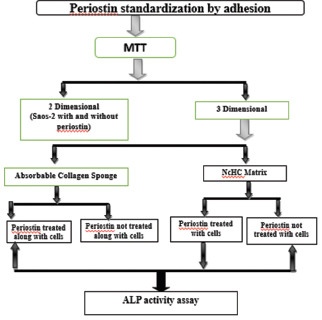
Study protocol in a flowchart; MTT – 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide; Saos2 – Osteoblast cell line; NcHc – Nanocrystalline hydroxyapatite collagen; ALP – Alkaline phosphatase assay
Statistical analysis
SPSS-software version 22 (IBM, India) was used for the statistical analyses. All data were presented as the mean value ± standard deviation. The normality tests such as Kolmogorov–Smirnov and Shapiro–Wilk tests results show that the variable MTT absorbance follows a normal distribution. Therefore to compare mean absorbance values, an independent sample t-test was applied. The ALP assay values did not follow the normal distribution, and therefore for comparison of groups, Mann–Whitney test was applied.
RESULTS
The mean assay value was greater in the Saos-2 cells not treated with periostin at day 2. However, at all other time points, there was a greater amount of MTT activity in the periostin-treated wells. This is indicative of greater amount of osteoblastic activity (adhesion and proliferation) on day 5 and day 7 in the periostin-treated wells [Figure 2a and b]. These differences between the groups were statistically significant (P < 0.22, P < 0.017, respectively) [Table 1].
Figure 2.

(a) Saos-2 cells not treated with Periostin on Day 7; (b) Saos-2 cells treated with periostin on Day 7
Table 1.
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay values of Saos-2 cells treated and not treated with periostin
| Cells | MTT absorbance at 570 nm | ||
|---|---|---|---|
|
| |||
| 2nd day | 5th day | 7th day | |
| Saos-2 cells | 0.10±0.010 | 0.17±0.009 | 0.27±0.005 |
| Saos-2 cells with periostin | 0.06±0.006 | 0.21±0.014 | 0.30±0.008 |
| P | 0.006 | 0.022 | 0.017 |
P≤0.05% is considered significant. MTT – 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; Saos-2 – Osteoblast cell line; P – Probability
MTT assay showed increased osteoblast adhesion for periostin impregnated ACS [Figure 3] compared to ACS group not treated with periostin [Figure 4] and it was significantly higher on days 5 and 7 (P < 0.029, P < 0.002), respectively. Similarly, periostin-treated NcHC matrix showed slightly higher osteoblast adhesion on all days compared to NcHC group not treated with periostin. (P < 0.701, P < 0.931, P < 0.133, respectively). However, it is not statistically significant at any time point [Table 2].
Figure 3.
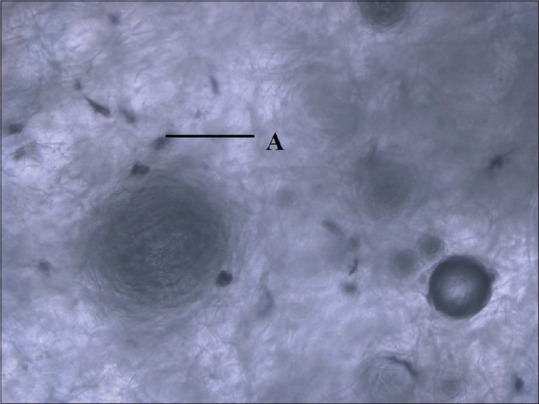
Osteoblast cells in absorbable collagen sponge treated with periostin
Figure 4.
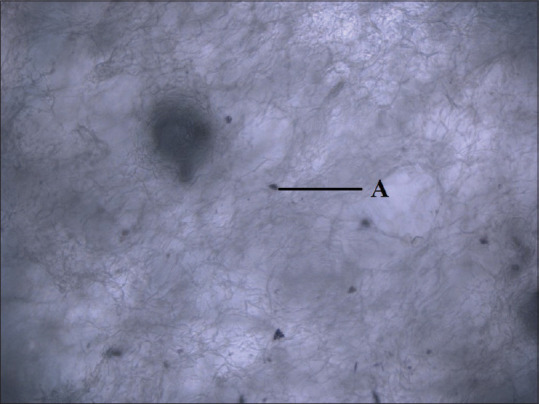
Osteoblast cells in absorbable collagen sponge not treated with periostin
Table 2.
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay values of matrices treated and not treated with periostin
| Matrices | MTT absorbance at 570 nm | ||
|---|---|---|---|
|
| |||
| 2nd day | 5th day | 7th day | |
| ACS | 0.06±0.004 | 0.08±0.008 | 0.10±0.009 |
| ACS - Periostin | 0.07±0.005 | 0.11±0.009 | 0.15±0.008 |
| P | 0.115 | 0.029 | 0.002 |
| NcHC | 0.014±0.006 | 0.026±0.008 | 0.041±0.006 |
| NcHC - Periostin | 0.016±0.009 | 0.027±0.009 | 0.051±0.006 |
| P | 0.701 | 0.931 | 0.133 |
P≤0.05% is considered significant. MTT – 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; ACS – Absorbable collagen sponge; NcHC – Nanocrystalline hydroxyapatite collagen; P – Probability
Among the two matrices not modified with periostin, that is between Group A and C, ACS showed a higher and statistically significant osteoblast adhesion than the NcHC matrix. Particularly, on day 2, the difference is highly significant. Similarly, when the matrices treated with periostin (Group B and D) were compared, ACS groups showed increased osteoblast adhesion than NcHC matrix group on all days on 2, 5, and 7. A highly significant difference was observed on days 5 and 7 (P < 0.001) [Table 3].
Table 3.
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay values of Osteoblast cell line cells on matrices treated and not treated with periostin
| Periostin | MTT absorbance at 570 nm | Membrane | Mean±SD | P |
|---|---|---|---|---|
| Cells not treated with periostin | Day 2 | ACS | 0.06±0.004 | <0.001 |
| NcHC | 0.01±0.006 | |||
| Day 5 | ACS | 0.08±0.008 | 0.001 | |
| NcHC | 0.02±0.008 | |||
| Day 7 | ACS | 0.10±0.009 | 0.001 | |
| NcHC | 0.04±0.006 | |||
| Cells treated with periostin | Day 2 | ACS | 0.07±0.005 | 0.001 |
| NcHC | 0.01±0.009 | |||
| Day 5 | ACS | 0.11±0.009 | <0.001 | |
| NcHC | 0.02±0.009 | |||
| Day 7 | ACS | 0.15±0.008 | <0.001 | |
| NcHC | 0.05±0.006 |
P≤0.05% is considered significant. MTT – 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; ACS – Absorbable Collagen Sponge; NcHC – Nanocrystalline hydroxyapatite collagen; SD – Standard deviation; P – Probability
Among the ACS and NcHC groups, there is a higher ALP activity in periostin-treated matrices compared to the matrices not treated with periostin on days 7 and 14 yet with no statistical significance. The ACS group treated with periostin demonstrated an increased ALP activity in the 2nd week compared to the periostin-treated NcHC group [Table 4].
Table 4.
Alkaline phosphatase assay values of cells on matrices treated and not treated with periostin
| Variable | Not treated with periostin | Treated with periostin | P |
|---|---|---|---|
| ACS - 7th day | 75±8.49 | 117±14.85 | 0.101 |
| ACS - 14th day | 160±15.56 | 227±14.14 | 0.47 |
| P | 0.039 | 0.017 | |
| NcHC - 7th day | 2.5±0.71 | 4±1.41 | 0.349 |
| NcHC - 14th day | 3.5±2.12 | 8.5±2.12 | 0.142 |
| P | 0.625 | 0.148 | |
| ACS - 14th day | 160±15.56 | 227±14.14 | |
| NcHC - 14th day | 3.5±2.12 | 8.5±2.12 | |
| P | 0.041 | 0.026 |
P≤0.05% is considered significant. ACS – Absorbable collagen sponge; NcHC – Nanocrystalline hydroxyapatite collagen; P – Probability
DISCUSSION
Appropriate signals, cells, blood supply, and scaffolds are required for the regeneration of the alveolar bone. Current clinical strategies involve the incorporation of biomolecules such as growth factors or proteins into the scaffolds so as to make them more osteopromotive. Several bioactive proteomic molecules such as bone morphogenetic proteins (BMP) −2, −4, −7,[7,8,9] and enamel matrix derivative[10] and periostin have been shown to act as signaling molecules that potentiate the activity of targeted cells and have shown positive results in promoting periodontal wound repair.
In that context, periostin promotes cell mobility and adhesion in a number of cell types including periodontal ligament fibroblasts and osteoblasts.[11] It binds to integrins expressed by osteoblasts namely like αvβ3 and αvβ5 and regulates cell adhesion and mobility, through the Akt/protein kinase B pathway.[4,5,6] Nevertheless, periostin also enhances osteoblastic proliferation and differentiation in vitro and bone formation in vivo.[12] All these cellular events make periostin a promsing molecule in bone development and bone metabolism.[3] In the present study, the role of recombinant human periostin in osteoblastic proliferation, adhesion, and activity when impregnated onto ACS and nanocrystalline hydroxyapatite has been investigated. It was observed that with progressive incubation in periostin, there was a greater osteoblastic proliferation and adhesion in the periostin-treated cells compared to the cells not treated with periostin. This indicated that there was a temporal progression in the action of periostin on osteoblast activity.
When the ACS groups were compared, periostin-treated ACS showed significantly increased osteoblast adhesion on days 5 and 7 with P < 0.029 and 0.002, respectively. Similarly, between the NcHC matrices, increased osteoblast adhesion was noted in periostin-treated group at all time points. However, it was not statistically significant. From these observations, it is clear that the improved osteoblastic adhesion in periostin-treated matrices is probably mediated by periostin adsorbed onto the biomaterials' surface. This is almost certainly due to the ability of periostin to modulate cell–matrix interactions. The findings of this study are in line with the previous studies which demonstrated that periostin promoted osteoblast cell motility and adhesion.[13,14]
When ACS and NcHC matrices not treated with periostin were compared, ACS had greater adhesion of cells than NcHC at all time points. These observations suggest that ACS provided the necessary support for osteoblastic cells to adhere and proliferate. It has been suggested that the porous collagen sponge supports the infiltration and adhesion of osteoblastic cells.[15] Further, ACS provides an appropriate three-dimensional scaffold for tissue formation and angioblast proliferation.
The highly porous ACS facilitated cell in growth and an accurate cell distribution and that it was capable of supporting neovascularization.[16] Hence, the ACS has important implications, as a carrier device, for the development, differentiation, and regeneration of alveolar bone. A further advantage of the ACS is that they easily fit into alveolar bone defects and are easily molded for use in various tissue disorders such as periodontal bone defects, cyst cavities, and alveolar bone augmentation.[17]
When the periostin-impregnated matrices were compared, periostin-treated ACS showed significantly increased osteoblast adhesion on all days compared to periostin-treated NcHC. The incorporation of recombinant human periostin into ACS showed a synergestic effect in favoring osteoblastic cell behavior like proliferation and adhesion. ACS has been regarded as the gold standard for the carrier of growth factors in the periodontal regeneration as it enhances cellular activity. Incorporation of rhBMP-2 into ACS demonstrated clinically relevant alveolar bone regeneration in supra-alveolar periodontal bone defects,[18] 3-walled intrabony periodontal defect,[19] and ridge augmentation.[20] Although ACS is regarded as the benchmark scaffold, to carry a biological agent, and has been approved for various clinical indications, it also exhibits certain shortcomings. ACS lacks the greater structural integrity, required for larger nonbone supported defects, like one wall or sites with vertical augmentation sites.
Among the ACS groups, there was a higher ALP activity in periostin-treated group than the group not treated with periostin but with no statistical significance. The ACS group treated with periostin demonstrated an increased ALP activity in 1st and 2nd week compared to the periostin-treated NcHC group. The key role played by the structure of ACS in regulating osteoblast adhesion explains the increased ALP activity.
The ability of periostin to guide the cellular response on these collagen matrices is a significant step, in the area of periodontal bone regeneration. The matrices used in this study despite being primarily collagen matrices were different in their micro- and macrostructure. The impregnation of nanohydroxyapatite into collagen probably made the NcHC matrix impervious to the periostin and therefore resulted in the lesser bioactivity of the periostin impregnated onto NcHC matrices. Periostin impregnation into matrices seems to be a viable option to regulate the activity of host osteoblasts. This however needs to go through the next stage of the trial, in assessing the action of periostin in appropriately conducted animal studies that are longitudinally monitored, before it may be considered for the next rung of the trial process.
CONCLUSION
The results of this in vitro study support the hypothesis that recombinant human periostin impregnated onto ACS favorably influences the viability, proliferation, and adhesion of osteoblast-like Saos-2 cells. Periostin could represent a novel biological agent with significant potential for periodontal therapeutic purposes. However, a complete understanding, of the bioactivity of periostin, in the periodontium, is essential for periodontal applications.
Financial support and sponsorship
This was a self-funded study.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–49. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki H, Amizuka N, Kii I, Kawano Y, Nozawa-Inoue K, Suzuki A, et al. Immunohistochemical localization of periostin in tooth and its surrounding tissues in mouse mandibles during development. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1264–75. doi: 10.1002/ar.a.20080. [DOI] [PubMed] [Google Scholar]
- 3.Merle B, Garnero P. The multiple facets of periostin in bone metabolism. Osteoporos Int. 2012;23:1199–212. doi: 10.1007/s00198-011-1892-7. [DOI] [PubMed] [Google Scholar]
- 4.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouyang G, Liu M, Ruan K, Song G, Mao Y, Bao S. Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB Pathway. Cancer Let. 2009;281:213–9. doi: 10.1016/j.canlet.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–9. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Xiao G, Berry JE, Franceschi RT, Reddi A, Somerman MJ. Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J Bone Miner Res. 2002;17:1441–51. doi: 10.1359/jbmr.2002.17.8.1441. [DOI] [PubMed] [Google Scholar]
- 8.Ahn SH, Kim CS, Suk HJ, Lee YJ, Choi SH, Chai JK, et al. Effect of recombinant human bone morphogenetic protein-4 with carriers in rat calvarial defects. J Periodontol. 2003;74:787–97. doi: 10.1902/jop.2003.74.6.787. [DOI] [PubMed] [Google Scholar]
- 9.Jin QM, Zhao M, Economides AN, Somerman MJ, Giannobile WV. Noggin gene delivery inhibits cementoblast-induced mineralization. Connect Tissue Res. 2004;45:50–9. doi: 10.1080/03008200490278142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyngstadaas SP, Lundberg E, Ekdahl H, Andersson C, Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001;28:181–8. doi: 10.1034/j.1600-051x.2001.028002181.x. [DOI] [PubMed] [Google Scholar]
- 11.Padial-Molina M, Volk SL, Rios HF. Periostin increases migration and proliferation of human periodontal ligament fibroblasts challenged by tumor necrosis factor -α and Porphyromonas gingivalis lipopolysaccharides. J Periodontal Res. 2014;49:405–14. doi: 10.1111/jre.12120. [DOI] [PubMed] [Google Scholar]
- 12.Heo SC, Shin WC, Lee MJ, Kim BR, Jang IH, Choi EJ, et al. Periostin accelerates bone healing mediated by human mesenchymal stem cell-embedded hydroxyapatite/tricalcium phosphate scaffold. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116698. doi:10.1371/journal.pone.0116698:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu S, Barbe MF, Liu C, Hadjiargyrou M, Popoff SN, Rani S, et al. Periostin-like-factor in osteogenesis. J Cell Physiol. 2009;218:584–92. doi: 10.1002/jcp.21633. [DOI] [PubMed] [Google Scholar]
- 14.Rani S, Barbe MF, Barr AE, Litivn J. Role of TNF alpha and PLF in bone remodeling in a rat model of repetitive reaching and grasping. J Cell Physiol. 2010;225:152–67. doi: 10.1002/jcp.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HL, Miyauchi M, Takata T. Initial attachment of osteoblasts to various guided bone regeneration membranes: An in vitro study. J Periodontal Res. 2002;37:340–4. doi: 10.1034/j.1600-0765.2002.01625.x. [DOI] [PubMed] [Google Scholar]
- 16.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–29. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Matsuno T, Nakamura T, Kuremoto K, Notazawa S, Nakahara T, Hashimoto Y, et al. Development of beta-tricalcium phosphate/collagen sponge composite for bone regeneration. Dent Mater J. 2006;25:138–44. doi: 10.4012/dmj.25.138. [DOI] [PubMed] [Google Scholar]
- 18.Sigurdsson TJ, Nguyen S, Wikesjo UM. Alveolar ridge augmentation with rhBMP-2 and bone-to-implant contact in induced bone. Int J Periodontics Restorative Dent. 2001;21:461–73. [PubMed] [Google Scholar]
- 19.Chi SH, Kim CK, Cho KS, Huh JS, Sorensen RG, Wozney JM, et al. Effect of recombinant human morphogenetic protein-2/Absorbable Collagen Sponge on healing in 3-wall intrabony defect in dogs. J periodontol. 2002;73:63–72. doi: 10.1902/jop.2002.73.1.63. [DOI] [PubMed] [Google Scholar]
- 20.Barboza EP, Caula AL, de Oliveria Caula F, de Souza RO, Neto LG, Sorensen RG, et al. Effect of recombinant human morphogenetic protein-2 in an Absorbable Collagen Sponge with space-providing biomaterials on the augmentation of chronic alveolar ridge defect. J Periodontol. 2004;75:702–8. doi: 10.1902/jop.2004.75.5.702. [DOI] [PubMed] [Google Scholar]


