Abstract
Background:
There were global concerns and predictions that Coronavirus disease 2019 (COVID-19) would severely affect tuberculosis (TB) care and treatment services in resource-constrained countries. This study aimed to assess the real-time impact of COVID-19 on clinical care and treatment of patients with TB in Addis Ababa, Ethiopia.
Methods:
This was a facility-based, multicenter, cross-sectional study conducted in 10 health centers with high TB clients in Addis Ababa, Ethiopia. Participants were patients with TB who have been attending TB clinical care and treatment in the COVID-19 pandemic period. Data were collected using adapted, interviewer-administered questionnaires to investigate the impact of COVID-19 in their routine care and treatment.
Result:
The study included a total of 212 consented participants. Study participants who missed appointments for medication refill were 40 (18.9%). The most important predictors of missed appointments were fear of COVID-19 [AOR = 4.25, 95% CI (1.710–25.446)], transport disruption [AOR = 8.88, 95% CI (1.618–48.761)], lockdown [AOR = 6.56, 95% CI (1.300–33.131)], traveling costs [AOR = 10.26, 95% CI (1.552–67.882)], and personal protective equipment costs [AOR = 11.15, 95% CI (2.164–57.437)]. The most costly COVID-19 preventive measures that caused financial burden to the patients were face mask [107 (50.5%)], disinfectant [106 (50%)], and sop [50 (23.6%)]. The participants were well aware of the recommended COVID-19 preventive measures. Their perceived most effective preventive measures were the use of face mask (90.1%), frequent hand washing with soap and use of disinfectant (83.0%), avoid touching eyes, nose and mouth with unwashed hands (77.8%), and stay at home (75.5%).
Conclusions:
COVID-19 significantly hampered the clinical care and treatment of patients with TB. The impact was primarily on their appointments for scheduled medication refills, clinical follow-ups, and laboratory follow-ups. Fear of getting infected with COVID-19, limited access to transportation, reduced income for traveling to health facilities, costs for personal protective equipment and traveling to healthcare facilities, and the lockdown were the major determinants. The impact could be mitigated by reducing the number of visits, rationing personal protective equipment as feasible, compensating travel expenses, providing health educations and community-based TB services, and maintaining TB services.
Background
The Coronavirus disease 2019 (COVID-19) outbreak has caused severe disruptions to healthcare systems and the overall well-being of people around the world [1,2,3]. There is a global concern that the world’s robust response and investment against the pandemic would impact the already overburdened healthcare system [4,5,6,7]. In Ethiopia, a country in sub-Saharan Africa, the burden of infectious diseases, coupled with shortage of healthcare facilities, healthcare workforce, and personal protective equipment could trigger the spread of the pandemic in the country [8,9,10,11,12,13,14,15]. The country confirmed its first case of COVID-19 on March 13, 2020, and as of June 11, 2021, there have been 273 678 people confirmed positive, of whom 4 231 died (case fatality rate = 1.55%) and 249 028 (90.99%) recovered [16]. Ethiopia has documented success in reducing common infectious diseases in the last two-and-a-half decades, while diseases such as tuberculosis (TB), HIV, lower respiratory infections, and diarrheal diseases are still the major causes of morbidity and mortality in the country [17,18,19,20,21,22,23].
TB is still one of the top 10 causes of death globally. According to the WHO global TB report 2020 [24], an estimated 10 million people fell ill with TB annually. In Ethiopia, although there is a major decline in TB-associated death and incidence, the country is still among the 30 high TB, TB/HIV, and MDR-TB burden countries [24]. The Ethiopian government has been taking key measures to fight TB, and as a result, Ethiopia is among the seven high-burden countries that reached the first milestone of the End TB Strategy: a 20% reduction of TB incidence between 2015 and 2020. However, facilitated by co-infections like HIV/AIDS, and socioeconomic situations including poverty and inequality, TB remains a major challenge in Ethiopia.
Studies conducted in different countries indicated a potential bidirectional link between TB and COVID-19. Patients with TB are more likely to experience poor outcomes from COVID-19 [25] and patients co-infected with COVID-19 and TB are more likely to suffer severe disease or death than patients with COVID-19 only [26]. The simultaneity of COVID-19 and pulmonary TB can issue a diagnostic dilemma [27] and new diagnostic challenges for clinicians [28]. As both TB and COVID-19 share respiratory symptoms, similar infrastructure and expertise are needed for the diagnosis, management and containment of both diseases [29,30]. COVID-19 creates stress among TB patients to go to healthcare facilities for diagnosis and treatment and the pandemic’s extensive demand for healthcare providers challenges the provision of routine TB case follow-ups [31,32,33]. As a result, different countries have attempted to adjust their TB care and treatment procedures based on their local demands and contexts, including multi-month dispensing and postal delivery of TB medications, home supply of TB treatments, outreach services to reach TB patients, and the use of digital health technologies to monitor medication intakes [34,35,36,37]. However, patient-level studies have been limited to inform how effective and useful these interventions were for the patients.
In Ethiopia, there have been limited studies conducted in the area, reporting that the series of COVID-19 containment measures that the government had taken, including state of emergency, had a significant impact on the overall TB care and treatment services in the country including a reduction in the flow of TB patients [38,39]. However, there has been no study that documented the impact of COVID-19 on patients with TB using real-time patient-level data.
Thus, this study aimed to assess the real-time impact of COVID-19 on the clinical care and treatment of patients with TB in Addis Ababa, Ethiopia.
Methods
Design and setting
A health facility-based, multicenter, cross-sectional study was conducted in ten primary healthcare facilities in Addis Ababa, Ethiopia, from January 15 to February 30, 2021. According to United Nations population estimates, Addis Ababa’s population is estimated at 4 793 699 in 2020. The city is administratively divided into ten sub-cities and 116 districts. Addis Ababa Health Bureau is responsible for the overall health activity in the city. There are numerous healthcare facilities in the city, including six public hospitals and 106 public health centers in the city. COVID-19 pandemic and its transmission potential has been relatively higher in Addis Ababa [39]. As a lot of people live in the city under crowded housing conditions, the risk for TB transmission is high in the city [18].
The public health centers were stratified by sub-city and one site with high TB patient’s load was taken from each sub-city, with a total of 10 facilities included. The study facilities were Addis Raey Health Center (Addisketema), Akaki Health Center (Akaki kality), Kebena Health Center (Arada), Goro Health Center (Bole), Adisu Gebeya Health Center (Gulele), Kazanchis Health Center (Kirkos), Alem Bank Health Center (Kolfe), Teklehaymanot Health Center (Lideta), Woreda 02 Health Center (Nifasilk lafto), and Woreda 13 Health Center (Yeka).
Participants
The source population was all TB outpatients of age >18 years attending care and treatment in the selected health facilities, and the study population was patients who had a confirmed diagnosis of any types of TB, regardless of whether the TB is drug-susceptible or drug-resistant, and attending TB clinical care and treatment in the study facilities during the data collection period. Thus, participants were included if they were 1) patients with TB, as confirmed within the study facilities or result referred from another health facility, attending care and treatment services in the study facilities during the data collection period; 2) man or woman aged ≥ 18 years; 3) volunteers to participate in the study. All participants who have been attending their TB clinical care and treatment in the study sites during the data collection period were considered using a predetermined sampling procedure (Figure 1).
Figure 1.
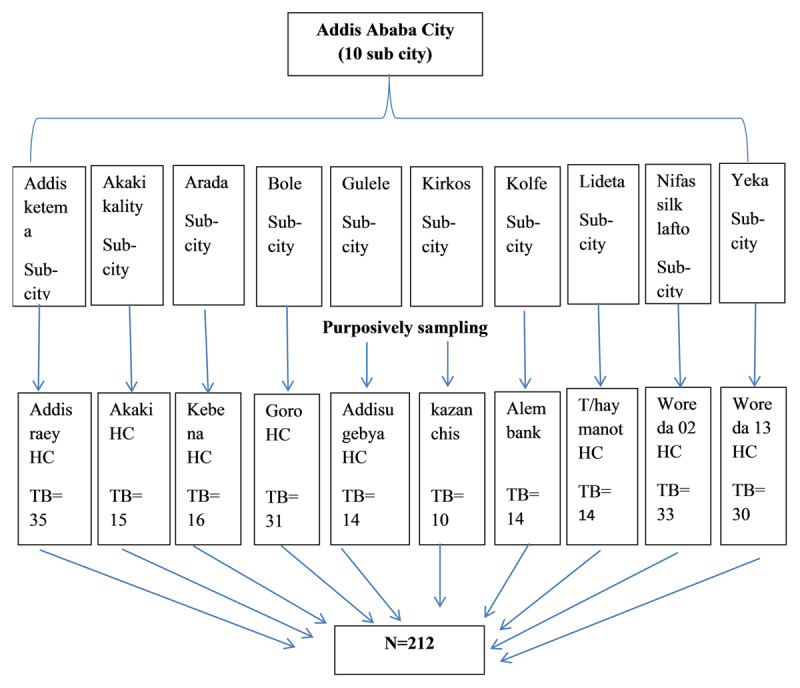
Schematic presentation of sampling procedure.
Data collection
We used an adapted, pre-tested, close-ended, interviewer-administered questionnaire to collect primary data from the study participants for the assessment of the overall impact of COVID-19. It had sections relevant to socio-demographic characteristics, awareness on COVID-19, patient-level preventive measures, patient-level financial burdens, TB care and treatment services, barriers to access healthcare, precaution measures in the healthcare facilities, medication and follow-ups, and non-medical supports during the COVID-19 period. The questionnaire was developed in English and translated to the local national language (Amharic), and later be back-translated to English. When they came to their routine TB care services, the participants were given information about the study through an information sheet and to sign a consent form if they agree and involve in the study. Ten data collectors and two supervisors were recruited and trained for a half-day on data collection techniques and involved accordingly.
Data analysis and interpretation
The data collection tool was checked for completeness and internal consistency. Then, data entry and cleaning were done by the principal investigator. The analysis was done using bivariate and multivariate logistic regression to observe the effects of independent variables on the outcome variable while simultaneously controlling for other potential confounding factors. The raw data was entered to Epi Info version 7 and exported to SPSS 26 for analysis.
Results
Socio-demographic characteristics
A total of 212 TB patients were enrolled in the study, with a response rate of 100%, and 110 (51.9%) were female. Of the total, 97 (45.8%) and 64 (30.2%) were in the age group 35–54 and 18–34 years, respectively. Ninty-five (44.8%) were married and 57 (26.9%) had attended primary education. One hundred and twenty-seven (59.9%) were Orthodox Christian, and fifty-two (24.5%) were unemployed (Table 1).
Table 1.
Sociodemographic characteristics of participants, Addis Ababa, Ethiopia.
|
| |||
|---|---|---|---|
| VARIABLE | CATEGORY | FREQUENCY | % |
|
| |||
| Sex | Male | 102 | 48.1% |
|
| |||
| Female | 110 | 51.9% | |
|
| |||
| Health center name | Addis raey | 35 | 16.5% |
|
| |||
| Akaki | 15 | 7.1% | |
|
| |||
| Kebena | 16 | 7.5% | |
|
| |||
| Goro | 31 | 14.6% | |
|
| |||
| Addisu gebya | 14 | 6.6% | |
|
| |||
| Kazanchis | 10 | 4.7% | |
|
| |||
| Alembank | 14 | 6.6% | |
|
| |||
| T/haymanot | 14 | 6.6% | |
|
| |||
| Woreda 02 | 33 | 15.6% | |
|
| |||
| Woreda 13 | 30 | 14.2% | |
|
| |||
| Age | 18–34 | 64 | 30.2% |
|
| |||
| 35–54 | 97 | 45.8% | |
|
| |||
| ≥55 | 51 | 24.1% | |
|
| |||
| Marital status | Single | 59 | 27.8% |
|
| |||
| Married | 95 | 44.8% | |
|
| |||
| Widowed | 25 | 11.8% | |
|
| |||
| Divorced | 21 | 9.9% | |
|
| |||
| Separated | 12 | 5.7% | |
|
| |||
| Level of education | No formal education, cannot read or write | 28 | 13.2% |
|
| |||
| No formal education, can read or write | 41 | 19.3% | |
|
| |||
| Primary education | 57 | 26.9% | |
|
| |||
| Secondary education | 44 | 20.8% | |
|
| |||
| Diploma and above | 42 | 19.8% | |
|
| |||
| Religion | Orthodox | 127 | 59.9% |
|
| |||
| Muslim | 51 | 24.1% | |
|
| |||
| Protestant | 21 | 9.9% | |
|
| |||
| Catholic | 8 | 3.8% | |
|
| |||
| Others | 5 | 2.4% | |
|
| |||
| Occupation | Student | 13 | 6.1% |
|
| |||
| Daily laborer | 42 | 19.8% | |
|
| |||
| Merchant | 38 | 17.9% | |
|
| |||
| Governmental employee | 36 | 17.0% | |
|
| |||
| Private/NGO employee | 29 | 13.7% | |
|
| |||
| Farmer | 2 | 0.9% | |
|
| |||
| Unemployed | 52 | 24.5% | |
|
| |||
| Family size | One | 46 | 21.7% |
|
| |||
| Two | 54 | 25.5% | |
|
| |||
| ≥ three | 112 | 52.8% | |
|
| |||
| Average monthly income | ≤1000 Eth. Birr | 16 | 7.5% |
|
| |||
| 1001–3000 Eth. Birr | 49 | 23.1% | |
|
| |||
| 3001–10000 Eth. Birr | 112 | 52.8% | |
|
| |||
| >10000 Eth. Birr | 35 | 16.5% | |
|
| |||
NB: NGO- Non-governmental organization; Eth. Birr-Ethiopian Birr.
Most effective preventive measure of COVID-19
Of the total study participants, 160 (75.5%) agreed “stay at home” is the most effective preventive measure of COVID-19. According to the participants, the most effective COVID-19 preventive measures were the use of facemask (90.1%), frequent hand washing with soap and use disinfectant (83.0%), avoid touching eyes, nose and mouth with unwashed hands (77.8%), and stay at home (75.5%) (Table 2).
Table 2.
Respondents’ awareness on COVID-19 preventive measures, Addis Ababa, Ethiopia.
|
| |||
|---|---|---|---|
| VARIABLE | CATEGORY | FREQUENCY | % |
|
| |||
| Stay at home | Disagree | 52 | 24.5% |
|
| |||
| Agree | 160 | 75.5% | |
|
| |||
| Maintain physical distancing | Disagree | 72 | 34.0% |
|
| |||
| Agree | 140 | 66.0% | |
|
| |||
| Avoid close contact | Disagree | 70 | 33.0% |
|
| |||
| Agree | 142 | 67.0% | |
|
| |||
| Cover mouth and nose with a facemask | Disagree | 21 | 9.9% |
|
| |||
| Agree | 191 | 90.1% | |
|
| |||
| Frequent handwashing with soap | Disagree | 36 | 17.0% |
|
| |||
| Agree | 176 | 83.0% | |
|
| |||
| Avoid touching of eyes, nose and mouth | Disagree | 47 | 22.2% |
|
| |||
| Agree | 165 | 77.8% | |
|
| |||
| Avoid mass gathering | Disagree | 78 | 36.8% |
|
| |||
| Agree | 134 | 63.2% | |
|
| |||
| Restrict movement | Disagree | 88 | 41.5% |
|
| |||
| Agree | 124 | 58.5% | |
|
| |||
| Use disinfectants | Disagree | 36 | 17.0% |
|
| |||
| Agree | 176 | 83.0% | |
|
| |||
Financial burden of COVID-19
The most costly COVID-19 preventive measures that cause financial burden to the patients were costs for buying facemasks [107 (50.5%)], sops for handwashing [50 (23.6%)], and disinfectants [106 (50.0%)] (Table 3).
Table 3.
Participants’ financial burden of COVID-19 preventive measures, Addis Ababa, Ethiopia.
|
| |||
|---|---|---|---|
| VARIABLE | CATEGORY | FREQUENCY | % |
|
| |||
| Stay at home | No | 212 | 100.0% |
|
| |||
| Yes | 0 | 0.0% | |
|
| |||
| Cover mouth and nose with facemask | No | 105 | 49.5% |
|
| |||
| Yes | 107 | 50.5% | |
|
| |||
| Wash hands with soap frequently | No | 162 | 76.4% |
|
| |||
| Yes | 50 | 23.6% | |
|
| |||
| Use disinfectants as asppropriate | No | 106 | 50.0% |
|
| |||
| Yes | 106 | 50.0% | |
|
| |||
TB care and treatment services during COVID-19
All of the participants have gone to a healthcare facility during the high COVID-19 time. Of these, 173 (81.6%) were treated differently. One hundred and thirty-one (61.8%) passed through new procedures, and for most participants, the procedure was a COVID-19 PCR test (52.7%). Six (2.8%) participants were obliged to change the health facility, and 33 (15.6%) have ever been denied health services because of this pandemic. Almost all participants said healthcare providers are polite and respectful (99.5%), willing to listen and answer their questions (99.5%), give attention to their individual needs (98.1%), and never physically assaulted (99.5%) (Table 4).
Table 4.
Participants’ response on health care facility and service delivery during COVID-19, Addis Ababa, Ethiopia.
|
| |||
|---|---|---|---|
| VARIABLE | CATEGORY | FREQUENCY | % |
|
| |||
| Visits the facility during high COVID-19 time | No | 0 | 0.0% |
|
| |||
| Yes | 212 | 100.0% | |
|
| |||
| Treated differently | Yes | 173 | 81.6% |
|
| |||
| No | 39 | 18.4% | |
|
| |||
| Unusual procedures were in place | Yes | 131 | 75.7% |
|
| |||
| No | 42 | 24.3% | |
|
| |||
| Unusual procedure (if any) | COVID-19 creening | 62 | 47.3% |
|
| |||
| COVID-19 PCR | 69 | 52.7% | |
|
| |||
| COVID-19 chest CT | 0 | 0.0% | |
|
| |||
| Others | 0 | 0.0% | |
|
| |||
| Obliged to change the health center because of COVID-19 | Yes | 6 | 2.8% |
|
| |||
| No | 206 | 97.2% | |
|
| |||
| Denied healthcare services | Yes | 33 | 15.6% |
|
| |||
| No | 179 | 84.4% | |
|
| |||
| Obtained polite and respectful services | Yes | 211 | 99.5% |
|
| |||
| No | 1 | 0.5% | |
|
| |||
| Listened well and got satisfactory answers to queries | Yes | 211 | 99.5% |
|
| |||
| No | 1 | 0.5% | |
|
| |||
| Gained considerable attention regarding individual needs | Yes | 208 | 98.1% |
|
| |||
| No | 4 | 1.9% | |
|
| |||
| Physically assaulted by providers | Yes | 1 | 0.5% |
|
| |||
| No | 211 | 99.5% | |
|
| |||
| Received prompt action for your health conditions or comorbidities | Yes | 128 | 60.4% |
|
| |||
| No | 84 | 49.6% | |
|
| |||
NB: PCR- polymerase chain reaction; CT- computed tomography; COVID-19- coronavirus disease 2019.
COVID-19 precaution measures in healthcare facilities
From the total study participants, 208 (98.1%) responded that health professionals provide health education on COVID-19, and 133 (62.7%) said the health centers provide COVID-19 screening. According to the participants, almost all health professionals wear gloves (99.5%) and mask (99.5%) when providing care, and there was frequent access to water (95.8%) and soap (95.3%) at the gate of the healthcare facilities, but not sanitizer (62.3%) (Table 5).
Table 5.
Response of study participants on health care facility and service delivery during COVID-19, Addis Ababa, Ethiopia.
|
| |||
|---|---|---|---|
| VARIABLE | CATEGORY | FREQUENCY | % |
|
| |||
| Providers give health education on COVID-19 | Agree | 208 | 98.1% |
|
| |||
| Disagree | 4 | 1.9% | |
|
| |||
| Health center provides COVID-19 screening | Agree | 133 | 62.7% |
|
| |||
| Disagree | 79 | 37.3% | |
|
| |||
| Providers wear gloves during caregiving | Agree | 211 | 99.5% |
|
| |||
| Disagree | 1 | 0.5% | |
|
| |||
| Providers wear mask during caregiving | Agree | 211 | 99.5% |
|
| |||
| Disagree | 1 | 0.5% | |
|
| |||
| Health center has water at entry for handwashing | Agree | 203 | 95.8% |
|
| |||
| Disagree | 9 | 4.2% | |
|
| |||
| Health center has soap at entry for handwashing | Agree | 202 | 95.3% |
|
| |||
| Disagree | 10 | 4.7% | |
|
| |||
| Health center has sanitizer at entry for hand cleaning | Agree | 80 | 37.7% |
|
| |||
| Disagree | 132 | 62.3% | |
|
| |||
Medications and follow-ups during COVID-19
In this study, 199 (93.9%) of the participants responded the pharmacy was accessible, and all participants had taken their drugs. Participants who missed appointments, follow-up tests, and counseling services were 40 (18.9%), 41 (19.3%), and 37 (17.5%) respectively according to their response (Table 6).
Table 6.
Response of study participants on medications and follow-up during COVID-19, Addis Ababa, Ethiopia.
|
| |||
|---|---|---|---|
| VARIABLE | CATEGORY | FREQUENCY | % |
|
| |||
| Accessed pharmacy readily | Yes | 199 | 93.9% |
|
| |||
| No | 13 | 6.1% | |
|
| |||
| Ordered with drugs and supplies | Yes | 212 | 100.0% |
|
| |||
| No | 0 | 0.0% | |
|
| |||
| Missed appointments or visits | Yes | 40 | 18.9% |
|
| |||
| No | 172 | 81.1% | |
|
| |||
| Obtained follow-up laboratory tests | Yes | 171 | 80.7% |
|
| |||
| No | 41 | 19.3% | |
|
| |||
| Obtained follow-up counseling on medication or health status | Yes | 175 | 82.5% |
|
| |||
| No | 37 | 17.5% | |
|
| |||
Missing appointments/visits for medication refill
On bivariate and multivariate logistic regression analysis, the independent variables marital status, education, monthly income, fear of COVID- 19, transport disruption, reduced income, immediate access to provider, soap available, pharmacy accessible, shortage of medicine and non-medical support since COVID-19 had significant associations with missing appointments/visits for medication refill during the COVID-19 pandemic (Tables 7, 8, 9).
Table 7.
Bivariate and multivariate logistic regression analysis of appointments for refill during COVID-19, Addis Ababa, Ethiopia.
|
| ||||||
|---|---|---|---|---|---|---|
| MISSED APPOINTMENTS | ODDS RATIO | |||||
|
| ||||||
| VARIABLES | CATEGORY | NO | YES | COR (CI) | AOR (CI) | P VALUE |
|
| ||||||
| Fear of COVID-19 | No | 45 (26.2%) | 1 (2.5%) | 1 | 1 | |
|
| ||||||
| Yes | 127 (73.8%) | 39 (97.5%) | 40.93 (13.575–123.437) | 4.25 (1.710–25.446) | 0.013* | |
|
| ||||||
| Transport disruption | No | 87 (50.6%) | 4 (10.0%) | 1 | 1 | |
|
| ||||||
| Yes | 85 (49.4%) | 36 (90.0%) | 9.21 (3.143–27.000) | 8.88 (1.618–48.761) | 0.012* | |
|
| ||||||
| Partial lockdown | No | 68 (39.5%) | 1 (2.5%) | 1 | 1 | |
|
| ||||||
| Yes | 104 (60.5%) | 39 (97.5%) | 47.73 (16.896–134.815) | 6.56 (1.300–33.131) | 0.023* | |
|
| ||||||
| Reduced income to travel | No | 53 (30.8%) | 1 (2.5%) | 1 | 1 | |
|
| ||||||
| Yes | 119 (69.2%) | 39 (97.5%) | 50.53 (11.725–217.789) | 10.26 (1.552–67.882) | 0.016* | |
|
| ||||||
| Unable to access mask | No | 81 (47.1%) | 2 (5.0%) | 1 | 1 | |
|
| ||||||
| Yes | 91 (52.9%) | 38 (95.0%) | 145.80 (0.283–491.13) | 11.15 (2.164–57.437) | 0.004* | |
|
| ||||||
| Staff seem uncomfortable | No | 120 (69.8%) | 21 (52.5%) | 1 | 1 | |
|
| ||||||
| Yes | 52 (30.2%) | 19 (47.5%) | 36.00 (12.972–99.909) | 5.69 (1.174–27.602) | 0.031* | |
|
| ||||||
Table 8.
Bivariate and multivariate logistic regression analysis of follow-up tests during COVID-19, Addis Ababa, Ethiopia.
|
| ||||||
|---|---|---|---|---|---|---|
| FOLLOW-UP TEST | ODDS RATIO | |||||
|
| ||||||
| VARIABLES | CATEGORY | NO | YES | COR (CI) | AOR (CI) | P VALUE |
|
| ||||||
| Age | 18–34 | 7 (17.1%) | 57 (33.3%) | 1 | 1 | |
|
| ||||||
| 35–54 | 8 (19.5%) | 89 (52.1%) | 1.56 (0.433–5.635) | 3.28 (0.483–22.327) | 0.224 | |
|
| ||||||
| ≥55 | 26 (63.4%) | 25 (14.6%) | 0.07 (0.025–0.203) | 0.14 (0.022–0.836) | 0.031* | |
|
| ||||||
| Partial | No | 1 (2.4%) | 68 (39.8%) | 1 | 1 | |
|
| ||||||
| Lockdown | Yes | 40 (97.6%) | 103 (60.2%) | 0.03 (0.010–0.067) | 0.16 (0.028–0.903) | 0.038* |
|
| ||||||
| Transport disruption | No | 5 (12.2%) | 86 (50.3%) | 1 | 1 | |
|
| ||||||
| Yes | 36 (87.8%) | 85 (49.7%) | 0.14 (0.051–0.367) | 0.18 (0.043–0.800) | 0.024* | |
|
| ||||||
| Fear of COVID-19 | No | 2 (4.9%) | 44 (25.7%) | 1 | 1 | |
|
| ||||||
| Yes | 39 (95.1%) | 127 (74.3%) | 0.03 (0.011–0.085) | 0.12 (0.019–0.779) | 0.026* | |
|
| ||||||
| Unable to access mask | No | 3 (7.3%) | 80 (46.8%) | 1 | 1 | |
|
| ||||||
| Yes | 38 (92.7%) | 91 (53.2%) | 0.01 (0.003–0.027 | 0.06 (0.009–0.374) | 0.003* | |
|
| ||||||
| Denied health services | No | 15 (36.6%) | 159 (93.0%) | 1 | 1 | |
|
| ||||||
| Yes | 26 (63.4%) | 12 (7.0%) | 0.04 (0.018–0.103) | 0.11 (0.020–0.641) | 0.014* | |
|
| ||||||
* Statistically significant at p-value < 0.05, COR = crude odds ratio at 95% confidence interval; AOR = adjusted odds ratio at 95% confidence interval.
Table 9.
Bivariate and multivariate logistic regression analysis of counseling during the COVID-19 pandemic, Addis Ababa, Ethiopia.
|
| ||||||
|---|---|---|---|---|---|---|
| COUNSELING DONE | ODDS RATIO | |||||
|
| ||||||
| VARIABLE | CATEGORY | NO | YES | COR (CI) | AOR (CI) | P VALUE |
|
| ||||||
| Age | 18–34 | 3 (8.1%) | 59 (33.7%) | 1 | 1 | |
|
| ||||||
| 35–54 | 5 (13.5%) | 89 (50.9%) | 0.90 (0.208–3.931) | 0.33 (0.022–4.805) | 0.414 | |
|
| ||||||
| ≥55 | 29 (78.4%) | 27 (15.4%) | 0.05 (0.013–0.169) | 0.06 (0.005–0.905) | 0.042* | |
|
| ||||||
| Partial lockdown | No | 1 (2.7%) | 68 (38.9%) | 1 | 1 | |
|
| ||||||
| Yes | 36 (97.3%) | 107 (61.1%) | 0.03 (0.009–0.073) | 0.03 (0.001–0.772) | 0.034* | |
|
| ||||||
| Fear of COVID-19 | No | 1 (2.7%) | 45 (25.7%) | 1 | 1 | |
|
| ||||||
| Yes | 36 (97.3%) | 130 (74.3%) | 0.02 (0.006–0.071) | 0.03 (0.002–0.532) | 0.017* | |
|
| ||||||
| Stigma | No | 14 (37.8%) | 173 (98.9%) | 1 | 1 | |
|
| ||||||
| Yes | 23 (62.2%) | 2 (1.1%) | 0.01 (0.002–0.033) | 0.01 (0.000–0.115) | 0.002* | |
|
| ||||||
* Statistically significant at p-value < 0.05, COR = crude odds ratio at 95% confidence interval; AOR = adjusted odds ratio at 95% confidence interval.
Discussion
This study assessed the real-time, patient-level impact of COVID-19 on the clinical care and treatment of patients with TB in Addis Ababa, Ethiopia. With a total of 212 patients with TB included, 18.9% of the participants missed appointments for medication refill in the COVID-19 outbreak for reasons including fear of COVID-19 infection, transport disruption, partial lockdown, reduced income for traveling to a healthcare facility, unable to get face masks, and pressure placed on providers. The participants were well aware of the COVID-19 preventive measures, where the most effective COVID-19 preventive measures they considered were the use of facemask, frequent hand washing with soap and use disinfectant, avoid touching eyes, nose and mouth with unwashed hands, and stay at home. The most costly COVID-19 preventive measures that caused a financial burden to the patients were facemask, disinfectant, and sop. To the best of our knowledge, this study was the first of its kind to assess the impact of COVID-19 on TB care and treatment services in Ethiopia using real-time, patient-level data.
The analysis indicated that fear of getting COVID-19 infection within the healthcare facilities a strong predictor of missing medication appointments. TB treatment disruption could abate patient outcomes and lead to TB drug resistance. In many sub-Saharan African countries, the COVID-19 pandemic has added burdens to already overwhelmed health systems and the burden of TB may aggravate as a result [40]. Applying a combination of standard infection control measures, healthcare facilities need to improve patients’ trust and keep them safe from the pandemic.
Transport disruption, particularly in the partial lockdown period, was among the main barriers for the TB patients, limiting their access to healthcare facilities thereby missing follow-up visits. The finding was in agreement with previous studies conducted in Ethiopia [11,39,41,42,43] and elsewhere in Africa [44,45,46,47,48] that COVID-19 lockdowns had a significant impact on patients’ access to transportation to healthcare facilities. In the case of TB medication follow-up visits, direct-observed therapy procedure necessitates patients’ daily visits to healthcare facilities for medication intake this procedure is susceptible to transport disruptions [6,39,40]. An appropriate people-centered model of care needs to be developed in consultation with TB patients and TB survivors to mitigate the challenges and upsurge the TB services [49]. To minimize the challenge, many countries, including Ethiopia, had modified their TB care service delivery by reducing patients’ health facility visits during the COVID-19. More advanced COVID-19 mitigation strategies, multilateral collaborations, international joint-venture, and innovative technologies are essential to address the double burden of the COVID-19 pandemic [50,51,52,53].
In the early days of the COVID-19 pandemic, there was limited access to facemasks for public use in Ethiopia. This was reverted shortly through huge donor and commercial supply and local production. However, our analysis demonstrates that despite understanding the protective effect of masks, patients with TB could not afford to buy facemask. This may be mitigated by rationing of masks for the patients until they complete their medications successfully.
There were some limitations to this study. The study was limited to healthcare facilities in Addis Ababa, and therefore may not be representative of Ethiopia. As the study design was a cross-sectional study, it does not show a causal relationship and only provides a view of the impacts of COVID-19 in a specific period. Otherwise, the study was based on real-time, patient-level primary data and it was conducted in a resource-constrained, high TB burden country context.
Conclusion
COVID-19 significantly hampered the clinical care and treatment of patients with TB. The impact was primarily on their appointments for scheduled medication refills, clinical follow-ups, and laboratory follow-ups. Fear of getting infected with COVID-19, limited access to transportation, reduced income for traveling to health facilities, costs for personal protective equipment and traveling to healthcare facilities, and the lockdown were the major determinants. The impact could be mitigated by reducing the number of visits, rationing personal protective equipment as feasible, compensating travel expenses, providing health educations and community-based TB services, and maintaining TB services.
Acknowledgements
The authors acknowledge the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University, for supporting the study. The authors acknowledge all health centers from where data were collected and study participants for their cooperation.
Funding Statement
This study was supported by the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa). TM was supported in part by the Fogarty International Center and National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (D43TW009127). The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDT-Africa or the National Institutes of Health.
Abbreviations
AAU, Addis Ababa University; COVID-19, Coronavirus Disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TB, Tuberculosis; DOT, Directly observed therapy; HC, Health center; ICU, Intensive care unit; MDR, Multidrug-resistant tuberculosis; PPE, Personal protective equipment; WHO, World Health Organization; AOR, Adjusted Odds Ratio; CI, Confidence Interval.
Ethics and Consent
Ethical clearance was obtained from the Scientific and Ethics Review Committee of the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University. Ethical clearance and support letters were obtained from Addis Ababa public health research and emergency directorate, Addis Ababa City Government Health Bureau. Informed consent was obtained from the study participants and their privacy and confidentiality were maintained strictly.
Funding Information
This study was supported by the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa). TM was supported in part by the Fogarty International Center and National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (D43TW009127). The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDT-Africa or the National Institutes of Health.
Competing Interests
The authors have no competing interests to declare.
Author Contributions
DC collected the primary data, conducted the analyses, and draft the manuscript. TM and YW contributed to the data collection and analysis and reviewed the manuscript. All authors have read and approved the manuscript.
Publisher’s Note
This paper underwent peer review using the Cross-Publisher COVID-19 Rapid Review Initiative.
References
- 1.Chan EYY, Gobat N, Dubois C, Bedson J, de Almeida JR. Bottom-up citizen engagement for health emergency and disaster risk management: Directions since COVID-19 [published online ahead of print, June 4, 2021]. Lancet; 2021. DOI: 10.1016/S0140-6736(21)01233-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haldane V, De Foo C, Abdalla SM, et al. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries [published online ahead of print, May 17, 2021]. Nat Med. 2021. DOI: 10.1038/s41591-021-01381-y [DOI] [PubMed] [Google Scholar]
- 3.Shah R, Ali FM, Nixon SJ, Ingram JR, Salek SM, Finlay AY. Measuring the impact of COVID-19 on the quality of life of the survivors, partners and family members: A cross-sectional international online survey. BMJ Open. 2021; 11(5): e047680. DOI: 10.1136/bmjopen-2020-047680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimsrud A, Wilkinson L. Acceleration of differentiated service delivery for HIV treatment in sub-Saharan Africa during COVID-19. J Int AIDS Soc. 2021; 24(6): e25704. DOI: 10.1002/jia2.25704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endriyas M, Kawza A, Alano A, Hussen M, Shibru E. COVID-19 prevention practices in urban setting during early introduction of the disease: Results from community survey in SNNP Region, Ethiopia. BMJ Open. 2021; 11(5): e047373. DOI: 10.1136/bmjopen-2020-047373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manyazewal T, Woldeamanuel Y, Blumberg HM, Fekadu A, Marconi VC. The fight to end tuberculosis must not be forgotten in the COVID-19 outbreak. Nat Med. 2020; 26(6): 811–812. DOI: 10.1038/s41591-020-0917-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odey GO, Alawad AGA, Atieno OS, et al. COVID-19 pandemic: Impacts on the achievements of Sustainable Development Goals in Africa. Pan Afr Med J. 2021; 38: 251. DOI: 10.11604/pamj.2021.38.251.27065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teshome A, Shegaze M, Glagn M, et al. Perceived stress and associated factors among health care professionals working in the context of COVID-19 pandemic in public health institutions of southern Ethiopia 2020. PLoS One. 2021; 16(6): e0252809. DOI: 10.1371/journal.pone.0252809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feyissa GT, Tolu LB, Ezeh A. Impact of COVID-19 Pandemic on Sexual and Reproductive Health and Mitigation Measures: The Case of Ethiopia. Afr J Reprod Health. 2020; 24(s1): 24–26. [DOI] [PubMed] [Google Scholar]
- 10.Bekele F, Mechessa DF, Sefera B. Prevalence and associated factors of the psychological impact of COVID-19 among communities, health care workers and patients in Ethiopia: A systematic review. Ann Med Surg (Lond). 2021; 66: 102403. DOI: 10.1016/j.amsu.2021.102403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girma A, Ayalew E, Mesafint G. Covid-19 Pandemic-Related Stress and Coping Strategies Among Adults with Chronic Disease in Southwest Ethiopia. Neuropsychiatr Dis Treat. 2021; 17: 1551–1561. DOI: 10.2147/NDT.S308394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassie A, Wale A, Yismaw W. Impact of Coronavirus Diseases-2019 (COVID-19) on Utilization and Outcome of Reproductive, Maternal, and Newborn Health Services at Governmental Health Facilities in South West Ethiopia, 2020: Comparative Cross-Sectional Study. Int J Womens Health. 2021; 13: 479–488. DOI: 10.2147/IJWH.S309096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birhanu Z, Ambelu A, Fufa D, et al. Risk perceptions and attitudinal responses to COVID-19 pandemic: An online survey in Ethiopia. BMC Public Health. 2021; 21(1): 981. DOI: 10.1186/s12889-021-10939-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haftom M, Petrucka PM. Determinants of Face Mask Utilization to Prevent Covid-19 Pandemic among Quarantined Adults in Tigrai Region, Northern Ethiopia, 2020 [published online ahead of print, May 6, 2021]. Clin Nurs Res. 2021. DOI: 10.1177/10547738211013219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estifanos AS, Kazmi K, Morris SK. Could COVID-19 Reverse the Modest Gains Made in Newborn Health in Ethiopia? Matern Child Health J. 2021; 25(6): 849–854. DOI: 10.1007/s10995-021-03175-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corona Scanner. Realtime coronavirus statistics, Ethiopia; June 11, 2021. https://corona-scanner.com/country/ethiopia.
- 17.Mussie KM, Gradmann C, Yimer SA, Manyazewal T. Pragmatic Management of Drug-Resistant Tuberculosis: A Qualitative Analysis of Human Resource Constraints in a Resource-Limited Country context-Ethiopia. Int J Public Health. 2021; 66: 633917. DOI: 10.3389/ijph.2021.633917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manyazewal T, Woldeamanuel Y, Holland DP, Fekadu A, Blumberg HM, Marconi VC. Electronic pillbox-enabled self-administered therapy versus standard directly observed therapy for tuberculosis medication adherence and treatment outcomes in Ethiopia (SELFTB): Protocol for a multicenter randomized controlled trial. Trials. 2020; 21(1): 383. DOI: 10.1186/s13063-020-04324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed H, Oljira L, Roba KT, et al. Burden of tuberculosis and challenges related to screening and diagnosis in Ethiopia. J Clin Tuberc Other Mycobact Dis. 2020; 19: 100158. DOI: 10.1016/j.jctube.2020.100158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsegaye Sahle E, Blumenthal J, Jain S, et al. Bacteriologically-confirmed pulmonary tuberculosis in an Ethiopian prison: Prevalence from screening of entrant and resident prisoners. PLoS One. 2019; 14(12): e0226160. DOI: 10.1371/journal.pone.0226160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mussie KM, Yimer SA, Manyazewal T, Gradmann C. Exploring local realities: Perceptions and experiences of healthcare workers on the management and control of drug-resistant tuberculosis in Addis Ababa, Ethiopia. PLoS One. 2019; 14(11): e0224277. DOI: 10.1371/journal.pone.0224277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manyazewal T, Mekonnen A, Demelew T, et al. Improving immunization capacity in Ethiopia through continuous quality improvement interventions: A prospective quasi-experimental study. Infect Dis Poverty. 2018; 7(1): 119. DOI: 10.1186/s40249-018-0502-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manyazewal T, Marinucci F, Belay G, et al. Implementation and Evaluation of a Blended Learning Course on Tuberculosis for Front-Line Health Care Professionals. Am J Clin Pathol. 2017; 147(3): 285–291. DOI: 10.1093/ajcp/aqx002 [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO). Global Tuberculosis Report 2020. Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 25.Beyene NW, Sitotaw AL, Tegegn B, Bobosha K. The impact of COVID-19 on the tuberculosis control activities in Addis Ababa. The Pan African Medical Journal. 2021; 38. DOI: 10.11604/pamj.2021.38.243.27132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song WM, Zhao JY, Zhang QY, et al. COVID-19 and Tuberculosis Coinfection: An Overview of Case Reports/Case Series and Meta-Analysis. Front Med (Lausanne). 2021; 8: 657006. DOI: 10.3389/fmed.2021.657006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selgado MB, Abramov AY, Kicha DI, Zuenkova JA. The double diseases burdens trends in Ethiopia: COVID-19 aggravate the burdens of diseases and health system. Problems of Social Hygiene, Public Health and History of Medicine. December 15, 2021; 29(S1): 813–7. DOI: 10.32687/0869-866X-2021-29-s1-813-817 [DOI] [PubMed] [Google Scholar]
- 28.Desta AA, Woldearegay TW, Gebremeskel E, et al. Impacts of COVID-19 on essential health services in Tigray, Northern Ethiopia: A pre-post study. Plos one. August 27, 2021; 16(8): e0256330. DOI: 10.1371/journal.pone.0256330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatia V, Mandal PP, Satyanarayana S, Aditama TY, Sharma M. Mitigating the impact of the COVID-19 pandemic on progress towards ending tuberculosis in the WHO South-East Asia Region. WHO South East Asia J Public Health. 2020; 9(2): 95–99. DOI: 10.4103/2224-3151.294300 [DOI] [PubMed] [Google Scholar]
- 30.Togun T, Kampmann B, Stoker NG, Lipman M. Anticipating the impact of the COVID-19 pandemic on TB patients and TB control programmes. Ann Clin Microbiol Antimicrob. 2020; 19(1): 21. DOI: 10.1186/s12941-020-00363-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A, Singla R, Caminero JA, Singla N, Mrigpuri P, Mohan A. Impact of COVID-19 on tuberculosis services in India. Int J Tuberc Lung Dis. 2020; 24(6): 637–639. DOI: 10.5588/ijtld.20.0212 [DOI] [PubMed] [Google Scholar]
- 32.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: A modelling study [published correction appears in Lancet Glob Health. January 2021; 9(1): e23]. Lancet Glob Health. 2020; 8(9): e1132–e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louie JK, Reid M, Stella J, et al. A decrease in tuberculosis evaluations and diagnoses during the COVID-19 pandemic. Int J Tuberc Lung Dis. 2020; 24(8): 860–862. DOI: 10.5588/ijtld.20.0364 [DOI] [PubMed] [Google Scholar]
- 34.Adewole OO. Impact of COVID-19 on TB care: Experiences of a treatment centre in Nigeria. Int J Tuberc Lung Dis. 2020; 24(9): 981–982. DOI: 10.5588/ijtld.20.0418 [DOI] [PubMed] [Google Scholar]
- 35.Kwak N, Hwang SS, Yim JJ. Effect of COVID-19 on Tuberculosis Notification, South Korea. Emerg Infect Dis. 2020; 26(10): 2506–2508. DOI: 10.3201/eid2610.202782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visca D, Tiberi S, Pontali E, Spanevello A, Migliori GB. Tuberculosis in the time of COVID-19: quality of life and digital innovation. Eur Respir J. 2020; 56(2): 2001998. DOI: 10.1183/13993003.01998-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Udwadia ZF, Vora A, Tripathi AR, Malu KN, Lange C, Sara Raju R. COVID-19 -Tuberculosis interactions: When dark forces collide. Indian J Tuberc. 2020; 67(4S): S155–S162. DOI: 10.1016/j.ijtb.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beyene NW, Sitotaw AL, Tegegn B, Bobosha K. The impact of COVID-19 on the tuberculosis control activities in Addis Ababa. Pan Afr Med J. 2021; 38: 243. DOI: 10.11604/pamj.2021.38.243.27132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammed H, Oljira L, Roba KT, Yimer G, Fekadu A, Manyazewal T. Containment of COVID-19 in Ethiopia and implications for tuberculosis care and research. Infect Dis Poverty. 2020; 9(1): 131. DOI: 10.1186/s40249-020-00753-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nachega JB, Kapata N, Sam-Agudu NA, et al. Minimizing the impact of the triple burden of COVID-19, tuberculosis and HIV on health services in sub-Saharan Africa [published online ahead of print, March 20, 2021]. Int J Infect Dis. 2021; S1201-9712(21)00256-3. DOI: 10.1016/j.ijid.2021.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanyero B, Edea ZA, Musa EO, et al. Readiness and early response to COVID-19: Achievements, challenges and lessons learnt in Ethiopia. BMJ Glob Health. 2021; 6(6): e005581. DOI: 10.1136/bmjgh-2021-005581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adefris D, Moges B. The psychological impact and coping of Covid-19 pandemic among Arsi University students -Ethiopia [published online ahead of print, June 12, 2021]. Curr Psychol. 2021; 1–7. DOI: 10.1007/s12144-021-01886-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woday Tadesse A, Mihret ST, Biset G, Kassa AM. Psychological problems and the associated factors related to the COVID-19 pandemic lockdown among college students in Amhara Region, Ethiopia: a cross-sectional study. BMJ Open. 2021; 11(9): e045623. DOI: 10.1136/bmjopen-2020-045623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogunkola IO, Adebisi YA, Imo UF, Odey GO, Esu E, Lucero-Prisno DE 3rd. Impact of COVID-19 pandemic on antenatal healthcare services in Sub-Saharan Africa. Public Health Pract (Oxf). 2021; 2: 100076. DOI: 10.1016/j.puhip.2021.100076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elbany M, Elhenawy Y. Analyzing the ultimate impact of COVID-19 in Africa. Case Stud Transp Policy. 2021; 9(2): 796–804. DOI: 10.1016/j.cstp.2021.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akande-Sholabi W, Adebisi YA. The impact of COVID-19 pandemic on medicine security in Africa: Nigeria as a case study. Pan Afr Med J. 2020; 35(Suppl 2): 73. DOI: 10.11604/pamj.supp.2020.35.2.23671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oaku I, Anaba EL. The impact of COVID-19 on the practice of dermatology in sub-Saharan Africa. Dermatol Ther. 2020; e14642. DOI: 10.1111/dth.14642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris D, Rogers M, Kissmer N, Du Preez A, Dufourq N. Impact of lockdown measures implemented during the Covid-19 pandemic on the burden of trauma presentations to a regional emergency department in Kwa-Zulu Natal, South Africa. Afr J Emerg Med. 2020; 10(4): 193–196. DOI: 10.1016/j.afjem.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmer AJ, Heitkamp P, Malar J, et al. Facility-based directly observed therapy (DOT) for tuberculosis during COVID-19: A community perspective. J Clin Tuberc Other Mycobact Dis. 2021; 24: 100248. DOI: 10.1016/j.jctube.2021.100248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunasekeran DV, Tseng RMWW, Tham YC, Wong TY. Applications of digital health for public health responses to COVID-19: A systematic scoping review of artificial intelligence, telehealth and related technologies. NPJ Digit Med. 2021; 4(1): 40. DOI: 10.1038/s41746-021-00412-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manyazewal T, Woldeamanuel Y, Blumberg HM, Fekadu A, Marconi VC. The potential use of digital health technologies in the African context: A systematic review of evidence from Ethiopia. NPJ Digit Med. 2021; 4(1): 125. DOI: 10.1038/s41746-021-00487-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yassin N, Saleh S. The World after COVID-19: Reflections on Global Health and Policy. Ann Glob Health. 2021; 87(1): 72. DOI: 10.5334/aogh.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wada YH, Musa MK, Musa SS, Khalid GM, Lucero Prisno DE, 3rd. Dual burden of COVID-19 and TB in Africa. Clin Epidemiol Glob Health. 2021; 12: 100847. DOI: 10.1016/j.cegh.2021.100847 [DOI] [PMC free article] [PubMed] [Google Scholar]


