Abstract
The incidence density trend of the carbapenem-resistant Gram-negative bacteria was analysed in device-associated infections and antimicrobial consumption in 99 critical care facilities in a low/middle-income country, between January 2019 and December 2020. Carbapenem-resistant Acinetobacter baumannii (CRAB) per 1000 patient-days increased in 2020 and this finding had a strong positive correlation with the incidence density of COVID-19 by the Spearman test. Polymyxin consumption also increased in 2020 but without significant correlation with CRAB or COVID-19 incidence density, presumably due to empirical and untargeted prescribing as a consequence of concern about CRAB infections. These findings are a warning to infection control programmes and antimicrobial stewardship.
Keywords: Healthcare-associated infections, Acinetobacter baumannii, Antimicrobial drug resistance, COVID-19, Carbapenems, Polymyxins
Introduction
The impact of the COVID-19 pandemic goes beyond the individual level (death and disease sequelae), causing several public health issues, including in antimicrobial resistance [1].
In Brazil, the first case of COVID-19 was reported on February 26th, 2020. According to the World Health Organization (WHO) COVID-19 dashboard, Brazil has 10,013 cases and 278.66 deaths per 100,000 inhabitants (September 26th, 2021), and is among the highest incidence countries for the pandemic [2,3].
In Paraná, a state located in southern Brazil, the first confirmed COVID-19 case was reported on March 12th, 2020. Since then, according to data made available by the Brazilian Ministry of Health on the COVID-19 panel, the number of new cases reported per quarter in 2020 were: 358 (first quarter); 45,396 (second quarter); 312,042 (third quarter); 475,336 (fourth quarter).
One of the major concerns regarding hospitalized patients with COVID-19 is bacterial superinfections, especially in the intensive care setting and in patients using invasive devices as well as in situations of outbreaks by multidrug-resistant bacteria resulting from poorer adherence to infection control practices [4,5].
Lower-middle-income countries have higher rates of antimicrobial resistance than developed countries [6]. The pandemic and the consequent overload on health services have further affected infection control programmes and antimicrobial stewardship [7].
Since publications on this subject are still limited, we aimed to analyse the trend of the incidence density of carbapenem-resistant Gram-negative bacteria (CR-GNB) in device-associated infections (DAIs), besides the profile of antimicrobial agent consumption between January 2019 and December 2020, in adult intensive care units (AICUs).
Methods
Study design and data source
This is an aggregated data study of CR-GNB notified in DAIs as well as antimicrobial consumption in AICUs, reported monthly in the online hospital infection notification system of the State Health Department – Paraná, Brazil, from January 2019 through December 2020. The validation of the data notified by the hospitals is carried out by the professionals responsible for monitoring this information in the state health department (Supplementary Appendix 1).
Data on reverse transcription–polymerase chain reaction (RT–PCR)-confirmed cases of COVID-19 admitted to AICUs were extracted from the open notification database available on the Brazilian Ministry of Health Website, downloaded on August 15th, 2021 (Supplementary Appendix 2).
Setting and participants
This study was conducted in the state of Paraná, south of Brazil, using aggregate data from 99 hospitals that reported 11,248 device-associated infections in 234,631 patients admitted to AICUs between January 2019 and December 2020; 6982 cases of COVID-19 were notified in patients admitted to these units. In all, 33.3% of the institutions were exclusively private; the other 66.7% were public or mixed profile; and 55.5% of the centres were teaching institutions.
Variables
Data on CR-GNB were collected regarding the notification of Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae in central line-associated bloodstream infection (CLABSI), mechanical ventilator-associated pneumonia (VAP), and catheter-associated urinary tract infection (CAUTI).
To define DAI, hospitals are recommended to use the criteria of the National Health Surveillance Agency criteria, which are based on those of the US Centers for Disease Control and Prevention. Notifications of colonization were not requested since they did not meet the infection criteria. All hospitals are instructed to report each aetiologic agent per infection episode only once.
Regarding susceptibility detection methods, either conventional (Kirby–Bauer) or any automated method following the breakpoints of the Clinical and Laboratory Standards Institute (CLSI) or European Committee on Antimicrobial Susceptibility Testing (EUCAST), were accepted.
Data related to the consumption of each antimicrobial (in grams) were collected to calculate the defined daily dose (DDD) using the updated WHO Collaborating Centre of Drug Statistics Methodology index (https://www.whocc.no/atc_ddd_index/). The antimicrobials included in the analysis were polymyxin B, colistin (polymyxin E), meropenem, piperacillin/tazobactam, cefepime, ceftazidime, and intravenous quinolones.
The variables included from the COVID-19 database were COVID-19 RT–PCR confirmation, date of notification and ICU admission. For the analysis, the database of COVID-19 notifications and hospital-acquired infections was cross-referenced by identifying the healthcare facility.
Statistical analysis
To calculate the incidence density of the DAIs, the specific denominator of each device-day was used (central line-day, mechanical ventilation-day, and urinary catheter-day).
The incidence density of CR-GNB per 1000 patient-days was calculated using the number of occurrences of each pathogen notified in the DAIs divided by the patient-day of the corresponding period.
To calculate the DDD per 1000 patient-days, the monthly antimicrobial consumption notification was divided by the corresponding DDD and then by the number of patient-days in the corresponding period. Both calculations were adjusted using the 1000 multiplier.
For trend analysis, the Joinpoint regression available from the National Cancer Institute (https://surveillance.cancer.gov/help/joinpoint) was applied. This is a method of time trend analysis (e.g. incidence, prevalence, survival rates) that identifies segments that best explain a trend over time using a sequence of permutation tests to select the number of join points, inflection points, or trend changes in the analysed series and to ensure that the approximate probability of the Type I error is less than the specified significance level (alpha level). In this study, the parameters set as a standard by the model were used, including the logarithmic transformation of the data, the number of permutations (4499), the minimum and the maximum number of junction points (0 and 4), and P < 0.05, with a 95% confidence interval. The defined independent variable was the time interval stratified into monthly periods, and the model then provided the corresponding monthly percent change (MPC) values between the identified segments.
The Spearman test was used to analyse the correlation between the incidence density of CR-GNB per 1000 patient-days versus reported cases of COVID-19 per 1000 patient-days and antimicrobial consumption in DDD per 1000 patient-days in the AICUs.
Results
Among the 11,248 device-associated infections reported during the study period, 58% (6515) were VAP, 23% (2622) CLABSI, and 19% (2111) CAUTI.
CLABSI and VAP incidence density presented join points with a significant increase in the second defined segment by the model, being for CLABSI the period of April 2019 to December 2020 (MPC: 1.9; 95% CI: 1.1–2.8; P < 0.001) and for VAP the period of November 2019 to December 2020 (MPC: 2.1; 95% CI: 1.2–2.9; P < 0.001) (Table I ).
Table I.
Joinpoint regression for DAI indicators, CR-GNB incidence density per 1000 patient-days and antibiotic consumption in DDD per 1000 patient-days in AICU, Paraná, Brazil, 2019–2020
| Variable | Period | MPC | Joinpoint | 95% CI | P-value |
|---|---|---|---|---|---|
| CLABSI/1000 cl-days | Jan 2019–Apr 2019 | –15.5 | 1 | –28.7, 0.1 | 0.051 |
| Apr 2019–Dec 2020 | 1.9 | 1.1, 2.8 | <0.001 | ||
| PAV/1000 mv-days | Jan 2019–Nov 2019 | –1.8 | 1 | –3.2, –0.3 | 0.019 |
| Nov 2019–Dec 2020 | 2.1 | 1.2, 2.9 | <0.001 | ||
| CAUTI/1000 uc-days | Jan 2019–Sep 2019 | –2.8 | 1 | –7.1, 1.7 | 0.209 |
| Sep 2019–Dec 2020 | 1.3 | –0.3, 3.0 | 0.115 | ||
| CRAB/1000 pt-days | Jan 2019–Apr 2020 | 1.1 | 1 | –1.0, 3.4 | 0.286 |
| Apr 2020–Dec 2020 | 14.2 | 10.1, 18.5 | <0.001 | ||
| CRKP/1000 pt-days | Jan 2019–Dec 2020 | 2.4 | 0 | 0.7, 4.1 | 0.008 |
| CRPA/1000 pt-days | Jan 2019–Dec 2020 | 0.5 | 0 | –1.0, 2.1 | 0.478 |
| PMB/1000 pt-days | Jan 2019–Jul 2020 | –1.6 | 1 | –4.2, 1.0 | 0.200 |
| Jul 2020–Dec 2020 | 17.5 | 4.6, 31.9 | 0.009 | ||
| COL/1000 pt-days | Jan 2019–Apr 2020 | –3.1 | 1 | –9.8, 4.0 | 0.362 |
| Apr 2020–Dec 2020 | 30.7 | 17.8, 44.9 | <0.001 | ||
| MEM/1000 pt-days | Jan 2019–Nov 2019 | 1.8 | 2 | 4.5, 1.5 | 0.155 |
| Nov 2019–Aug 2020 | –4.7 | –8.2, –1.0 | 0.015 | ||
| Aug 2020–Dec 2020 | 10.2 | 0.3, 21.1 | 0.043 | ||
| PTZ/1000 pt-days | Jan 2019–Dec 2020 | –0.2 | 0 | –0.8, 0.4 | 0.496 |
| CPM/1000 pt-days | Jan 2019–Dec 2020 | –2.5 | 0 | –3.4, –1.5 | <0.001 |
| CAZ/1000 pt-days | Jan 2019–Nov 2019 | 3.9 | 1 | –0.3, 8.2 | 0.067 |
| Nov 2019–Dec 2020 | –6.4 | –9.1, –3.6 | <0.001 | ||
| CIP/1000 pt-days | Jan 2019–Dec 2020 | –2.5 | 0 | –3.5, –1.5 | <0.001 |
| LVX (P)/1000 pt-days | Jan 2019–Dec 2020 | 2.0 | 0 | 0.1, 3.8 | 0.038 |
| MOX (P)/1000 pt-days | Jan 2019–Dec 2020 | –0.4 | 0 | –2.5, 1.8 | 0.728 |
AICU, adult intensive care unit; CAUTI, catheter-associated urinary tract infection; CAZ (ceftazidime); CIP, ciprofloxacin; cl, central line; CLABSI, central line-associated bloodstream infection; COL, colistin; CPM, cefepime; CRAB, carbapenem-resistant A. baumannii; CR-GNB, carbapenem-resistant Gram-negative bacteria; CRKP, carbapenem-resistant K. pneumoniae; CRPA, carbapenem-resistant P. aeruginosa; DAIs, device-associated infections; DDD, defined daily dose; LVX, levofloxacin; MEM, meropenem; MOX, moxifloxacin; MPC, monthly percent change; mv, mechanical ventilation; (P), parenteral; PMB, polymyxin B; pt, patient; PTZ, piperacillin/tazobactam; uc, urinary catheter; VAP, ventilator-associated pneumonia.
Carbapenem-resistant A. baumannii (CRAB) was notified in 7.9% (373/4734) of DAI notifications in 2019 and 12.4% (805/6514) in 2020. Meanwhile, carbapenem-resistant K. pneumoniae (CRKP) was reported in 6.4% (304/4734) and 6.4% (420/6514) in both periods. Carbapenem-resistant P. aeruginosa (CRPA) was reported in 3.3% (157/4734) and 2.6% (167/6514) in 2019 and 2020 respectively.
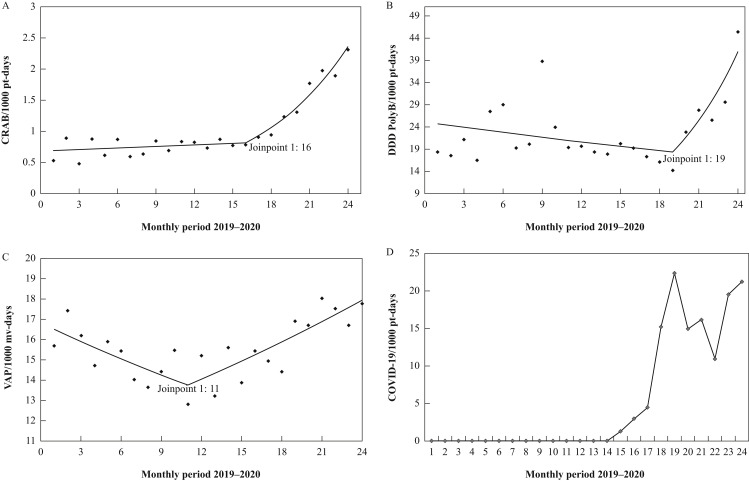
According to the trend analysis, the monthly incidence density of CRAB per 1000 patient-days (Figure 1 A) increased significantly after April 2020 (MPC: 14.2; 95% CI: 10.1–18.5; P < 0.001), while CRKP per 1000 patient-days showed a gradual increase during the entire observed period, but with no change in trend (MPC: 2.4; 95% CI: 0.7–4.1; P = 0.008). And for CRPA incidence density per 1000 patient-days, there was no significant change in the study period.
Figure 1.
Trend analysis for incidence density of CRAB, VAP, and PolyB consumption in DDD per 1000 patient-days concerning COVID-19 cases in AICU, Paraná, Brazil, 2019–2020. (A) Trend analysis for CRAB per 1000 patient-days (April 2020 to December 2020: MPC: 14.2; 95% CI: 10.1–18.5; P < 0.001). (B) Trend analysis for PolyB DDD per 1000 patient-days (July 2020 to December 2020: MPC: 17.5; 95% CI: 4.6–31.9; P = 0.009). (C) Trend analysis for VAP/1000 mv-days (November 2019 to December 2020: MPC: 2.1; 95% CI: 1.2–2.9; P < 0.001). (D) Confirmed COVID-19 cases per 1000 patient-days in AICU. AICU, adult intensive care unit; CRAB, carbapenem-resistant A. baumannii; CI, confidence interval; DDD, defined daily dose; PolyB, polymyxin B; MPC, monthly percent change; VAP, ventilator-associated pneumonia; pt, patient.
Interestingly, some antimicrobials revealed an increasing trend in consumption in DDD per 1000 patient-days. Polymyxin B increased in the period from July to December 2020 (MPC: 17.5; 95% CI: 4.6–31.9; P = 0.009), as did colistin from April 2020 (MPC: 30.7; 95% CI: 17.8–44.9; P < 0.001); however, this increase did not correlate significantly with the incidence density of infections caused by CR-GNB. On the other hand, other antimicrobials showed a reduction in consumption – for example, cefepime and ceftazidime (Table I).
Figure 1A–C shows a Joinpoint regression of the shift in trend in the incidence density of CRAB per 1000 patient-days, VAP per 1000 mv-days and polymyxin B in DDD per 1000 patient-days, following the curve of incidence density of new COVID-19 cases per 1000 patient-days reported per month in patients admitted to the AICU in the state of Paraná (Figure 1D).
To investigate the possible relationship between the increased incidence density of CRAB and related factors, Spearman's correlation analysis was performed. By this test there was a strong positive correlation between CRAB and COVID-19 (r 2 = 0. 760; P < 0. 001), and moderate correlation for VAP (r 2 = 0.517; P = 0.010) and CLABSI (r 2 = 0.569; P = 0.004), but no correlation was detected with polymyxin B and colistin consumption (r 2 = 0.128; P = 0.552 and r 2 = 0.372; P = 0.073). On the other hand, there was a negative correlation with cefepime consumption (r 2 = –0.609; P = 0.002).
Discussion
One of the main findings of our study is that the incidence density of A. baumannii per 1000 patient-days increased significantly in 2020, and this finding had a strong positive correlation with the incidence density of COVID-19 and moderate correlation for VAP, and CLABSI. The incidence density of CRKP per 1000 patient-days showed a gradual increase during the entire observed period, but with no change in trend.
Our results possibly signal persistent clonal spread of CRAB with significant trend change after the COVID-19 pandemic [8]. CRKP also showed a slight increase during the study period, but gradually, with no change of trend detected, which may also be related to clonal dissemination [9]. Violation of infection control protocols – favoured by the increased workload and reduced availability of healthcare workers during the pandemic – combined with institutional inability to sustain prevention and control measures may have contributed to this finding [5].
Increased consumption of polymyxins was observed in 2020, although it showed no significant correlation with CRAB or COVID-19 incidence density by Spearman test. This finding may be related to the increase in empiric prescribing of this antimicrobial, presumably driven by the increase in CRAB infections, increasing the expectation that patients would have CRAB. Consequently, there may be increased selection pressure for microbial resistance, in addition to the lack of this resource on the market forcing the use of suboptimal therapies for the treatment of these infections [10,11].
Some changes in the pattern of antimicrobial consumption regarding cefepime, ceftazidime, ciprofloxacin, levofloxacin, and meropenem (Table I) may not presently be fully clarified as this study did not include information on customized institutional protocols nor a complete profile of agents causing hospital-acquired infections in intensive care units.
The limitations of this study lie in the analysis of the pooled data, the possibility of underreporting, as well as possible typing and data submission errors. Furthermore, information on occupancy rates, as well as distribution of exclusive beds for COVID-19 in the studied ICUs, was not available. There are also methodological strengths. We used a valid time-series analysis, instead of the usual ‘before-and-after’ comparisons. Also, we chose to use Joinpoint regression, which identifies moments of trend change (join points), rather than interrupted time-series analysis, which requires the researchers to identify the ‘turning point’. This is coherent with the gradual (though rapid) increase of COVID-19 incidence in the study area.
We believe that our study identifies a marked trend of change in the resistance profile in DAIs as well as in antimicrobial consumption since the onset of the COVID-19 pandemic, which is an alarm for healthcare institutions and government agencies regarding the need to focus efforts for investigation and control, especially against a scenario of scarce resources.
Conflict of interest statement
None declared.
Funding sources
This study was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), Ministry of Education, Brazil.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.11.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rawson T.M., Ming D., Ahmad R., Moore L.S.P., Holmes A.H. Antimicrobial use, drug-resistant infections and COVID-19. Nat Rev Microbiol. 2020;18:409–410. doi: 10.1038/s41579-020-0395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/?gclid=Cj0KCQjwtMCKBhDAARIsAG-2Eu-HciJsbL9zoQLmRuqjZ4OIipukC9SSVRWdiY-iRG9IjgB9fqxMi1YaAhKSEALw_wcB [last accessed December 2021].
- 3.Neiva M.B., Carvalho I., Filho E.D.S.C., Barbosa-Junior F., Bernardi F.A., Sanches T.L.M., et al. Brazil: the emerging epicenter of COVID-19 pandemic. Rev Soc Bras Med Trop. 2020;53:1–8. doi: 10.1590/0037-8682-0550-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharifipour E., Shams S., Esmkhani M., Khodadadi J., Fotouhi-ardakani R. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20:1–7. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez S., Innes G.K., Walters M.S., Mehr J., Arias J., Greeley R., et al. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions – New Jersey, February–July. Morb Mortal Wkly Rep. 2020;69:1827–1831. doi: 10.15585/mmwr.mm6948e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia R., Katoch V.M., Inoue H. Creating political commitment for antimicrobial resistance in developing countries. Ind J Med Res. 2021:83–86. doi: 10.4103/ijmr.IJMR_1980_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiri B., Sensi E., Marsiliani V., Cantarini M., Priante G., Vernelli C., et al. Antimicrobial stewardship program, COVID-19, and infection control: spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J Clin Med. 2020;9:2744. doi: 10.3390/jcm9092744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cieslinski J.M., Arend L., Tuon F.F., Silva E.P., Ekermann R.G.S., Dalla-Costa L.M., et al. Molecular epidemiology characterization of OXA-23 carbapenemase-producing Acinetobacter baumannii isolated from 8 Brazilian hospitals using repetitive sequence-based PCR. Diagn Microbiol Infect Dis. 2013;77:337–340. doi: 10.1016/j.diagmicrobio.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Arend L.N., Toledo P., Pilonetto M., Tuon F.F. Molecular epidemiology of Klebsiella pneumoniae carbapenemase-producing Entero-bacteriaciae in different facilities in Southern Brazil. Am J Infect Control. 2015;43:137–140. doi: 10.1016/j.ajic.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Tuon F.F., Rymsza A.M., Penteado-Filho S.R., Pilonetto M., Arend L.N., Levin A.S. Should polymyxin be used empirically to treat infections in patients under high risk for carbapenem-resistant Acinetobacter? J Infect. 2011;62:246–249. doi: 10.1016/j.jinf.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Tuon F.F., Rocha J.L., Merlini A.B. Combined therapy for multi-drug-resistant Acinetobacter baumannii infection – is there evidence outside the laboratory? J Med Microbiol. 2015;64:951–959. doi: 10.1099/jmm.0.000144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.