Abstract
Healthcare workers have been categorized among the priority groups for COVID-19 vaccination. However, post-vaccination infections have been identified. This study was conducted to investigate SARS-CoV-2 infection among healthcare workers (HCWs) who received the COVID-19 vaccine. A case series in a multicenter healthcare system in Saudi Arabia was created from HCWs who had (PCR-RT) confirmed SARS-CoV-2 infection after at least one dose of Pfizer-BioNTech vaccination. A total of 20 healthcare workers (HCWs) have been included. The majority (70.0%) were males and the average age was 39.4 ± 10.1 years. They included physicians (55.0%), nurses (25.0%) and other HCWs (20.0%). Eighteen (90%) HCWs had infection after the first dose; 47.1% within the first week, 41.2% within the second week, and 11.8% within the third week. Only two HCWs (10.0%) had infection one week after the second dose. The majority (63.2%) had mild (52.6%) or moderate (10.3%) disease with no severe disease or hospitalization. The majority of post-vaccination COVID-19 infections among HCWs occurred before the full protection of the vaccine is gained. Suspicion of COVID-19 infection should be considered even with a history of COVID-19 vaccination. Recently vaccinated HCWs should be advised to fully comply with all recommended precautions to prevent COVID-19 transmission.
Keywords: Coronavirus disease (COVID-19), Pandemic, Hospital, Healthcare, Saudi Arabia
Background
The current coronavirus disease (COVID-19) pandemic is considered a huge burden for healthcare workers (HCWs), both physically and mentally [1]. They suffer from a considerably higher risk of exposure to and infection with SARS-CoV-2 compared with the general population [2,3]. The WHO, the Centers for Disease Control and Prevention (CDC), and the Ministries of Health identified HCWs as a priority for COVID-19 vaccination even during the period of initial limited supply [4,5].
In Saudi Arabia, a COVID-19 vaccination campaign was started on the last week of December 2020, following approval of the Pfizer-BioNTech vaccine by the Saudi Food and Drug Administration (SFDA) [6]. Although Pfizer-BioNTech reported a high safety and efficacy vaccine profile [7], there have been some initial concerns among the population in Saudi Arabia including HCWs [8]. The current study was conducted to investigate post-vaccination SARS-CoV-2 infection among HCWs who received the Pfizer-BioNTech vaccine in a multihospital healthcare system in Saudi Arabia.
Methods
The current study was conducted at the six Ministry of National Guard Health Affairs (MNGHA) hospitals in Riyadh, Jeddah, Alahsaa, Dammam, and Madinah. MNGHA hospitals are tertiary care governmental hospitals with a total of 2510 beds. They provide healthcare services for Saudi National Guard soldiers, employees, and their families. MNGHA hospitals are served by 19,016 clinical HCWs; including 4605 physicians, 8701 nurses, and 5710 other clinical HCWs.
A case series study has been conducted based on prospective surveillance among HCWs who were working in MNGHA hospitals during the study period and had confirmed SARS-CoV-2 infection after COVID-19 vaccination. The duration covered was three months (from December 24, 2020, to March 23, 2021).
HCWs who had a positive RT-PCR test for COVID-19, irrespective of the presence of symptoms were identified. Data were collected on the HCW’s demographics, symptoms, exposure, and vaccination history.
Descriptive statistics were done using SPSS software (Version 22.0. Armonk, NY: IBM Corp).
Results
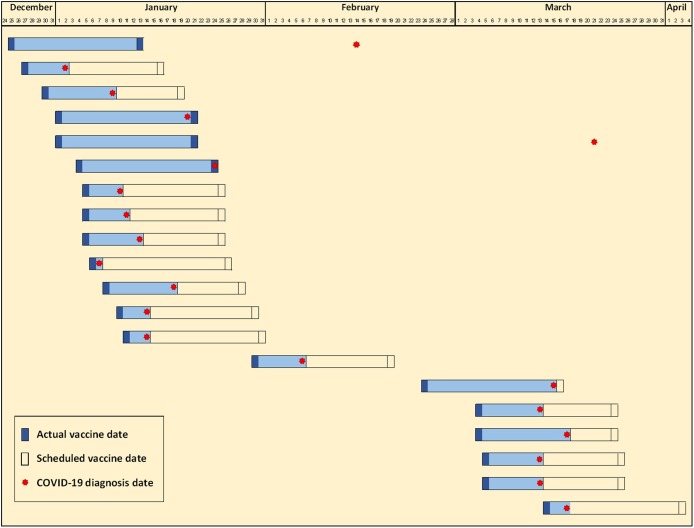
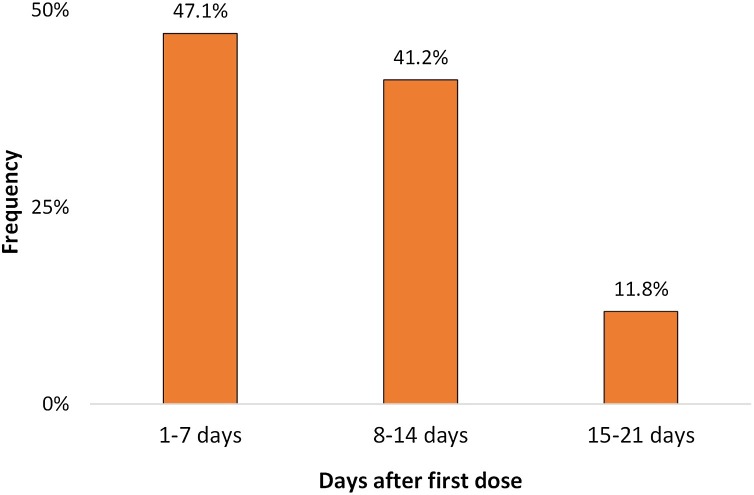
A total of 20 healthcare workers (HCWs) have been included in the current report. The majority (70.0%) were males and the average age was 39.4 ± 10.1 years (range between 25 and 61 years). Most of the studied HCWs were physicians (55.0%), followed by nurses (25.0%) and other HCWs (20.0%). All HCWs have taken the first dose while only four (20.0%) have taken the second dose of the Pfizer-BioNTech COVID-19 vaccine. All HCWs had PCR-RT confirmed SARS-CoV-2 infection after a median of 8 days (range 1–79 days) from the first dose and a median of 16 days (range −1 to 59 days) from the second dose. Individual post-vaccine durations arranged according to the date of administration of the first dose are shown in Fig. 1 . When considering those who had an infection before the second dose, 47.1% had the infection onset within the first week, 41.2% had it within the second week, and 11.8% had it within the third week (Fig. 2 ). Only two HCWs (10.0%) had confirmed infection after the suggested immunity cut point (one week after the second dose).
Fig. 1.
Duration between first and second doses of Pfizer-BioNTech COVID-19 vaccine and the diagnosis of COVID-19 in 20 healthcare workers.
Fig. 2.
Frequency of diagnosis of COVID-19 by the duration after first dose of Pfizer-BioNTech COVID-19 vaccine among 17 healthcare workers who had the diagnosis before the second dose.
The majority (63.2%) of the HCWs who reported clinical symptoms had either mild (52.6%) or moderate (10.5%) disease while 36.8% of the HCWs had asymptomatic disease. The most frequent symptoms included fever (36.8%), cough (36.8%), headache (21.1%), malaise (21.1%), sore throat (21.1%), runny nose (15.8%), and shortness of breath (10.5%). All HCWs were isolated in the home and required no hospital admission. The majority (65.0%) of HCWs could not recognize COVID-19 exposure with only 35.0% reported COVID-19 exposure related to the workplace (15.0%), family (15.0%), and community (5.0%). Moreover, no reports of COVID-19 clusters linked to the immunization process have been identified.
Discussion
In the current report, 20 HCWs with post-vaccination laboratory-confirmed SARS-CoV-2 infection were investigated. The majority (90%) had SARS-CoV-2 infection before the vaccine full protection is reached. Consistent with current findings, data published at the time of the study (early 2021) showed that 91%–96% of post-vaccination infections occurred before full protection, at least one week after the second dose [7,9].
The frequency of post-vaccination infection in this case series was decreasing as the duration from the first dose was increasing, indicating mounting protection. Consistent with these findings, an interim analysis of Pfizer-BioNTech vaccination data after excluding those who got the infection in the first two weeks after the first dose showed increased vaccine efficacy from 89% to 91% during the period between 15 and 28 days after the first dose [10].
A the time of this study, the original alpha variant was predominant. Over time an increased concern of surge of cases coinciding with the spread of other variants (i.e., beta and delta) has been raised globally. A test-negative case-control study in the UK showed modest differences in vaccine effectiveness against symptomatic disease with delta compared to the alpha variant [11]. Additionally, another case-control study among healthcare workers argued the role of initial immune response compared to the decay of antibody levels on the degree of protection against breakthrough infections [12].
Despite being among the first case series about post-vaccination COVID-19 in Saudi Arabia and the region, a couple of limitations should be acknowledged. As HCWs were vaccinated in centers outside the MNGHA hospital authority, the total number of vaccinated HCWs in MNGHA hospitals could not be obtained. This hindered our ability to estimate the vaccine effectiveness. Additionally, PCR testing after vaccination was based on self-request. Therefore, HCWs who got asymptomatic disease may pass unnoticed if testing was not sought. However, this most probably cannot be avoided in similar studies.
In conclusion, the current report reconfirms the possibility of post-vaccination COVID-19 infection among HCWs. The false sense of full protection immediately following vaccination might have increased undue exposure while adherence to precautionary measures was inconsistently followed. Efforts should be maintained to ensure completion of the recommended vaccine series among the general population, with special emphasis on the high-risk people including HCWs. Strategies that include sequencing of viral RNA and monitoring of neutralizing antibody titers, particularly in persons with breakthrough infection should continue to identify pandemic change and determine the effectiveness of the applied interventions.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
Author contributions
Majid M. Alshamrani: Conception, Design, Acquisition of data, Analysis and interpretation of data; Fayssal M Farahat: Conception, Design, Acquisition of data, Analysis and interpretation of data; Aiman El-Saed: Conception, Design, Acquisition of data, Analysis and interpretation of data; Mohammed Alzunitan: Acquisition of data and interpretation of data; Asim Alsaedi: Acquisition of data and interpretation of data; Ayman El Gammal: Acquisition of data and interpretation of data; Wafaa Al Nasser: Acquisition of data and interpretation of data; Syed Nazeer: Acquisition of data and interpretation of data; Saad A Almohrij: Conception and interpretation of data.
References
- 1.Cabarkapa S., Nadjidai S.E., Murgier J., Ng C.H. The psychological impact of COVID-19 and other viral epidemics on frontline healthcare workers and ways to address it: a rapid systematic review. Brain Behavior Immunity Health. 2020;8 doi: 10.1016/j.bbih.2020.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei J.te, Liu Z.D., Fan Z.W., Zhao L., Cao W.C. Epidemiology of and risk factors for COVID-19 infection among health care workers: a multi-centre comparative study. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17197149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alserehi H.A., Alqunaibet A.M., Al-Tawfiq J.A., Alharbi N.K., Alshukairi A.N., Alanazi K.H., et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis. 2021;99(3):115273. doi: 10.1016/j.diagmicrobio.2020.115273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . WHO; 2020. WHO sage roadmap for prioritizing uses of Covid 19 vaccines in the context of limited supply. [Google Scholar]
- 5.Dooling K., McClung N., Chamberland M., Marin M., Wallace M., Bell B.P., et al. The advisory committee on immunization practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine — united States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(49):1857–1859. doi: 10.15585/mmwr.mm6949e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saudi Ministry of Health . 2021. Al-Rabiah launches COVID-19 vaccination campaign.https://WwwMohGovSa/En/Ministry/MediaCenter/News/Pages/News-2020-12-17-007Aspx [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qattan A.M.N., Alshareef N., Alsharqi O., al Rahahleh N., Chirwa G.C., et al. Acceptability of a COVID-19 vaccine among healthcare workers in the Kingdom of Saudi Arabia. Front Med. 2021;8 doi: 10.3389/fmed.2021.644300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keehner J., Horton L.E., Pfeffer M.A., Longhurst C.A., Schooley R.T., Currier J.S., et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384 doi: 10.1056/nejmc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Public Health England . 2021. Annex A: Report to JCVI on estimated efficacy of a single dose of Pfizer BioNTech (BNT162b2 mRNA) vaccine and of a single dose of ChAdOx1 vaccine (AZD1222)https://AssetsPublishingServiceGovUk/Government/Uploads/System/Uploads/Attachment_data/File/949505/Annex-a-Phe-Report-to-Jcvi-on-Estimated-Efficacy-of-Single-Vaccine-DosePdf [Google Scholar]
- 11.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385 doi: 10.1056/nejmoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/nejmoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]