Abstract
Objective:
To determine whether hospital mortality (primary outcome) is associated with duration of bradycardia without chest compressions during delivery room (DR) resuscitation in a retrospective cohort study of randomized controlled trials (RCTs) in preterm infants assigned low versus high initial oxygen concentration.
Methods:
Medline and EMBASE were searched from 01/01/1990 to 12/01/2020. RCTs of low vs high initial oxygen concentration which recorded serial heart rate (HR) and oxygen saturation (SpO2) during resuscitation of infants <32 weeks gestational age were eligible. Individual patient level data were requested from the authors. Newborns receiving chest compressions in the DR and those with no recorded HR in the first 2 minutes after birth were excluded. Prolonged bradycardia (PB) was defined as HR <100 bpm for ≥ 2 min. Individual patient data analysis and pooled data analysis were conducted.
Results:
Data were collected from 720 infants in 8 RCTs. Neonates with PB had higher odds of hospital death before [OR3.8 (95% CI 1.5, 9.3)] and after [OR 1.7 (1.2, 2.5)] adjusting for potential confounders. Bradycardia occurred in 58% infants, while 38% had PB. Infants with bradycardia were more premature and had lower birth weights. The incidence of bradycardia in infants resuscitated with low (≤ 30%) and high (≥ 60%) oxygen was similar. Neonates with both, PB and SpO2<80% at 5 minutes after birth had higher odds of hospital mortality. [OR 18.6 (4.3, 79.7)]
Conclusion:
In preterm infants who did not receive chest compressions in the DR, prolonged bradycardia is associated with hospital mortality.
INTRODUCTION:
Intrauterine hypoxia or factors influencing the physiologic changes during transition can make a newborn limp, apneic or bradycardic.[1, 2] Many preterm infants experience suboptimal transition, producing bradycardia and/or apnea requiring resuscitation at birth.[3, 4] A rising heart rate (HR) is an important indicator of effective ventilation in a bradycardic newborn.[5-8] If the HR remains below 100 bpm after the initial steps, International Liaison Committee on Resuscitation (ILCOR) guidelines recommend positive pressure ventilation.[5, 7] If the HR remains below 60 bpm after attempting adequate ventilation, ILCOR guidelines recommend chest compressions[5, 7] Preterm infants who received chest compression in the delivery room (DR-CPR) have increased mortality or morbidity in survivors.[9-14] Fortunately, most preterm infants with bradycardia respond to adequate ventilation and few require DR-CPR.[4, 6] It remains unclear if the duration of bradycardia increases morbidity and mortality in preterm infants not requiring DR-CPR.
The largest clinical trial of initial oxygen (O2) concentration for preterm resuscitation in the DR so far showed a higher incidence of bradycardia in infants whose resuscitation began with room air.[15] It was unclear if the duration of bradycardia differed between preterm infants resuscitated with low vs high O2 concentration.[15] In a post-hoc analysis, O2 saturation (SpO2) < 80% at 5 minutes after birth was associated with increased mortality.[16] Along with O2 content of blood, cardiac output is important for adequate O2 delivery to tissues.[17] Prolonged bradycardia (PB) compromises cardiac output, causing inadequate O2 delivery and tissue hypoxia. Intermittent bradycardia in preterm neonates during their neonatal intensive care unit (NICU) stay has been associated with decreased cerebral O2 saturation and motor impairment.[18-20] It is unclear if prolonged bradycardia and low SpO2 in the DR have an additive significance on adverse outcomes.
We therefore obtained individual patient SpO2 and HR data from randomized controlled trials (RCT) that compared outcomes of high versus low inspired O2 resuscitation strategies in infants <32 weeks gestational age (GA). We hypothesized that infants <32 weeks GA who are bradycardic immediately after birth and remain bradycardic for two minutes or more will be at a higher risk of the primary outcome of neonatal mortality and secondary morbidities. We also hypothesized that preterm infants whose resuscitation started with low O2 concentration (21% - 30%) had longer bradycardia.
METHODS:
Protocol
This individual patient data analysis was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement for meta-analysis in health care interventions. [21, 22] The protocol was submitted with the Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO/CRD42020216231).
Eligibility Criteria
RCTs which recorded serial HR, SpO2, fraction of inspired oxygen (FiO2) during resuscitation of preterm neonates <32 weeks GA, titrated FiO2 to achieve a target SpO2 and reported neonatal morbidities and mortality were eligible for this study. Methods of HR assessment, pulse oximeter or an ECG monitor, were noted. Individual patient data for infants <32 weeks GA were obtained directly from the authors. Neonates without HR data by two minutes or who received chest compressions in the DR were excluded as per the protocol. Patients were divided into three groups based on their HR data in the DR during the first 10 minutes from birth. 1. No bradycardia (NB): HR ≥100 bpm throughout the first 10 minutes 2. Transient bradycardia (TB): HR below 100 bpm for < two minutes 3. Prolonged bradycardia (PB): HR below 100 bpm for ≥ two minutes.
Outcomes
The primary outcome of this study was neonatal in-hospital mortality. This and the secondary outcomes of bronchopulmonary dysplasia (BPD), severe retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), and severe intraventricular hemorrhage (IVH) were analysed in relation to the three groups of bradycardia. In-hospital mortality was defined as death before discharge from the NICU. The low O2 strategy was defined as starting resuscitation with 21%-30% O2 while high O2 strategy was defined as starting with 60%-100% O2. [23] BPD was defined as need for supplemental O2 and/or ventilatory support at 36 weeks postmenstrual age.[24, 25] Severe IVH was defined as grade 3 or higher on any head ultrasounds unilaterally or bilaterally as per Papile criteria.[26] NEC was defined ≥ Stage 2 based on the modified Bell criteria.[27] Severe ROP was defined as Stage 3 or higher based on the international classification of retinopathy of prematurity.[28]
Search strategy and data sources (Figure 1):
Figure 1:
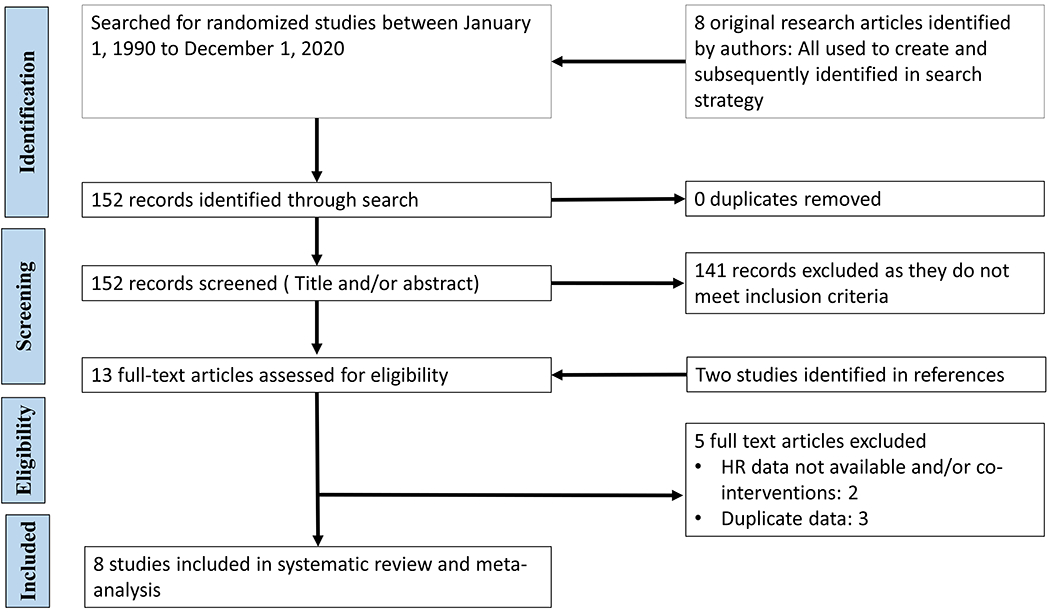
PRISMA flow diagram of study selection
Databases (Medline/PubMed, EMBASE, ClinicalTrials.gov, Cochrane controlled trial registers) and meeting abstracts (Pediatric Academic Societies, European Society of Paediatric Research, European Association of Paediatric Societies) were searched from 1990 to 1 November 2020 without language restrictions using index terms: preterm, resuscitation and O2. Studies in full manuscript or abstract forms were acceptable. An iterative approach was used to ensure that key articles (identified by VK and JO) were found.
Study Selection and Data Extraction
Two authors (VK and JO) independently screened titles and abstracts. In the event of a disagreement during abstract screening, the full text was reviewed. They subsequently completed full-text review for eligibility independently. Final decisions were determined by consensus. The reason for exclusion was captured according to a predetermined, ordered list of exclusions.
Data Collection, Risk of Bias, and Certainty of Evidence Assessment
For each study, two authors (VK and JO) independently extracted predetermined study characteristics and outcomes and then achieved consensus. They independently evaluated the risk of bias (RoB) in individual studies using the Cochrane Risk of Bias Tool for RCTs. The two authors also assessed the certainty of evidence (confidence in the estimate of effect) for each outcome based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework (GRADEpro Guideline Development Tool; McMaster University, Hamilton, Canada). Individual patient data were obtained directly from the authors including the HR and SpO2 data downloaded from the pulse oximeter.
Data analysis
A. Individual patient data analysis:
Individual patient data analysis was performed to assess the association of bradycardia with neonatal mortality and secondary morbidities. For this individual data analysis, categorical data were examined by chi-square for trend. Continuous variables were assessed by ANOVA or Kruskal Wallis test. To adjust for confounding variables, multivariate stepwise forward logistic regression was conducted, including GA, birth weight, gender, antenatal steroids, SpO2 < 80% at 5 minutes after birth and individual studies as independent variables.
B. Pooled data analysis:
Pooled analysis was conducted from aggregated data per study to compare neonates with PB and those without PB using Review Manager software 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark), which does not adjust for potential confounding variables. We used the standard methods of the Cochrane Collaboration. Individual studies were weighted. Random effects models were used to account for variation within and between studies and to compute the summary odds ratio (OR). We report unadjusted summary OR and corresponding 95% confidence intervals (CIs) using the Mantel-Haenszel (MH) method for dichotomous variables. Forest plots were used for the graphical representation of ORs generated from unadjusted data. Heterogeneity between studies was evaluated with I2 statistics and publication bias was assessed by the Egger’s test and by funnel plot inspection.
RESULTS:
Literature Search and Study Selection: (Figure 1)
In total 152 records were identified. As there were no duplicates, 152 records were screened by title and abstract. Two additional articles were found via reference searches and added to the full-text screening. [29, 30] A total of 13 full text articles were assessed for eligibility. Two articles were excluded as serial HR data were not available.[30, 31] Three articles were excluded as they represented a further analysis of the previous study.[32-34] After consensus between two authors (VK and JO), eight RCTs were included in the final quantitative synthesis. [15, 29, 35-40]
Study and patient characteristics (Table 1)
Table 1:
Study characteristics
| Study | Enrollment period | Location | FiO2 groups | Total eligible | Total included, N (%) | Total excluded as no HR available for the first two minutes after birth | No bradycardia | Transient bradycardia | Prolonged bradycardia |
|---|---|---|---|---|---|---|---|---|---|
| Wang et al | 2005-2007 | United States | 0.21 vs 1.0 | 37 | 27 (73%) | 10 (27%) | 14 | 5 | 8 |
| Escrig et al | 2005-2007 | Spain | 0.3 vs 0.9 | 42 | 42(100%) | 0 (0%) | 2 | 25 | 15 |
| Vento et al | 2007-2008 | Spain | 0.3 vs 0.9 | 78 | 78 (100%) | 0 (0%) | 18 | 2 | 58 |
| Rabi et al | 2005-2007 | Canada | 0.21 vs 1.0 | 26 | 24 (92%) | 4 (8%) | 7 | 4 | 13 |
| Aguar et al | 2010-2012 | Spain | 0.3 vs 0.6 | 60 | 60 (100%) | 0 (0%) | 14 | 24 | 22 |
| Rook et al | 2018-2012 | Netherlands | 0.3 vs 0.6 | 139 | 61 (44%) | 78 (54%) | 44 | 4 | 13 |
| Kapadia et al | 2010-2011 | United states | 0.21 vs 1.0 | 51 | 51 (100%) | 0 (0%) | 28 | 11 | 12 |
| Oei et al | 2009-2014 | Australia Malaysia Qatar | 0.21 vs 1.0 | 287 | 262 (91%) | 25 (9%) | 127 | 47 | 88 |
| Total | 720 | 605 (84%) | 115 (16%) | 254 | 122 | 229 |
No bradycardia: HR ≥ 100 bpm throughout the first 10 minutes. Transient bradycardia: HR below 100 bpm for < two minutes. Prolonged bradycardia: HR below 100 bpm for ≥ two minutes.
In total, 720 preterm infants < 32 weeks GA were enrolled in the eight RCTs. Most infants had serial HR data in the first 2 minutes after birth except in the RCTs of Rook et al[38] (44% of infants) and Wang et al[40] (73% of infants). As pre-specified, 115 infants (16%) were excluded, as no HR were data available for the first 2 minutes after birth. In total, 605 infants (84% of the eligible infants) were included. All studies used pulse oximetry to collect HR data. Fifty eight percent of preterm infants were bradycardic when first assessed after birth. Thirty eight percent of preterm infants had PB. Amongst bradycardic preterm infants, 93% were bradycardic when first assessed.
RoB Assessment (Supplemental table 1)
All studies except Rabi et al were classified as unclear RoB.[37] Caregivers were blinded to the intervention in only three of the included RCTs.[35, 37, 38] Five RCTs reported that researchers assessing trial end points were blind to randomized group.[29, 35, 37-39] In two RCTs, many infants had no HR in the first 2 minutes after birth making them at high risk of bias because of incomplete outcome data.[38, 40] The study by Oei et al was terminated early due to low recruitment rate.[15] More infants <32 weeks GA in the RCT by Vento et al had PB compared to the average in the study (74% vs 38%) and their mean (SD) GA was 26 ± 1 weeks compared with 28 ± 2 weeks GA (p<0.05) in the other studies.
Individual patient data analysis (Table 2)
Table 2:
Pooled data analysis: Infant characteristics and outcomes
| No Bradycardia N= 254 (42%) | Transient Bradycardia N= 122 (20%) | Prolonged Bradycardia N= 229 (38%) | P value | Adjusted P value for GA | |
|---|---|---|---|---|---|
| Gestational age, wks | 28 ± 2a | 27 ± 2b | 27 ± 2b | < 0.01 | NA |
| Birth weight, gms | 1152 ± 352a | 976 ± 257b | 980 ± 270b | < 0.01 | NA |
| Male | 114 (45%) | 65 (53%) | 113 (49%) | NS | NS |
| Antenatal Steroids | 216 (85%) | 101 (83%) | 198 (87%) | NS | NS |
| Cesarean section | 152 (60%) | 64 (52%) | 131 (58%) | NS | NS |
| Starting low FiO2 | 112 (44%) | 58 (48%) | 122 (53%) | NS | NS |
| SpO2 < 80% at 5 minutes | 89 (35%)a | 51 (43%)a | 149 (65%)b | <0.01 | <0.01 |
| Apgar 1 min | 7 (6,8)a | 5 (3,6)b | 5 (3,6)b | <0.01 | <0.01 |
| Apgar 5 min | 9 (8,9)a | 7 (7,9)b | 7 (6,9)b | <0.01 | <0.01 |
| IVH | 10 (4%)a | 14 (12%)b | 29 (14%)b | <0.01 | 0.03 |
| NEC | 4 (2%) | 1 (2%) | 4 (4%) | NS | NS |
| BPD | 49 (20%)a | 38 (32%)b | 58 (27%)a,b | 0.03 | NS |
| ROP | 34 (14%) | 20 (17%) | 37 (17%) | NS | NS |
| Death | 6 (3%)a | 9 (7%)a,b | 37 (16%)b | <0.01 | <0.01 |
| Death or BPD | 54 (22%)a | 45 (38%)b | 88 (41%)b | <0.01 | NS |
| Death or IVH | 16 (6%)a | 23 (20%)b | 61 (28%)b | <0.01 | <0.01 |
Superscripts in columns signify pairwise comparison of columns with different letters (a or b) signify statistical difference with Bonferroni correction and similar letters are not statistically different.
Bradycardic infants in the DR were more premature, with lower birth weights, than infants without bradycardia. There was no association between initial FiO2 and bradycardia in the DR. More infants with bradycardia in the DR had SpO2 <80% at 5 minutes after birth. They also had lower 1 minute and 5 minute Apgar scores. As the duration of bradycardia increased, the incidence of IVH, BPD and in-hospital mortality also increased. This was also true for a composite outcome of in-hospital mortality and/or IVH but not true for in-hospital mortality and/or BPD. These associations remained significant even after adjusting for GA. Even after adjusting for these potential confounders, prolonged bradycardia remained associated with in-hospital mortality, the primary outcome. [OR 1.7 (1.2, 2.5), p<0.01] (Table 3) There was an exposure response relationship between duration of bradycardia and in-hospital mortality (figure 2).
Table 3:
Multiple regression analysis reporting the association of in-hospital mortality as primary outcome (dependent variable) - with seven pre-specified exposures (independent variables).
| Independent variable | Odds Ratio (95% CI) | p value | Coefficients |
|---|---|---|---|
| Prolonged Bradycardia* | 1.7 (1.2,2.5) | 0.004 | 0.552 |
| Gestational Age** | 0.6 (0.4,0.8) | 0.003 | −0.513 |
| Male gender† | 2 (1,4) | 0.06 | 0.671 |
| Birth Weight | 0.1 (0.1,1) | 0.2 | −0.00189 |
| Antenatal Steroids | 1 (0.2,5.2) | 0.9 | 0.032 |
| Individual Study | 0.9 (0.8,1) | 0.1 | −0.111 |
| SPO2 < 80% at 5 minutes after birth | 1.4 (0.6,3) | 0.4 | 0.327 |
Exposure to prolonged bradycardia vs non-exposure is associated with a statistically significant increase in odds of in-hospital mortality of 1.7 (95% CI 1.2 to 2.5)
Each increase in gestation of one week is associated with a statistically significant reduction in the odds of hospital mortality of 0.6 (95% CI 0.4 to 0.8).
Relative to being female, being male is associated with a non-statistically significant twofold increase in the odds of hospital mortality
Figure 2:
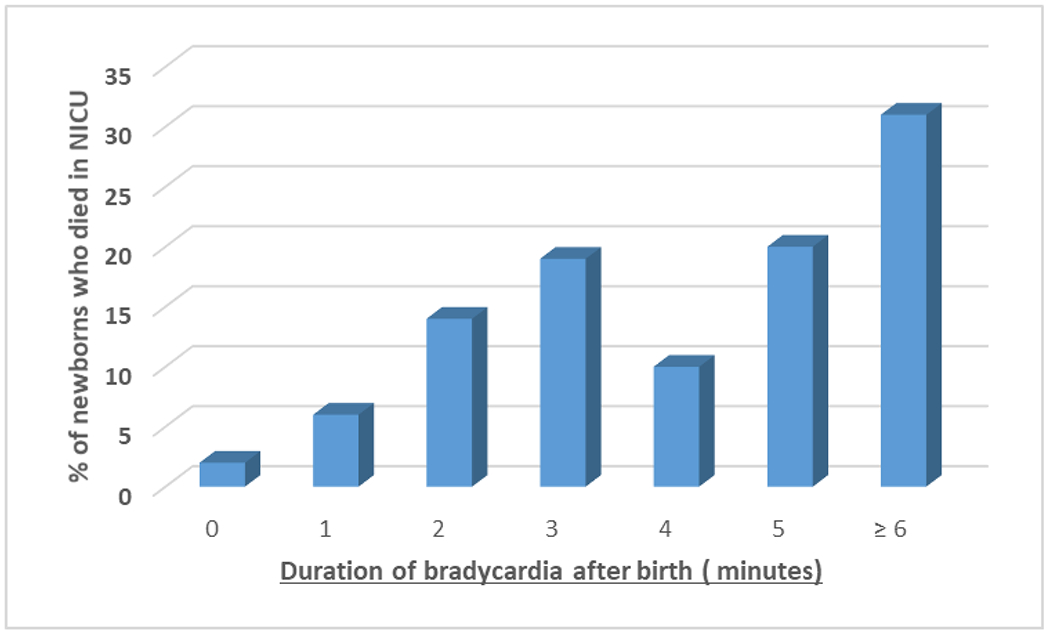
Association of mortality with duration of bradycardia during resuscitation in preterm infants
Pooled data analysis (Figure 3)
Figure 3:
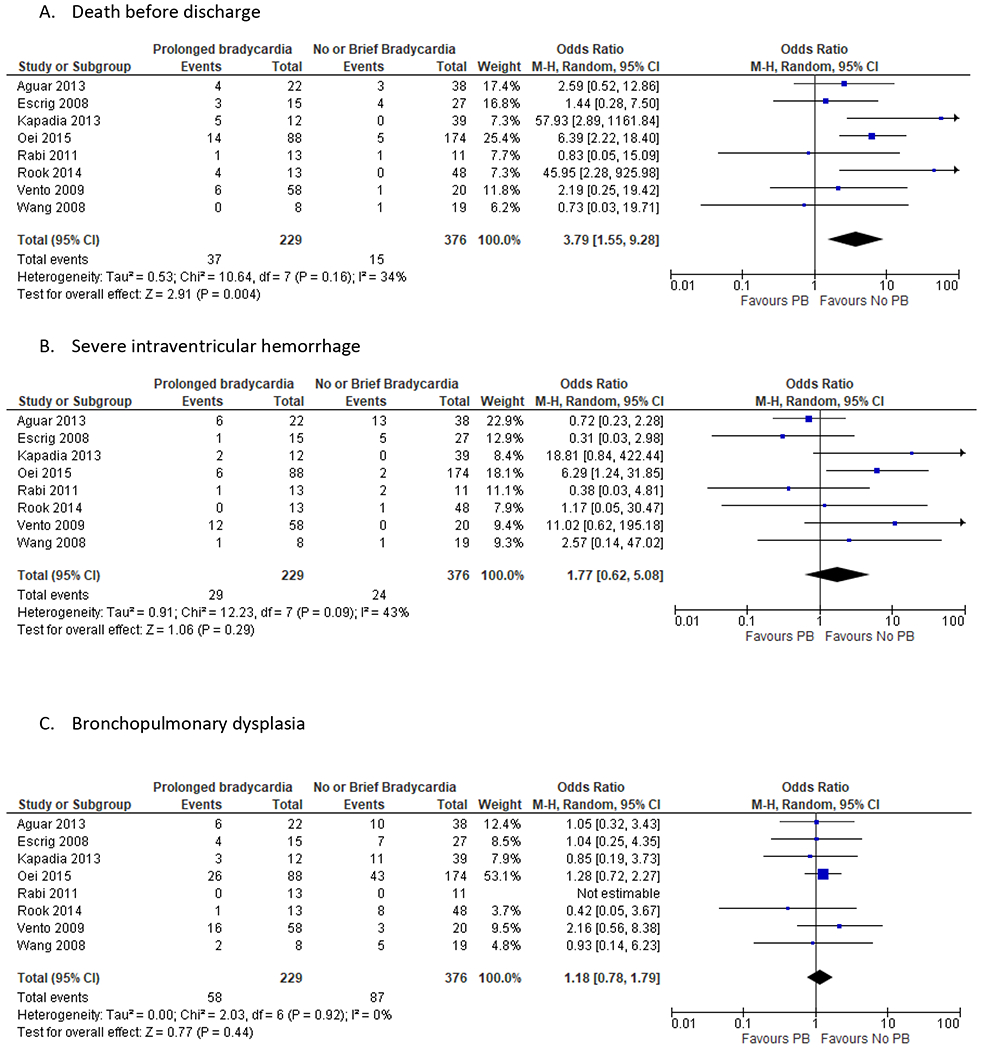
Summary of results: Prolonged bradycardia versus no prolonged bradycardia at birth. The following forest plots are graphic representation of the unadjusted data. A. Death before discharge B. Severe Intraventricular hemorrhage C. Bronchopulmonary dysplasia
The primary outcome of in-hospital mortality was reported in eight RCTs involving 605 preterm infants (Figure 3A). The pooled data analysis found higher summary odds of in-hospital mortality for infants with PB [Summary OR 3.57, 95% CI (1.34, 9.5)]. Pre-specified sensitivity analysis was conducted by excluding two studies, which had a large number of infants with no HR recorded by 2 minutes of life. The summary odds ratio was 3.49 [95% CI (1.45, 8.44)] (Supplementary figure 1). The pooled data analysis did not find any statistical difference between the PB and no PB groups for IVH [Summary OR 1.77, 95% CI (0.62, 5.08)] or BPD [Summary OR 1.18, 95% CI (0.78, 1.79)] (Figure 3B and 3C). The summary OR of low initial FiO2 was not different between the PB and no PB groups [Summary OR 1.28, 95% CI (0.71, 2.29)] (Supplementary figure 2A). Similarly, the summary OR of having SpO2 < 80 % at 5 minutes was no different between the PB and no PB groups [Summary OR 1.89, 95% CI (0.82, 4.36)] (Supplementary figure 2B). The certainty of evidence was down-graded to low due to serious concerns with RoB, imprecision and plausible confounding.
Interaction between SpO2 and Bradycardia (Supplementary table 2)
For this analysis, the group of preterm infants who neither had bradycardia nor had SpO2 <80% at 5 minutes was used as the reference group. Compared to this reference group, preterm infants who had PB without SpO2 <80% at 5 minutes were 10 times more likely to die before discharge. [OR 10.2, 95% CI (2.1, 48.4)] If preterm infants had both, PB and SpO2 < 80% at 5 minutes after birth, the odds of in-hospital mortality were 18 times higher. [ OR 18.6, 95% CI (4.3, 79.7)] This positive additive interaction between SpO2<80% at 5 minutes and PB in the DR for mortality persists after controlling for GA.
DISCUSSION:
In this study, thirty-eight percent of preterm infants < 32 weeks GA experienced prolonged bradycardia, which was associated with increased mortality and severe IVH. To focus on PB not receiving chest compressions, infants receiving chest compressions were excluded from the study. There was no association between duration of bradycardia and low vs high O2 strategy. Neonates who were bradycardic were more likely to have SpO2 < 80 % at 5 minutes after birth. There was an exposure response relationship between duration of bradycardia and mortality. The highest odds of in-hospital mortality were in preterm infants with PB and SpO2 < 80 % at 5 minutes after birth.
Studies have shown that infants who receive higher intensity of resuscitation have a higher incidence of adverse outcomes.[41-43] A recent study showed that low 5 minute and 10 minute Apgar scores are associated with an increased risk of mortality in preterm infants.[44] Apgar score at 5 minutes < 3 has been linked with higher mortality in term infants.[8, 10] None of these studies have examined the association between the duration of bradycardia and neonatal mortality. Intermittent hypoxemia in the NICU has been associated with adverse outcomes. Study by Walter et al showed that in preterm infants admitted to the NICU, bradycardic episodes were associated with clinically significant cerebral desaturations.[20] This effect was more prominent in preterm infants < 32 weeks GA. Interestingly, post hoc analysis of a large randomized controlled trial by Poets et al showed percentage time with bradycardia was associated with motor impairment at 18 months of age, but it did not add any significant prognostic information when added in the model with exposure to intermittent hypoxemia.[19] We have shown in our previous publication that HR < 100 bpm at 5 minutes is associated with mortality and infants who have SpO2 <80% at 5 minutes are more likely to have bradycardia too.[16]
The current study was designed to examine the association between duration of bradycardia in preterm infants not needing CPR and adverse outcomes The PB may reflect in-utero insult and perhaps in some cases, inadequate resuscitation. In the fetus, HR is the most important determinant of cardiac output.[17, 45] In a depressed preterm neonate at birth, PB can interfere with O2 delivery and may contribute to tissue hypoxia, which in turn may cause adverse outcomes. Alternatively PB may just be a surrogate marker for severity of intrauterine insult or a neonate with significantly impaired transition at birth. Similarly, the additive interaction between SpO2< 80% at 5 minutes and PB for mortality could reflect severe tissue hypoxia or suggest severity of illness. Low SpO2 and bradycardia may not be independent of each other. Given its retrospective nature, this study cannot prove causation. Even so, this study shows that HR is the most important vital sign during neonatal resuscitation. It remains to be seen if DR interventions that decrease duration of bradycardia improve clinical outcomes.
This study, analyzed individual data on 605 preterm infants in 8 RCTs using pre-specified, uniform definitions for bradycardia and outcomes. Its strengths include use of individual patient data analysis, pooled data analysis[46, 47] and inclusion of a biostatistician as a co-author.[48] To our knowledge, this is the only study of the association between duration of bradycardia in the DR and adverse outcomes in infants < 32 weeks who did not need CPR. As one objective was to explore the interaction between PB and low SpO2, only trials collecting serial FiO2, HR and SpO2 data were included. The study demonstrates an exposure response relationship between duration of bradycardia and mortality, which strengthens the association between PB and mortality. Also, pre-specified confounders were included in the logistic regression to reduce bias.
This study has several limitations. All RCTs used pulse oximetry to record HR data. Pulse oximeters frequently underestimate HR in the first few minutes after birth, likely due to technical limitation.[49-52] It is unclear if HR measured by ECG, based on cardiac electrical activity, or by pulse oximetry, based on the peripheral pulse, is a better measure of a cardiac contraction and circulation of blood to peripheral tissues. Our findings should be confirmed by observations where ECG is used for HR measurement. Similarly, individual studies do not describe if delayed cord clamping was practiced on vigorous preterm infants. Studies have shown that immediate cord clamping results in bradycardia.[45] Healthy newborns who underwent delayed cord clamping had lower HRs and higher SpO2 in the delivery room.[53, 54] Our findings should be confirmed in preterm infants who have undergone delayed cord clamping as recommended by current guidelines.[5, 7, 55] Inadequate ventilation during neonatal resuscitation may lead to prolonged bradycardia. Details of respiratory interventions during neonatal resuscitation were not available for the individual patient data analysis. Future studies should include the amount of DR respiratory support provided and its duration. None of the RCTs were designed to investigate the duration of bradycardia and adverse neonatal outcomes. Despite collecting individual patient data from 8 RCTs, the sample size is relatively small. The studies included in this study were heterogeneous and used different O2 strategies, which may have affected results. The random effect model was used for the pooled analysis to account for this. In addition, the study did not find any association between starting FiO2 and incidence of PB. Due to missing HR data for the first 2 minutes after birth, 16% of eligible infants were excluded from the analysis based on the pre-specified study protocol. Although this seems to be missing data at random, a sensitivity analysis of the primary outcome by excluding these two studies was conducted. Although Forest Plots are increasingly used in observational analyses like ours, we used them as only graphic representation of the unadjusted pooled data analysis.[56]. Even though logistic regression was performed to control for known confounders, it is possible that some variables, which may have influenced mortality and intraventricular hemorrhage in this population, are unaccounted for.
In conclusion, PB in the DR was associated with mortality and IVH in preterm infants < 32 weeks GA. PB and low SpO2 in the DR increased the odds of mortality. The quality of evidence was judged to be low due to risk of bias and imprecision. Future studies involving resuscitation in preterm infants should report duration of bradycardia and 5 minute SpO2 as an outcome. Larger prospective studies should be done to evaluate the association between PB and adverse outcome in neonates.
Supplementary Material
Supplementary Figure 1: Summary of results: prolonged bradycardia versus no prolonged bradycardia at birth. The following forest plots are graphic representation of the unadjusted data. Sensitivity analysis of death before discharge: Excluding two studies with large number of infants without recorded HR by 2 minutes of life
Supplementary Figure 2: Summary of results: Prolonged bradycardia versus no prolonged bradycardia at birth. The following forest plots are graphic representation of the unadjusted data. A. Low FiO2 B. SpO2 < 80% at 5 minutes of life
Funding Source:
V Kapadia acknowledges support by K23HD083511 grant by NIH. M Vento acknowledges RETICS funded by the PN 2018-2011 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (FEDER), reference RD16/0022/0001.
Abbreviations:
- BPD
Bronchopulmonary Dysplasia
- DR
Delivery Room
- DR-CPR
Chest compressions and/or epinephrine use in the DR
- FiO2
Fraction of inspired oxygen
- GA
Gestational age
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HR
Heart rate
- ILCOR
International Liaison Committee on Resuscitation
- IVH
Intraventricular Hemorrhage
- NB
No bradycardia
- NEC
Necrotizing enterocolitis
- NICU
Neonatal intensive care unit
- O2
Oxygen
- PB
Prolonged bradycardia
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- RCT
Randomized controlled trial
- RoB
Risk of bias
- ROP
Retinopathy of prematurity
- SpO2
Oxygen saturation
- TB
Transient bradycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Y Rabi has patents for technology to guide oxygen titration during newborn resuscitation. He did not contribute to any aspects of the manuscript related to the targeting of oxygen saturations.
Contributor’s Statement:
Dr Kapadia prepared the protocol, screened studies, abstracted data, completed risk-of-bias and Grading of Recommendations Assessment, Development and Evaluation evaluations, completed the analysis, and prepared the first draft and the final draft of the manuscript.
Dr Oei reviewed the protocol, screened studies, abstracted data, completed risk-of-bias and Grading of Recommendations Assessment, Development and Evaluation evaluations, reviewed the analysis, and prepared the first draft of the manuscript.
Steve Brown reviewed the protocol and conducted the statistical analysis. He was involved in writing and editing the manuscript.
Drs Finer, Rich, Rabi, Wright, Rook, Vermeulen, Tarnow-Mordi, Smyth, Lui, Saugstad and Vento were involved in reviewing the protocol, reviewing the analysis, and writing and editing the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References:
- [1].Kattwinkel J e. Textbook of Neonatal Resuscitation 5th ed: American Academy of Pediatrics; 2006. [Google Scholar]
- [2].Saugstad OD. 60 - Physiology of Resuscitation A2 - Polin, Richard A. In: Abman SH, Rowitch DH, Benitz WE, Fox WW, editors. Fetal and Neonatal Physiology (Fifth Edition): Elsevier; 2017. p. 619–26.e1. [Google Scholar]
- [3].Aziz K, Chadwick M, Baker M, Andrews W. Ante- and intra-partum factors that predict increased need for neonatal resuscitation. Resuscitation. 2008;79:444–52. [DOI] [PubMed] [Google Scholar]
- [4].Kakkilaya V, Jubran I, Mashruwala V, Ramon E, Simcik VN, Marshall M, et al. Quality Improvement Project to Decrease Delivery Room Intubations in Preterm Infants. Pediatrics. 2019;143:e20180201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: Neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2015;132:S204–S41. [DOI] [PubMed] [Google Scholar]
- [6].Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S543–60. [DOI] [PubMed] [Google Scholar]
- [7].Wyckoff MH, Wyllie J, Aziz K, de Almeida MF, Fabres J, Fawke J, et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2020;142:S185–S221. [DOI] [PubMed] [Google Scholar]
- [8].Saugstad OD, Ramji S, Rootwelt T, Vento M. Response to resuscitation of the newborn: early prognostic variables. Acta Paediatr. 2005;94:890–5. [DOI] [PubMed] [Google Scholar]
- [9].Handley SC, Sun Y, Wyckoff MH, Lee HC. Outcomes of extremely preterm infants after delivery room cardiopulmonary resuscitation in a population-based cohort. J Perinatol. 2015;35:379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kumar VH, Skrobacz A, Ma C. Impact of bradycardia or asystole on neonatal cardiopulmonary resuscitation at birth. Pediatr Int. 2017;59:891–7. [DOI] [PubMed] [Google Scholar]
- [11].Shah PS. Extensive cardiopulmonary resuscitation for VLBW and ELBW infants: a systematic review and meta-analyses. J Perinatol. 2009;29:655–61. [DOI] [PubMed] [Google Scholar]
- [12].Shah PS, Shah P, Tai KF. Chest compression and/or epinephrine at birth for preterm infants <32 weeks gestational age: matched cohort study of neonatal outcomes. J Perinatol. 2009;29:693–7. [DOI] [PubMed] [Google Scholar]
- [13].Wyckoff MH, Salhab WA, Heyne RJ, Kendrick DE, Stoll BJ, Laptook AR, et al. Outcome of extremely low birth weight infants who received delivery room cardiopulmonary resuscitation. J Pediatr. 2012;160:239–44 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Haumont D, Modi N, Saugstad OD, Antetere R, NguyenBa C, Turner M, et al. Evaluating preterm care across Europe using the eNewborn European Network database. Pediatr Res. 2020;88:484–95. [DOI] [PubMed] [Google Scholar]
- [15].Oei JL, Saugstad OD, Lui K, Wright IM, Smyth JP, Craven P, et al. Targeted Oxygen in the Resuscitation of Preterm Infants, a Randomized Clinical Trial. Pediatrics. 2017;139. [DOI] [PubMed] [Google Scholar]
- [16].Oei JL, Finer NN, Saugstad OD, Wright IM, Rabi Y, Tarnow-Mordi W, et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2018;103:F446–F54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McNamara PJ, El-Khuffash A. 71 - Oxygen Transport and Delivery A2 - Polin, Richard A. In: Abman SH, Rowitch DH, Benitz WE, Fox WW, editors. Fetal and Neonatal Physiology (Fifth Edition): Elsevier; 2017. p. 724–37.e2. [Google Scholar]
- [18].Poets CF. Intermittent hypoxemia/bradycardia and the developing brain: how much is too much? Commentary on M.B. Schmid et al.: Cerebral oxygenation during intermittent hypoxemia and bradycardia in preterm infants (Neonatology 2015;107:137-146). Neonatology. 2015;107:147–9. [DOI] [PubMed] [Google Scholar]
- [19].Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, et al. Association Between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA. 2015;314:595–603. [DOI] [PubMed] [Google Scholar]
- [20].Walter LM, Ahmed B, Odoi A, Cooney H, Horne RSC, Wong FY. Bradycardias are associated with more severe effects on cerebral oxygenation in very preterm infants than in late preterm infants. Early Hum Dev. 2018;127:33–41. [DOI] [PubMed] [Google Scholar]
- [21].Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Cochrane 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Welsford M, Nishiyama C, Shortt C, Weiner G, Roehr CC, Isayama T, et al. Initial Oxygen Use for Preterm Newborn Resuscitation: A Systematic Review With Meta-analysis. Pediatrics. 2019;143:e20181828. [DOI] [PubMed] [Google Scholar]
- [24].Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American journal of respiratory and critical care medicine. 2001;163:1723–9. [DOI] [PubMed] [Google Scholar]
- [25].Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal Outcomes of Extremely Preterm Infants From the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Papile L-A, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. The Journal of Pediatrics. 1978;92:529–34. [DOI] [PubMed] [Google Scholar]
- [27].Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal Necrotizing Enterocolitis - Therapeutic Decisions Based Upon Clinical Staging. Ann Surg. 1978;187:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9. [DOI] [PubMed] [Google Scholar]
- [29].Escrig R, Arruza L, Izquierdo I, Villar G, Saenz P, Gimeno A, et al. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics. 2008;121:875–81. [DOI] [PubMed] [Google Scholar]
- [30].Ezaki S, Suzuki K, Kurishima C, Miura M, Weilin W, Hoshi R, et al. Resuscitation of Preterm Infants with Reduced Oxygen Results in Less Oxidative Stress than Resuscitation with 100% Oxygen. J Clin Biochem Nutr. 2009;44:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Harling AE, Beresford MW, Vince GS, Bates M, Yoxall CW. Does the use of 50% oxygen at birth in preterm infants reduce lung injury? Arch Dis Child Fetal Neonatal Ed. 2005;90:F401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Boronat N, Aguar M, Rook D, Iriondo M, Brugada M, Cernada M, et al. Survival and Neurodevelopmental Outcomes of Preterms Resuscitated With Different Oxygen Fractions. Pediatrics. 2016;138. [DOI] [PubMed] [Google Scholar]
- [33].Thamrin V, Saugstad OD, Tarnow-Mordi W, Wang YA, Lui K, Wright IM, et al. Preterm Infant Outcomes after Randomization to Initial Resuscitation with FiO2 0.21 or 1.0. The Journal of Pediatrics. 2018;201:55–61.e1. [DOI] [PubMed] [Google Scholar]
- [34].Tataranno M, Oei J, Perrone S, Wright I, Smyth J, Lui K, et al. Resuscitating preterm infants with 100% oxygen is associated with higher oxidative stress than room air. Acta Paediatr. 2015;104:759–65. [DOI] [PubMed] [Google Scholar]
- [35].Aguar MBM, Escobar J,, et al. Resuscitation of ELBW infants with initial FiO2 of 30% vs. 60%, a randomized, controlled, blinded study: the REOX trial. PAS Annual Meeting. Washington, DC: 2013. [Google Scholar]
- [36].Kapadia VS, Chalak LF, Sparks JE, Allen JR, Savani RC, Wyckoff MH. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics. 2013;132:e1488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rabi Y, Singhal N, Nettel-Aguirre A. Room-air versus oxygen administration for resuscitation of preterm infants: the ROAR study. Pediatrics. 2011;128:e374–81. [DOI] [PubMed] [Google Scholar]
- [38].Rook D, Schierbeek H, Vento M, Vlaardingerbroek H, van der Eijk AC, Longini M, et al. Resuscitation of Preterm Infants with Different Inspired Oxygen Fractions. J Pediatr. 2014. [DOI] [PubMed] [Google Scholar]
- [39].Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–49. [DOI] [PubMed] [Google Scholar]
- [40].Wang CL, Anderson C, Leone TA, Rich W, Govindaswami B, Finer NN. Resuscitation of preterm neonates by using room air or 100% oxygen. Pediatrics. 2008;121:1083–9. [DOI] [PubMed] [Google Scholar]
- [41].Bajaj M, Natarajan G, Shankaran S, Wyckoff M, Laptook AR, Bell EF, et al. Delivery Room Resuscitation and Short-Term Outcomes in Moderately Preterm Infants. The Journal of Pediatrics. 2018;195:33–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bashir A, Bird B, Wu L, Welles S, Taylor H, Anday E, et al. Neonatal outcomes based on mode and intensity of delivery room resuscitation. Journal Of Perinatology. 2017;37:1103. [DOI] [PubMed] [Google Scholar]
- [43].DeMauro SB, Roberts RS, Davis P, Alvaro R, Bairam A, Schmidt B. Impact of Delivery Room Resuscitation on Outcomes up to 18 Months in Very Low Birth Weight Infants. The Journal of Pediatrics. 2011;159:546–50.e1. [DOI] [PubMed] [Google Scholar]
- [44].Cnattingius S, Johansson S, Razaz N. Apgar Score and Risk of Neonatal Death among Preterm Infants. N Engl J Med. 2020;383:49–57. [DOI] [PubMed] [Google Scholar]
- [45].Lakshminrusimha S, Steinhorn RH, Wedgwood S, Savorgnan F, Nair J, Mathew B, et al. Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21 and 100% oxygen. J Appl Physiol (1985). 2011;111:1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Clarke MJSL. Obtaining individual patient data from randomised controlled trials. In: Egger MSG, Altman DG, editor. Systematic reviews in health care: meta-analysis in context. 2nd ed. London: BMJ Publishing Group; 2009. p. 109–21. [Google Scholar]
- [47].Drennan S, Szyld E. Should we target higher or lower oxygen saturation targets in the preterm infant? J Perinatol. 2019;39:758–60. [DOI] [PubMed] [Google Scholar]
- [48].Tarnow-Mordi W, Kirby A. Current Recommendations and Practice of Oxygen Therapy in Preterm Infants. Clin Perinatol. 2019;46:621–36. [DOI] [PubMed] [Google Scholar]
- [49].Katheria A, Rich W, Finer N. Electrocardiogram provides a continuous heart rate faster than oximetry during neonatal resuscitation. Pediatrics. 2012;130:e1177–81. [DOI] [PubMed] [Google Scholar]
- [50].Mizumoto H, Tomotaki S, Shibata H, Ueda K, Akashi R, Uchio H, et al. Electrocardiogram shows reliable heart rates much earlier than pulse oximetry during neonatal resuscitation. Pediatr Int. 2012;54:205–7. [DOI] [PubMed] [Google Scholar]
- [51].Voogdt KG, Morrison AC, Wood FE, van Elburg RM, Wyllie JP. A randomised, simulated study assessing auscultation of heart rate at birth. Resuscitation. 2010;81:1000–3. [DOI] [PubMed] [Google Scholar]
- [52].Dawson JA, Kamlin CO, Wong C, te Pas AB, Vento M, Cole TJ, et al. Changes in heart rate in the first minutes after birth. Arch Dis Child Fetal Neonatal Ed. 2010;95:F177–81. [DOI] [PubMed] [Google Scholar]
- [53].Kc A, Singhal N, Gautam J, Rana N, Andersson O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth - randomized clinical trial. Matern Health Neonatol Perinatol. 2019;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mukherjee S, Bulsara JS, Das MK, Waratakar Y, Saha AK, Dubey S, et al. Is Delaying Cord Clamping until Placenta Delivery Beneficial? Oxygen Saturation and Heart Rate Transition during the Initial 5 Minutes after Delivery in Indian Healthy Newborns. Am J Perinatol. 2019. [DOI] [PubMed] [Google Scholar]
- [55].Padilla-Sanchez C, Baixauli-Alacreu S, Canada-Martinez AJ, Solaz-Garcia A, Alemany-Anchel MJ, Vento M. Delayed vs Immediate Cord Clamping Changes Oxygen Saturation and Heart Rate Patterns in the First Minutes after Birth. J Pediatr. 2020;227:149–56 e1. [DOI] [PubMed] [Google Scholar]
- [56].Li G, Zeng J, Tian J, Levine MAH, Thabane L. Multiple uses of forest plots in presenting analysis results in health research: A Tutorial. J Clin Epidemiol. 2020;117:89–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Summary of results: prolonged bradycardia versus no prolonged bradycardia at birth. The following forest plots are graphic representation of the unadjusted data. Sensitivity analysis of death before discharge: Excluding two studies with large number of infants without recorded HR by 2 minutes of life
Supplementary Figure 2: Summary of results: Prolonged bradycardia versus no prolonged bradycardia at birth. The following forest plots are graphic representation of the unadjusted data. A. Low FiO2 B. SpO2 < 80% at 5 minutes of life


