Abstract
The Bcl-2 family of proteins are involved in regulating the redox state of cells. However, the mode of action of Bcl-2 proteins remains unclear. This work analyzed the effects of Bcl-xL on the cellular redox state after treatment with tumor necrosis factor alpha (TNF-α) or exogenous oxidants. We show that in cells that undergo TNF-α-induced apoptosis, TNF-α induces a partial decrease in mitochondrial membrane potential (ΔΨm) followed by high levels of reactive oxygen species (ROS). ROS scavengers delay the progression of mitochondrial depolarization and apoptotic cell death. This indicates that ROS are important mediators of mitochondrial depolarization. However, ROS scavengers fail to prevent the initial TNF-α-induced decrease in ΔΨm. In contrast, expression of Bcl-xL prevents both the initial decrease in ΔΨm following TNF-α treatment and the subsequent induction of ROS. Bcl-xL itself does not act as a ROS scavenger. In addition, Bcl-xL does not block the initial decrease in ΔΨm following treatment with the oxidant hydrogen peroxide. However, unlike control-transfected cells, Bcl-xL-expressing cells can recover their mitochondrial membrane potential following the initial drop in ΔΨm induced by hydrogen peroxide. These data suggest that Bcl-xL plays a regulatory role in controlling the membrane potential of and ROS production by mitochondria rather than acting as a direct antioxidant.
The redox state of a cell is an important factor in determining its susceptibility to different apoptotic stimuli (for a recent review, see reference 18). Both reactive oxygen species (ROS), which are prooxidants, and thiol compounds, which act as antioxidants, play a critical role in regulating apoptosis following the application of apoptotic stimuli such as tumor necrosis factor alpha (TNF-α) (24), Fas signaling (1), glucocorticoids (31), thiol depletion (12, 16, 22), radiation (19), chemotherapeutic agents (4), ceramide (20), and lymphocyte activation (8). However, the mechanism by which the cellular redox state is regulated during apoptosis and its role in modulating cell death remain unclear.
All aerobic cells generate ROS in the form of superoxide anions, hydrogen peroxides, organic peroxides, and hydroxyl radicals (3). Since ROS are by-products of oxidative phosphorylation, most cellular ROS are produced by mitochondria. Superoxide radicals are produced when oxygen is reduced by a single electron; this occurs mostly at the ubiquinone site of complex III of the mitochondrial electron transport chain. However, electron transfer in other cellular organelles, such as the endoplasmic reticulum and nuclear and plasma membranes, can also produce superoxides. Moreover, several biochemical reactions other than electron transport chains can produce ROS. For example, the catabolism of purine nucleotides (xanthine oxidase reaction), the metabolism of fatty acids, in particular the production of prostaglandins and leukotrienes from arachidonic acid (lipoxygenase reaction), and many biochemical reactions that take place in peroxisomes can produce ROS (3). ROS produced at sites other than mitochondria have been reported to be involved in some apoptotic systems, but it is widely accepted that mitochondria are the predominant source of ROS produced in most apoptotic systems (14, 18).
Mitochondrial homeostasis is critical in regulating apoptosis (6, 14). In particular, it has been noted that some or all of the following mitochondrial changes are associated with apoptosis: mitochondrial membrane hyperpolarization and depolarization, matrix swelling, and permeability of the outer membrane, resulting in the release of proapoptotic proteins such as cytochrome c, apoptosis-inducing factor, and potentially other proteins. In many instances, such mitochondrial events are a prerequisite for the activation of a family of aspartic acid-specific, cysteine-containing proteases (caspases) that are known to be important mediators of apoptosis (21, 25). Other mitochondrion-related cellular alterations that are important in modulating the apoptotic process can also occur. These include a decreased ATP/ADP ratio, thiol depletion, and ROS induction (6, 14).
The Bcl-2 family of proteins are ubiquitous regulators of cell death (7, 28). The mechanism of action of several members of this family appears to involve mitochondria. Bcl-2 and Bcl-xL exert at least some of their antiapoptotic effect by regulating mitochondrial homeostasis, in particular by maintaining mitochondrial-cytosolic coupling of oxidative phosphorylation (7, 28). In addition, Bcl-2 proteins can modulate the release of apoptogenic factors, such as cytochrome c and apoptosis-inducing factor, from mitochondria. There are also some indications that Bcl-2 and Bcl-xL may function as antioxidants and in this way exert anti-apoptotic activity. Bcl-2 knockout (Bcl-2−/−) mice are hypopigmented due to a defect in melanin synthesis, which itself is redox regulated (29). Moreover, enhanced oxidative stress and a higher susceptibility to prooxidants are evident in the brains of Bcl-2−/− mice compared to control mice (9). Bcl-2 protects cells from hydrogen peroxide- or thiol depletion-induced death and suppresses lipid peroxidation (10, 12, 19, 26). There are two mechanisms that can explain the antioxidant activity of Bcl-2 proteins. In the first mechanism, Bcl-2 maintains cells in a more reduced state by scavenging ROS either directly or by up-regulating other ROS scavengers such as thiol compounds. Alternatively, or in addition, Bcl-2 may function to prevent the generation of ROS during apoptosis. It remains unclear how Bcl-2-like proteins antagonize oxidation. However, since Bcl-2 was shown to protect cells from death induced by exogenous oxidant compounds such as hydrogen peroxide or lipid peroxide (10, 26), it has been considered likely that Bcl-2 proteins act downstream of ROS formation.
Here we further elucidate the role of Bcl-2 proteins in determining the redox state of cells. In response to TNF-α treatment, cells produced elevated levels of ROS. However, this production of ROS occurred only after TNF-α induced a reduction in the mitochondrial membrane potential (ΔΨm). ROS scavengers did not interfere with the initial decrease in ΔΨm but significantly attenuated the subsequent mitochondrial collapse of ΔΨm and partially rescued the cells from apoptosis. These results indicate that ROS contribute to mitochondrial membrane depolarization. Expression of Bcl-xL blocked both the initial decrease in ΔΨm and the subsequent increase in ROS levels following TNF-α treatment. In contrast, Bcl-xL expression did not block the initial reduction in ΔΨm that follows hydrogen peroxide treatment. Still, Bcl-xL expression allowed cells to recover from the hydrogen peroxide-induced drop in ΔΨm and prevented progression to complete mitochondrial membrane depolarization. In this system, Bcl-xL did not have ROS scavenger activity. These data lead to the conclusion that Bcl-xL acts to promote mitochondrial recovery from oxidant-induced damage. Bcl-xL partially inhibits the decrease in mitochondrial respiration that begins shortly after TNF-α treatment. This activity of Bcl-xL can explain both the maintenance of mitochondrial ΔΨm and the prevention of ROS production in mitochondria. Therefore, it is proposed that following TNF-α-induced signal transduction, Bcl-xL plays a direct regulatory role in maintaining mitochondrial physiology. In addition, these data support the hypothesis that Bcl-xL is active in regulating mitochondrial physiology even in cells that ultimately succumb to TNF-α-induced death.
MATERIALS AND METHODS
Materials.
S-18 anti-Bcl-x monoclonal antibody was purchased from Santa Cruz Biotechnology. Propidium iodide (PI), tetramethylrhodamine ethyl ester (TMRE), and 2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were purchased from Molecular Probes. 1-Pyrrolidine dithiocarbamate (PDTC) was purchased from Calbiochem. N-Acetyl-l-cysteine (NAC), carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP), and cycloheximide (CHX) were purchased from Sigma. Recombinant murine TNF-α was purchased from Boehringer Mannheim Biochemicals, and the modified peptide benzyloxycarbonyl (z)-Val-Ala-Asp-fluoromethyl ketone (zVAD-fmk) was purchased from Enzyme Systems Products.
Cell culture and transfections.
The 2B4.11 (2B4) T-cell hybridoma line was maintained routinely at 37°C and 5% CO2 in RPMI medium supplemented with 10% bovine calf serum and 50 μM 2-mercaptoethanol. Cells were transfected by electroporation (250 V and 960 μF) with the pSFFV-neo plasmid (control cells) or with the plasmid containing an insert encoding the human Bcl-xL cDNA. Transfected cells were selected with G418 (1 mg/ml) to generate control 2B4/neo cells and several Bcl-xL-expressing clones. Clones were generated by limiting dilution. Bcl-xL protein levels in the different clones were analyzed by Western blot analysis using the S-18 monoclonal antibody.
Cell analysis.
Cells were split 1:10 a day before treatment. At 2 h before treatments, the cells were counted and replated at 105 cells/ml. To induce cell death, 200 U of TNF-α and 5 μg of CHX per ml were added. CHX was used to inhibit antiapoptotic pathways induced by TNF-α. When anti-oxidants (25 μM PDTC or 50 mM NAC) were used, they were added 1 h prior to the apoptotic stimuli.
Viability was determined by PI exclusion. Cells were incubated with PI (5 μg/ml) and analyzed immediately by fluorescence-activated cell sorter (FACS) analysis. For ΔΨm analysis, cells were incubated with 0.2 μM TMRE for 30 min at 37°C and then subjected to FACS analysis. TMRE fluorescence was detected in live cells as determined by forward-scatter and side-scatter criteria. The mitochondrial uncoupler FCCP (10 μM), added 3 h before TMRE staining, was used to depolarize mitochondria and serves as an indicator that TMRE staining is in proportion to the mitochondrial membrane potential.
ROS levels were detected by incubating the cells with 10 μM DCFH-DA for 30 min at 37°C. ROS analysis was performed together with either PI (to determine viability) or TMRE (to analyze ΔΨm). At each time point, cells were also stained with either PI or TMRE alone, and the mean FL1 fluorescence of these cells (FL1 background) was subtracted from that of cells that were double stained with DCFH-DA and either PI or TMRE, giving a corrected mean FL1 value. Untreated cells were used as a reference for ROS levels at each time point, and the corrected mean FL1 value of the untreated cells was defined as ROS = 1.
To detect the DCFH oxidation rate following hydrogen peroxide treatment, cells were incubated with 10 μM DCFH-DA for 30 min at 37°C and analyzed by FACS analysis for DCFH oxidation for 2 min (basal levels). This was followed by addition of hydrogen peroxide (50 nmol/105 cells) and continuous reading of DCFH oxidation for 30 min more.
Oxygen consumption assay.
Cells (5 × 106 in 30 ml) were either left untreated or treated with TNF-α as described above. At 2 h later, cells were centrifuged and resuspended in 3 ml of medium. The oxygen consumption rate was measured in a respirometer using a polarographic oxygen electrode (Cameron Instrument Co.).
RESULTS
Bcl-xL inhibits TNF-α-induced apoptosis in 2B4 T cells.
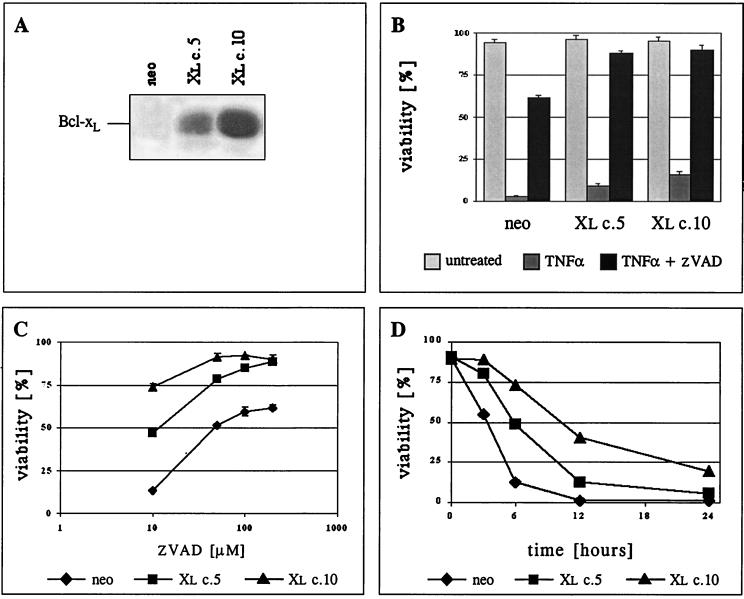
We examined the role that the cellular redox state plays during apoptosis, particularly the roles of ROS in initiating apoptotic events and of Bcl-xL in controlling such events. TNF-α increases cellular ROS levels during apoptosis (24), and lymphocytes are sensitive to ROS-mediated cell death (31). The TNF-α-sensitive murine T-cell hybridoma line 2B4 was used as a model to study redox state regulation during apoptosis. Several clones of 2B4 cells overexpressing Bcl-xL were established. The Bcl-xL protein levels of two representative Bcl-xL-expressing clones (2B4/Xl c.5 and 2B4/Xl c.10), as well as of the control cells transfected with the G418 resistance gene only (2B4/neo), are presented in Fig. 1A.
FIG. 1.
Bcl-xL can protect 2B4 cells from apoptosis induced by TNF-α. (A) Bcl-xL protein levels in 2B4-transfected cells were detected by Western blot analysis. The results for one control transfected clone (neo) and two Bcl-xL-transfected clones (Xl c.5 and Xl c.10) are presented. (B) Viability assay (PI exclusion) of the clones at 36 h following treatment. Cells were either left untreated or treated with TNF-α in the presence or absence of the caspase inhibitor zVAD-fmk (200 μM). (C) Survival assay as described in panel B for cells treated with TNF-α in the presence of increasing concentrations of zVAD-fmk. (D) Kinetics of cell death. Cells were treated with TNF-α, and viability was determined by PI exclusion at the indicated time points.
There is evidence to suggest that there are two types of response to death mediated by TNF receptor (TNFR) family members (23). Type I cells show high levels of caspase-8 activity within the “death-inducing signaling complex” (DISC) following Fas activation, and type II cells show lower levels of caspase-8 activity under the same conditions. Only type II cells are protected by the expression of Bcl-2. 2B4 cells are defined as type I, particularly since expression of Bcl-xL fails to block Fas-induced death (17). Indeed, when 2B4 cells were treated with TNF-α in the presence of the protein synthesis inhibitor CHX to prevent the induction of the antiapoptotic response induced by TNF-α-mediated NF-κB signaling, both control cells and Bcl-xL-overexpressing cells were killed at 36 h (Fig. 1B). This was a caspase-mediated cell death, since zVAD-fmk, a general caspase inhibitor, was able to protect cells from death (Fig. 1B). Although Bcl-xL expression failed to prevent TNF-α-induced death, Bcl-xL lowered the amount of zVAD-fmk necessary to protect the cells from death (Fig. 1C). The cooperative protection by Bcl-xL and zVAD-fmk is related to the Bcl-xL protein levels (Fig. 1A and C). This indicates that once caspase activity is limited, Bcl-xL can play a role in protecting even type I cells from TNF-α-mediated death. Consistent with this conclusion, examination of earlier time points following TNF-α treatment shows that Bcl-xL has a major effect on the kinetics of cell death even in the absence of caspase inhibitors (Fig. 1D), although the cells were not ultimately protected from death.
An increase in cellular ROS levels is associated with TNF-α-mediated apoptosis and is blocked by Bcl-xL expression.
We analyzed the involvement of ROS in apoptosis induced by TNF-α. Intracellular hydrogen peroxide levels were measured using the fluorescent probe DCFH-DA. This is a cell-permeant dye that, once inside the cells, is cleaved by endogenous esterase into its nonfluorescent form, DCFH. This form is no longer membrane permeant and is therefore trapped in the cells, where it is oxidized, mostly by hydrogen peroxide in the presence of peroxidase, into its fluorescent form, DCF. At different time points following treatment, the cells were stained with DCFH-DA for 30 min followed by PI and then subjected to FACS analysis. Only the population of living cells, as indicated by their ability to exclude PI, was analyzed, and the ratio of the mean fluorescence of TNF-α-treated to untreated live cells is presented (Fig. 2A).
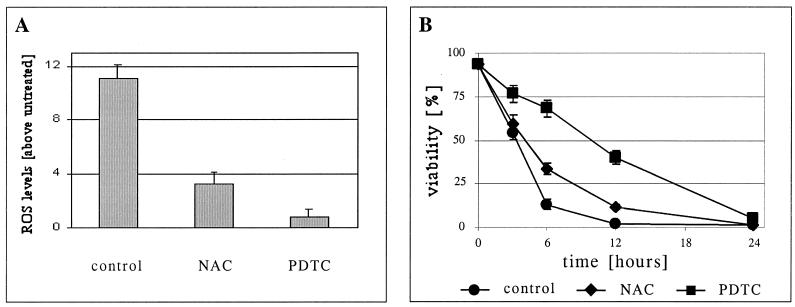
FIG. 2.
ROS scavengers can delay apoptosis of TNF-α-treated cells. (A) Parental 2B4 cells were incubated with TNF-α for 6 h without (control) or with ROS scavengers (NAC or PDTC). Untreated cells were incubated as a reference for ROS levels. DCFH-DA was then added to the cells, and the cells were further incubated for 30 min. PI was added just prior to FACS analysis. DCFH oxidation was analyzed only in live cells (PI negative). ROS levels are defined as the ratio between the mean fluorescence of treated and untreated cells (see Materials and Methods). (B) Kinetics of cell death. Cells were treated as in panel A, and viability was measured by PI exclusion at the indicated time points.
A more than 10-fold increase in ROS levels was observed in parental 2B4 cells at 6 h following TNF-α treatment (Fig. 2A). Although only live cells were analyzed in this assay, it could be argued that ROS are early by-products of cell death. Therefore, to establish that ROS contribute directly to this apoptotic process, 2B4 cells were treated with TNF-α in the presence or absence of antioxidants. NAC and PDTC are potent ROS scavengers. These two compounds reduced the ROS levels following TNF-α treatment (Fig. 2A) and attenuated the death induced by TNF-α (Fig. 2B). PDTC was a more effective antioxidant in this system and also a better protector from apoptosis (Fig. 2). These results suggest that ROS play a role in the regulation of 2B4 cell death following TNF-α treatment. Like the incomplete inhibition of cell death achieved by Bcl-xL expression (Fig. 1D), PDTC treatment did not completely block apoptosis but, rather, significantly delayed the process. These observations indicate that although ROS production is important for TNF-α-induced apoptosis in these cells, it is not essential, most probably due to a direct apoptotic signal that is mediated by caspases activated upon recruitment to TNFR (type I cells [see above]).
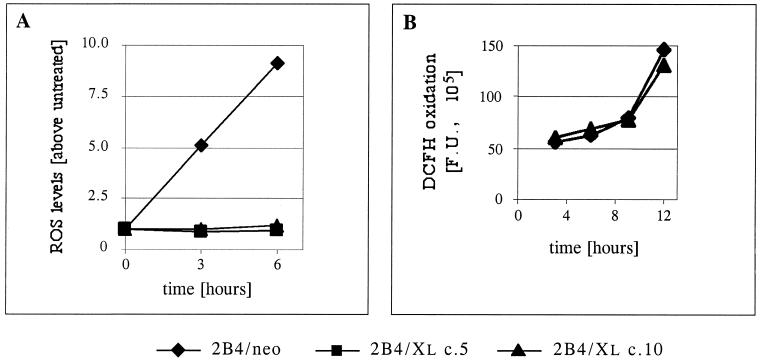
To check whether the ability of Bcl-xL to partially protect 2B4 cells from TNF-α-mediated apoptosis is correlated with a reduction in the level of TNF-α-induced ROS, we analyzed the ROS levels in control and Bcl-xL-expressing cells following TNF-α treatment. A significant and time-dependent increase in ROS levels was observed in control 2B4/neo cells at early time points following TNF-α treatment. This increase was completely blocked when Bcl-xL protein was expressed in these cells (Fig. 3A). These observations suggest that the partial protective effect of Bcl-xL may be due to its regulation of the cellular redox state.
FIG. 3.
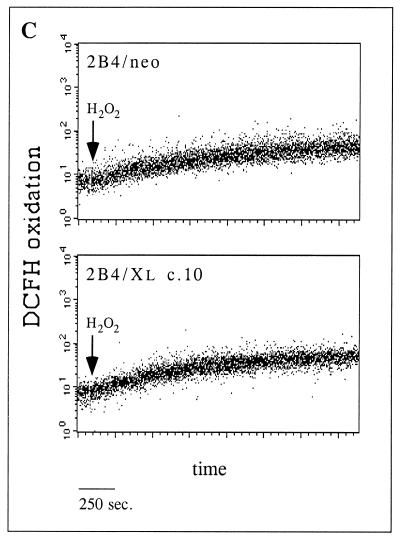
Bcl-xL blocks ROS induction in TNF-α-treated cells but has no ROS scavenging activity. (A) Control cells (2B4/neo) and Bcl-xL-expressing cells (2B4/Xl c.5 and 2B4/Xl c.10) were either left untreated or treated with TNF-α. At the indicated time points, the cells were stained with DCFH-DA and PI, and ROS levels were analyzed as in the experiment in Fig. 2A. (B) DCFH oxidation. Untreated cells were incubated in the presence of DCFH-DA (added at time zero), and DCFH oxidation was analyzed by FACS analysis at the indicated time points. Mean fluorescence units (F.U.) represent the level of DCFH oxidation at each time point. (C) Cells were incubated with DCFH-DA for 30 min, and DCFH oxidation was measured by FACS analysis in a continuous assay. After 2 min, H2O2 (50 nmol/105 cells) was added (arrow), and DCFH oxidation was measured for a further 30 min.
Bcl-xL is not a direct antioxidant but plays a regulatory role in preventing ROS production.
Two ways to explain the block of ROS induction by Bcl-xL are available. Bcl-xL can work downstream of the point of ROS production by promoting ROS scavenger activity either directly or by up-regulating other ROS scavengers. Alternatively, Bcl-xL can block the increase in ROS levels by altering upstream events that lead to ROS production. To determine whether Bcl-xL can interfere with the oxidation rate of DCFH simply by acting as a ROS scavenger, untreated 2B4/neo and Bcl-xL-overexpressing cells (2B4/Xl c.10) were incubated in the presence of DCFH-DA and the fluorescence level was analyzed over time (Fig. 3B). It is notable that Bcl-xL does not act as a direct inhibitor of DCFH oxidation since the rate of DCFH oxidation during the incubation of untreated cells was indistinguishable between 2B4/neo and 2B4/Xl c.10 cells. Moreover, these results also indicate that the basal redox state (DCFH fluorescence) of these cells is comparable and unaffected by the expressed Bcl-xL protein. To rule out the possibility that Bcl-xL may adapt an antioxidant activity following the generation of ROS, the response of 2B4/neo and 2B4/Xl c.10 cells to oxidative stress was analyzed. The kinetics of DCFH oxidation immediately after the addition of exogenous hydrogen peroxide (50 nmol/105 cells) was detected by FACS analysis over time. Both cell lines oxidized DCFH at the same rate over the first 30 min following the addition of hydrogen peroxide (Fig. 3C). These observations support the idea that Bcl-xL works upstream of the point of ROS production during apoptosis and are inconsistent with the hypothesis that Bcl-xL itself acts as a ROS scavenger or induces ROS scavenging activity.
An increase in ROS precedes mitochondrial depolarization during apoptosis.
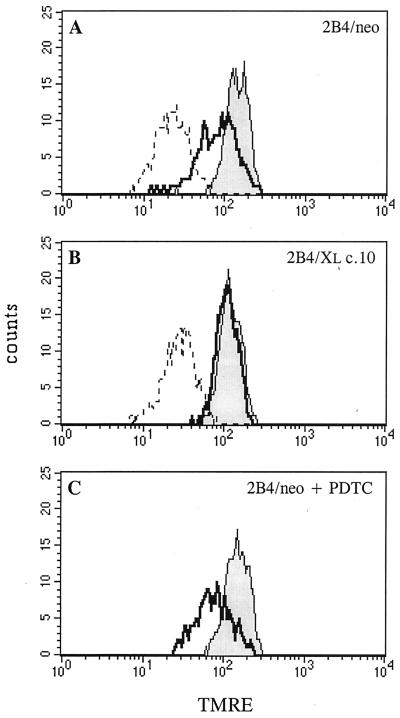
Bcl-2 and Bcl-xL block mitochondrial depolarization in many apoptotic systems. We sought to determine the relationship between Bcl-xL, mitochondrial depolarization and ROS levels. To quantify ΔΨm, TMRE, a potentiometric fluorescent dye that incorporates into mitochondria in a ΔΨm-dependent manner, was used. Cells were either left untreated or treated with TNF-α for 3 h in the presence or absence of the ROS scavenger PDTC. The cells were then stained with TMRE for 30 min and analyzed by FACS analysis. As can be seen in Fig. 4A, a decrease in ΔΨm was observed in 2B4/neo cells following TNF-α treatment. FCCP, a protonophore that induces a collapse of ΔΨm, was used as a control for TMRE staining (Fig. 4A and B). The decrease in ΔΨm observed 3 h after TNF-α treatment was only a partial mitochondrial depolarization compared to the complete mitochondrial depolarization observed in FCCP-treated cells. The expression of Bcl-xL completely blocked this decrease in ΔΨm at this time point (Fig. 4B). In contrast, the ROS scavenger, PDTC, which strongly protects these cells from apoptosis induced by TNF-α (Fig. 2B), could not protect them from the initial decrease in ΔΨm following the same treatment (Fig. 4C).
FIG. 4.
Unlike Bcl-xL, ROS scavengers cannot protect cells from the initial decrease in ΔΨm following TNF-α treatment. TMRE staining followed by FACS analysis was used to monitor the ΔΨm in untreated cells (shaded area), in cells treated with TNF-α for 3 h (thick line) or in cells treated with FCCP for 3 h (dashed line). Only live cells (as judged by forward-scatter and side-scatter criteria) were analyzed. (A) Control cells (2B4/neo). (B) Bcl-xL-expressing cells (2B4/Xl c.10). (C) 2B4/neo cells treated as above in the presence of the ROS scavenger PDTC.
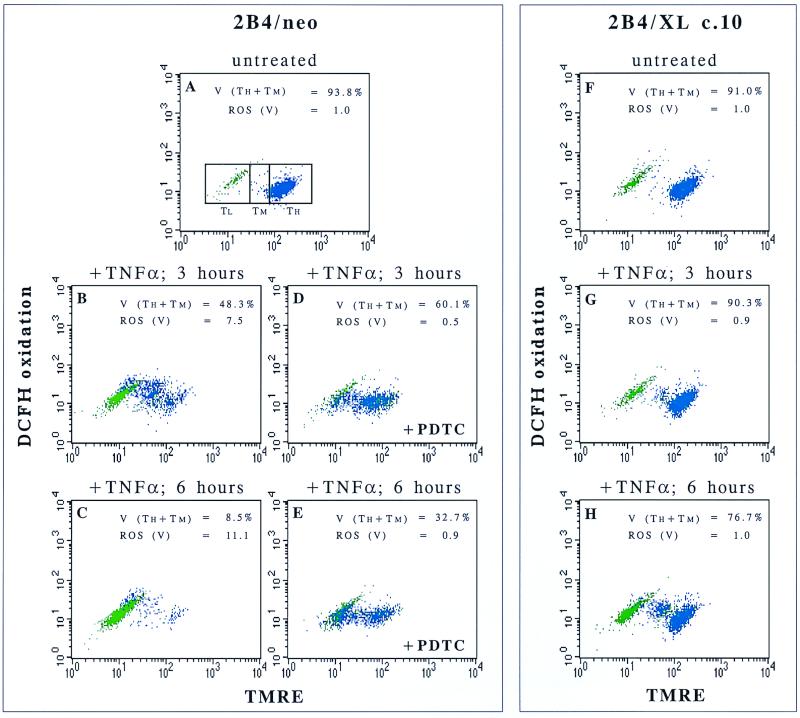
Since Bcl-xL can block both the decrease of ΔΨm (Fig. 4B) and the increase in ROS levels following TNF-α treatment (Fig. 3A), it is possible that these two events are related. To address this issue, cells were assayed simultaneously for both ROS levels and ΔΨm. When cells were doubly labeled with TMRE and DCFH-DA, the ROS levels in cells before and during mitochondrial depolarization could be differentiated and measured. The processes of mitochondrial depolarization and ROS induction following TNF-α treatment appeared to occur in several stages (Fig. 5). First, cells were shifted from a high-ΔΨm state (TH) into a decreased-ΔΨm state (TM). At this point, an increase in ROS production was observed (Fig. 5B). This state (TM) was followed by a further depolarization of the mitochondria into a low-ΔΨm state (TL). Then the cells lost viability as judged by size (forward-scatter) and granularity (side-scatter) criteria (green cells). It is noteworthy that in these cells, the extent of death measured by forward-scatter and side-scatter criteria was comparable to that assayed by PI exclusion (data not shown). This course of events in 2B4 cells suggested that ROS may play a role in the second depolarization (from TM to TL) during the apoptotic cascade following TNF-α treatment. This hypothesis is supported by the observation that the ROS scavenger PDTC significantly attenuated the escalation in mitochondrial depolarization but could not prevent the first stage of decrease in ΔΨm (Fig. 5A to E). Based on these results, it seems that ROS are not absolutely required for a complete mitochondrial depolarization but, rather, significantly accelerate this process. As early as 6 h after TNF-α treatment, only 8.5% of the control cells still maintained some ΔΨm (Fig. 5C), while on addition of PDTC, 32.7% of the cells still maintained a ΔΨm (Fig. 5E). However, a substantial number of cells were completely depolarized at this time point even in the presence of PDTC, and all the cells eventually died despite the ability of PDTC to keep ROS levels low (Fig. 2). On the other hand, unlike the results with PDTC, Bcl-xL inhibited the initial decrease in ΔΨm that preceded ROS induction and it completely blocked the increase in ROS levels (Fig. 5G and H). Therefore, it is likely that Bcl-xL modulates ROS production during TNF-α-induced apoptosis by regulating the initial drop in ΔΨm. Based on these results, it appears that ROS production increases coincidently with the initial drop in ΔΨm. Increased ROS levels appear to further facilitate the progression of mitochondrial depolarization of apoptotic cells.
FIG. 5.
Following TNF-α treatment, ROS are induced after a partial decrease in ΔΨm and are important mediators of mitochondrial membrane depolarization. Control cells (2B4/neo) and Bcl-xL-expressing cells (2B4/Xl c.10) were either left untreated (A and F) or treated with TNF-α (B to E, G, and H). PDTC, a ROS scavenger, was added to 2B4/neo cells (D and E). At the indicated time points, cells were doubly stained with TMRE and DCFH-DA to analyze the increase in ROS levels in parallel with loss of ΔΨm. Cells were divided by forward-scatter and side-scatter criteria into live cells (blue) and dead cells (green). Other subgroups were defined by ΔΨm levels: TH, TMRE high; TM, TMRE medium; TL, TMRE low. Viability (V) was defined in this assay as the percentage of cells in the TH + TM subgroups. ROS levels (fold above untreated cells [see Materials and Methods]) were analyzed for viable cells only [ROS (V)].
Bcl-xL partially relieves cells from a TNF-α-induced decrease in respiration.
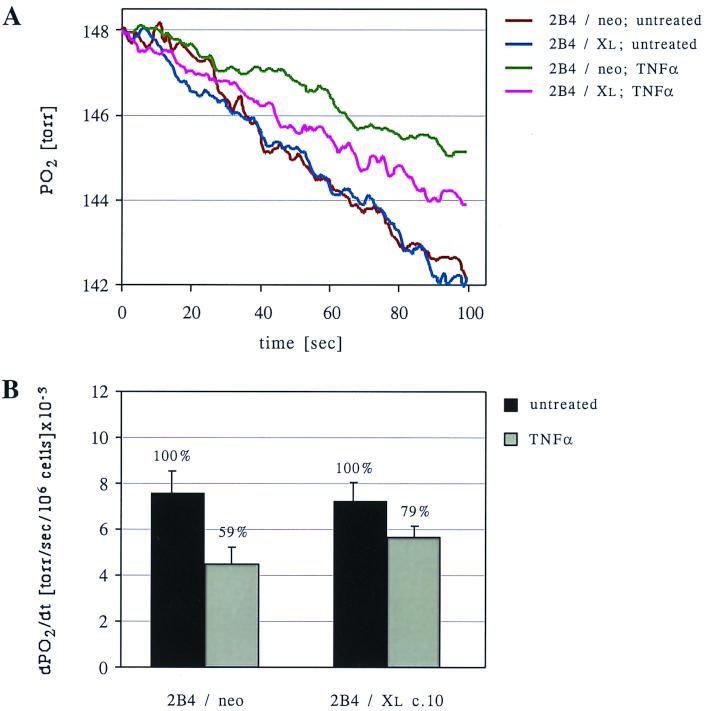
One way to produce high levels of ROS from mitochondria is to use electron transport inhibitors that block respiration downstream of complex III, leaving complex III in its reduced state. Under these conditions, the rate of electron transfer from the ubiquinone site of complex III directly to oxygen molecules will increase, resulting in high levels of partially reduced oxygen in the form of superoxide radicals. Furthermore, a blockage of respiration could explain the early reduction in ΔΨm following TNF-α treatment. To test whether treatment with TNF-α leads to a decrease in electron transport, we analyzed the respiration rates of TNF-α-treated cells compared to untreated cells. A significant decrease in oxygen consumption was observed in 2B4/neo cells as early as 2 h after TNF-α treatment (59% ± 12.5% of that in untreated cells) (Fig. 6). This observation supports the hypothesis that a respiration block develops following TNF-α treatment. This block was significantly alleviated by the expression of Bcl-xL (Fig. 6). The ability of Bcl-xL to maintain a higher rate of cellular respiration following TNF-α treatment suggests that by promoting coupled oxidative phosphorylation, Bcl-xL functions to sustain ΔΨm and to prevent ROS production.
FIG. 6.
Inhibition of respiration induced by TNF-α is reversed by Bcl-xL expression. (A) Control cells (2B4/neo) and Bcl-xL-expressing cells (2B4/Xl c.10) were either left untreated or treated with TNF-α for 2 h. Oxygen consumption was analyzed using an oxymeter (see Materials and Methods), and the decrease in oxygen pressure in the chamber over time is presented for each sample. (B) For each treatment, the average slopes of the graphs obtained in panel A, calculated from three independent experiments, are presented. This value describes the average rate of oxygen consumption by the cells (presented as the decrease in oxygen pressure per second per 106 cells).
Bcl-xL protects cells from exogenous oxidant-induced apoptosis by blocking the escalation in the mitochondrial depolarization process.
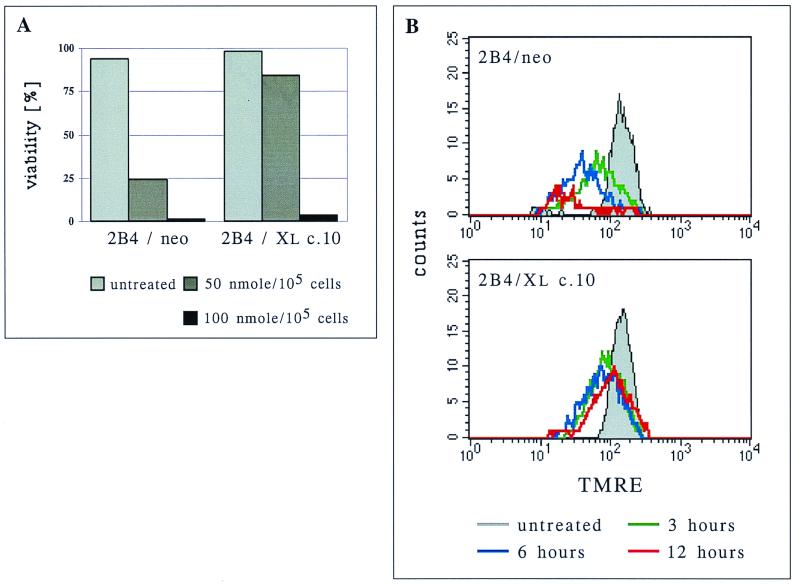
The above results indicate that Bcl-xL works upstream of ROS production and cannot reduce oxidation stress once ROS are produced or introduced into the cells. It is challenging to reconcile these observations with reports that Bcl-xL protects cells from death induced by exogenous hydrogen peroxide (10). In 2B4 cells, Bcl-xL protected cells from death induced by 50 nmol of exogenously added hydrogen peroxide per 105 cells (Fig. 7A) but provided no protection if twice the amount of hydrogen peroxide was used. The same restriction in the ability to protect cells from hydrogen peroxide-induced death was previously described for Bcl-2 and was attributed to its limited ROS scavenger capacity (10). However, at 50 nmol/105 cells, hydrogen peroxide induced an initial mitochondrial depolarization 3 h after treatment that was comparable in control transfected cells and Bcl-xL-expressing cells (Fig. 7B; green histograms), yet Bcl-xL expression prevented further depolarization in the subsequent 3 h whereas control cells continued to decrease their TMRE uptake. At 12 h posttreatment, ΔΨm began to recover in the Bcl-xL-expressing cells (Fig. 7B). When 100 nmol of hydrogen peroxide per 105 cells was used, Bcl-xL did not protect cells from further depolarization (data not shown), and rapid cell death occurred (Fig. 7A).
FIG. 7.
Bcl-xL protects 2B4 cells from hydrogen peroxide by preventing a complete drop in ΔΨm and allowing its recovery. (A) Control cells (2B4/neo) and Bcl-xL-overexpressing cells (2B4/Xl c.10) were treated with the indicated amounts of hydrogen peroxides, and viability was determined by PI exclusion 12 h later. (B) Cells were treated with hydrogen peroxide (50 nmol/105 cells) and at the indicated time points, stained with TMRE, and subjected to FACS analysis to determine their ΔΨm. Only live cells (as judged by forward-scatter and side-scatter criteria) were analyzed.
DISCUSSION
TNF-α alters ROS levels in many cellular systems (24, 30). ROS are important mediators of cell death in lymphocytes following different apoptotic stimuli, such as Fas signaling (1), glucocorticoid treatment (10, 31), and activation-induced cell death (8). We used the TNF-α-treated mouse T-cell hybridoma cell line 2B4 as a cellular model to study the role of Bcl-xL in regulating the redox state of cells in response to an apoptotic signal.
Bcl-xL-regulated pathways can contribute to the death induced by TNF-α in type I cells.
Both Bcl-2 and antioxidants protect some cell types from apoptosis initiated through death receptor signal transduction. Here we show that Bcl-xL protects even type I cells from mitochondrial changes and death induced by TNFR signaling. 2B4 cells are type I cells in their response to Fas signaling (17). This definition implies that the mitochondrial proapoptotic pathway is dispensable in these cells and that Bcl-xL does not protect them from Fas-induced apoptosis (23). Here we report that although Bcl-xL could not rescue 2B4 cells from apoptosis induced by TNF-α, both Bcl-xL-dependent and Bcl-xL-independent mechanisms are required for efficient induction of cell death. We demonstrate that Bcl-xL blocks TNF-α-induced mitochondrial changes (Fig. 4 and 5) and significantly attenuates the rate of death (Fig. 1D). Moreover, a potent ability of Bcl-xL to protect from TNF-α-induced apoptosis is revealed once caspase activity is restricted (Fig. 1C). This is in line with other results obtained with another lymphoid cell line, the pro-B cells FL5.12 (11). It therefore seems that the antiapoptotic Bcl-2-like proteins may function to protect mitochondria even in type I cells following TNF-α treatment. This raises the question whether, under physiological conditions, where death-inducing ligands are limiting, synergism between mitochondrion-dependent and mitochondrion-independent apoptotic machineries is critical to determine whether a cell will live or die. In such a scenario, Bcl-2 or Bcl-xL expression would confer a survival advantage in response to limited TNFR signal transduction.
Bcl-xL reduces ROS levels during apoptosis by blocking ROS production.
In TNF-α-treated cells, ROS are produced after a partial drop in ΔΨm and are important mediators of the complete mitochondrial depolarization (Fig. 5). Bcl-xL was shown in our system to prevent the induction of ROS levels following TNF-α treatment (Fig. 3A). When the basal levels of ROS in untreated cells were analyzed by DCFH oxidation, there was no detectable difference between the levels in control cells and in Bcl-xL-expressing cells (Fig. 3B). These results support the idea that Bcl-xL expression does not maintain more reduced conditions in these cells during normal proliferation. In addition, the observation that the expression of Bcl-xL did not reduce the initial rate of DCFH oxidation in hydrogen peroxide-treated cells (Fig. 3C) further supports the hypothesis that Bcl-xL regulates ROS levels at or upstream of the point of ROS production.
Since ROS were induced after the first decrease in ΔΨm (Fig. 5) and Bcl-xL blocked that decrease, it is possible that the decrease in ΔΨm is the cause of ROS production. A decrease in ΔΨm can occur by one of two mechanisms. In the first, making the mitochondrial inner membrane more permeable to protons will dissipate the proton gradient across this membrane. Alternatively, a decrease in electron transport will result in fewer protons being pumped from the matrix to the intermembrane space and will eventually lead to decreased ΔΨm. The first mechanism is more likely to lead to a complete collapse of ΔΨm and is probably important for the final mitochondrial depolarization, while the second mechanism could explain the initial partial reduction of ΔΨm. Theoretically, both mechanisms would lead to a drop of ΔΨm and could result in increased formation of ROS. For example, increased inner mitochondrial membrane permeability to protons would increase the rate of electron transport in this membrane, and thus the rate of partially reduced oxygen molecules (ROS production) would increase proportionately. However, since FCCP, a protonophore that induces a collapse of ΔΨm, does not significantly increase ROS levels in these cells (data not shown), it appears unlikely that this is the mechanism by which ROS is induced during apoptosis. Moreover, a decrease in respiration rate was observed early after TNF-α treatment (Fig. 6). Therefore, ROS production is probably due to the partial reduction of ΔΨm that occurs when the respiration rate decreases. A block in respiration downstream of complex III would result in a constitutively reduced complex III that would supply continuous single-electron transport directly to oxygen and so would induce high levels of superoxide radicals. Therefore, the ability of Bcl-xL to maintain respiration following TNF-α treatment could explain both the prevention of the initial decrease in ΔΨm and the prevention of ROS production.
Although the ability of TNF-α treatment to inhibit respiration has been reported (13, 15), the reason for the TNF-α-induced decrease in respiration is unclear. Fas signaling has been suggested to block respiration by inactivating or releasing cytochrome c (13). TNF-α signal transduction also induces ceramide production, and ceramide has been reported to inhibit complex III of the respiratory chain (5). More detailed bioenergetic studies are needed to determine the mechanism by which TNF-α signal transduction decreases respiration. However, the ability of Bcl-xL to at least partially inhibit TNF-α-induced changes in respiration suggest that Bcl-xL functions to maintain mitochondrial physiology following TNF-α treatment. This is consistent with the recent report that Bcl-xL functions to maintain mitochondrial metabolite exchange following growth factor withdrawal, thus maintaining efficient coupling of oxidative phosphorylation in response to changes in cellular bioenergetics (27). The more efficiently coupled these processes are, the less likely the rate of respiratory ROS production is to change.
How does Bcl-xL protect from death due to treatment with exogenous ROS?
Once ROS levels are induced, they appear to accelerate mitochondrial depolarization (Fig. 5 and 7B). The role of ROS in subsequent apoptotic steps is not clear. ROS may induce membrane peroxidation, leading to destabilization of the mitochondrial and/or other cellular membranes. ROS may be required to create a redox environment that facilitates the function of other proapoptotic proteins.
Adding exogenous ROS to 2B4 cells results in partial membrane depolarization regardless of Bcl-xL expression. This observation, along with the one that Bcl-xL does not induce ROS scavenging (Fig. 3), supports the hypothesis that in TNF-α-treated cells Bcl-xL regulates the membrane potential upstream of ROS production and that exogenous ROS therefore bypass the Bcl-xL effect. However, Bcl-xL protected cells from hydrogen peroxide-induced death (Fig. 7A), suggesting that Bcl-xL must somehow limit ROS toxicity even when ROS generation is beyond its control. Interestingly, it was recently reported that cells treated with hydrogen peroxide required further mitochondrial changes as a secondary death mediator in order to achieve full toxicity of the treatment (2). This observation supports our conclusion that Bcl-xL protects cells from certain levels of hydrogen peroxide by regulating mitochondrial functions and not by scavenging the exogenous ROS. Indeed, treatment with TNF-α, as well as with hydrogen peroxide, resulted in further mitochondrial depolarization before cell death was observed. The steps in mitochondrial depolarization could be part of an amplification loop that involves initial depolarization and ROS production that in turn leads to further mitochondrial dysfunction and depolarization. We have shown that Bcl-xL expression cannot reduce ROS levels in cells treated with exogenous hydrogen peroxides for the first 30 min after treatment (Fig. 3C). However, Bcl-xL may inhibit the amplification loop in hydrogen peroxide-treated cells, thus restricting depolarization of the mitochondria and allowing cells to eventually recover (Fig. 7B). Nevertheless, there is a point when the amount of exogenously supplied hydrogen peroxide is sufficient to cause complete depolarization, so that amplification is not required and Bcl-xL will fail to protect cells from death.
ACKNOWLEDGMENTS
Parental 2B4.11 cells were a gift from J. Ashwell. We thank Tanya Gottlieb and Ayala King for helpful discussions and expert editorial advice.
Eyal Gottlieb was supported by a fellowship from the European Molecular Biology Organization (EMBO).
REFERENCES
- 1.Banki K, Hutter E, Gonchoroff N J, Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- 2.Dumont A, Hehner S P, Hofmann T G, Ueffing M, Droge W, Schmitz M L. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-kappaB. Oncogene. 1999;18:747–757. doi: 10.1038/sj.onc.1202325. [DOI] [PubMed] [Google Scholar]
- 3.Freeman B A, Crapo J D. Biology of disease: free radicals and tissue injury. Lab Investig. 1982;47:412–426. [PubMed] [Google Scholar]
- 4.Friesen C, Fulda S, Debatin K M. Induction of CD95 ligand and apoptosis by doxorubicin is modulated by the redox state in chemosensitive- and drug-resistant tumor cells. Cell Death Differ. 1999;6:471–480. doi: 10.1038/sj.cdd.4400512. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa J C. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 6.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 7.Gross A, McDonnell J M, Korsmeyer S J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 8.Hildeman D A, Mitchell T, Teague T K, Henson P, Day B J, Kappler J, Marrack P C. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 9.Hochman A, Sternin H, Gorodin S, Korsmeyer S, Ziv I, Melamed E, Offen D. Enhanced oxidative stress and altered antioxidants in brains of Bcl-2-deficient mice. J Neurochem. 1998;71:741–748. doi: 10.1046/j.1471-4159.1998.71020741.x. [DOI] [PubMed] [Google Scholar]
- 10.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 11.Johnson B W, Boise L H. Bcl-2 and caspase inhibition cooperate to inhibit tumor necrosis factor-alpha-induced cell death in a Bcl-2 cleavage-independent fashion. J Biol Chem. 1999;274:18552–18558. doi: 10.1074/jbc.274.26.18552. [DOI] [PubMed] [Google Scholar]
- 12.Kane D J, Sarafian T A, Anton R, Hahn H, Gralla E B, Valentine J S, Ord T, Bredesen D E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 13.Krippner A, Matsuno-Yagi A, Gottlieb R A, Babior B M. Loss of function of cytochrome c in Jurkat cells undergoing fas-mediated apoptosis. J Biol Chem. 1996;271:21629–21636. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]
- 14.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 15.Lancaster J R, Jr, Laster S M, Gooding L R. Inhibition of target cell mitochondrial electron transfer by tumor necrosis factor. FEBS Lett. 1989;248:169–174. doi: 10.1016/0014-5793(89)80454-5. [DOI] [PubMed] [Google Scholar]
- 16.Marchetti P, Decaudin D, Macho A, Zamzami N, Hirsch T, Susin S A, Kroemer G. Redox regulation of apoptosis: impact of thiol oxidation status on mitochondrial function. Eur J Immunol. 1997;27:289–296. doi: 10.1002/eji.1830270142. [DOI] [PubMed] [Google Scholar]
- 17.Memon S A, Moreno M B, Petrak D, Zacharchuk C M. Bcl-2 blocks glucocorticoid- but not Fas- or activation-induced apoptosis in a T cell hybridoma. J Immunol. 1995;155:4644–4652. [PubMed] [Google Scholar]
- 18.Mignotte B, Vayssiere J L. Mitochondria and apoptosis. Eur J Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- 19.Mirkovic N, Voehringer D W, Story M D, McConkey D J, McDonnell T J, Meyn R E. Resistance to radiation-induced apoptosis in Bcl-2-expressing cells is reversed by depleting cellular thiols. Oncogene. 1997;15:1461–1470. doi: 10.1038/sj.onc.1201310. [DOI] [PubMed] [Google Scholar]
- 20.Quillet-Mary A, Jaffrezou J P, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- 21.Rathmell J C, Thompson C B. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- 22.Sato N, Iwata S, Nakamura K, Hori T, Mori K, Yodoi J. Thiol-mediated redox regulation of apoptosis. Possible roles of cellular thiols other than glutathione in T cell apoptosis. J Immunol. 1995;154:3194–3203. [PubMed] [Google Scholar]
- 23.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993;12:3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 26.Tyurina Y Y, Tyurin V A, Carta G, Quinn P J, Schor N F, Kagan V E. Direct evidence for antioxidant effect of Bcl-2 in PC12 rat pheochromocytoma cells. Arch Biochem Biophys. 1997;344:413–423. doi: 10.1006/abbi.1997.0201. [DOI] [PubMed] [Google Scholar]
- 27.Vander Heiden M G, Chandel N S, Li X X, Schumacker P T, Colombini M, Thompson C B. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci USA. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vander Heiden M G, Thompson C B. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 29.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 30.Wong G H, Goeddel D V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 31.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin S A, Petit P X, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]