Abstract
The combination of COVID-19 vaccination with immunotherapy by checkpoint inhibitors in cancer patients could intensify immunological stimulation with potential reciprocal benefits. Here, we examine more closely the possible adverse events that can arise in each treatment modality. Our conclusion is that caution should be exercised when combining both treatments.
Subject terms: Cancer, Viral infection
The persisting spreading of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; coronavirus disease 2019 [COVID-19]) has prompted the development of vaccine candidates at an accelerated rate with remarkable efficacy. To date, the most advanced vaccines are those that transfer nucleotide coding for the S protein as DNA (inactivated adenovirus-DNA delivered) such as the Astrazeneca vaccine or as lipid nanoparticle-encapsulated RNA forms such as those produced by Pfizer BioNTech and Moderna. The Johnson and Johnson (Janssen) and the Sputnik V vaccines as well as vaccines other than nucleic acid vectors are also joining the market in different regions. Given the disproportionate impact of COVID-19 in cancer patients, these vulnerable subjects should be included in the populations prioritised for early vaccination along with the transplanted patient, rheumatic disease patients and other immunosuppressed subjects [1].
There is a scarcity of data on the consequences of COVID-19 vaccination in cancer patients under specific treatments, as recently stressed by Korompoki et al. [2], especially those in phase III vaccine trials [3]. One recent short article by Waissengrin et al. [4] reports on the BNT162b2 messenger RNA (mRNA) COVID-19 vaccine administered in cancer patients under checkpoint inhibitors (CPIs). Based on a relatively limited number of cases, the authors note that when compared to matched controls, CPI therapy results in a constant and variable increase of all COVID-19 vaccination side effects, which is cause for alarm. The authors nevertheless consider their data as supporting the short-term safety of the mRNA COVID-19 vaccine in patients under CPIs. A larger set of patients would certainly be necessary to confirm their findings and deliver a clear message on this point. On the other hand, the report by Waissengrin et al. also mentions the apparent short-term absence of immunologically related adverse events (IRAEs) in a subgroup of 134 patients treated by CPIs who received the BNT162b2 mRNA COVID-19 vaccine [4]. However, the authors recognise the possibility that rare IRAEs [4] could be identified in larger cohorts of patients under COVID-19 vaccination.
This possibility was clinically confirmed in the recent case report by Au et al. describing a close temporal association between BNT162b2 vaccination and the onset of a harmful cytokine release syndrome (CRS) in a patient with colorectal cancer on long-standing anti-programmed death-ligand 1 (PD-1) monotherapy [5]. The authors suggest that this CRS could be due to the vaccine and could occur on the background of immune activation secondary to the PD-1 blockade with an increase in T cell proliferation and effector function.
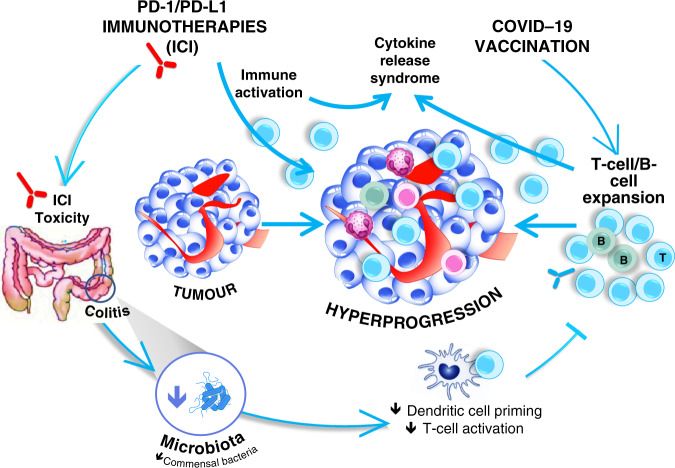
The reports by Waissengrin et al. [4] and Au et al. [5] illustrate the view that interaction between immunotherapy and COVID-19 vaccination should be considered from both sides, i.e. how vaccination could influence immunotherapy and, conversely, how immunotherapy could impact COVID-19 vaccination (Fig. 1). Both treatments cause their own stimulation of the immunological system, and more particularly, at the T cell and dendritic cell levels, their coexistence could therefore lead to final effects that potentiate their respective activity. In this respect, it has recently been reported that influenza vaccination improves the survival of patients under CPIs without having any detrimental effects in terms of safety [6, 7]. But overall, COVID-19 vaccination generates more severe side effects than influenza vaccines; consequently, its impact on CPI-related side effects cannot be overlooked.
Fig. 1. The reciprocal interaction between COVID-19 vaccination and cancer treatment by checkpoint inhibitors.
ICI: Immune Checkpoint Inhibitors.
CPIs induce IRAEs at a rate of 20–50% for any grade, and the risk for developing these toxicities is higher in elderly patients [8, 9]. Of note, one of these IRAEs is colitis, which can impact microbiota integrity with potential immune consequences. This could result from the complex interrelationship between the overall immune status and microbiota, which has been well elucidated [10]. Interestingly, the influence of microbiota on the immune response to vaccination has also been reported [11]. Therefore, more attention should be paid to a potential loss of COVID-19 vaccination efficacy in patients under CPI due to a possible occurrence of IRAEs and more particularly a case of colitis.
In the context of rare unexpected findings under CPIs, one should consider in addition the rare but nevertheless concerning phenomenon of tumour hyper-progression (THP) [12]. This form of tumour flare, although infrequent, can cause a potentially fatal locoregional progression of the disease. The pathophysiology of THP is not clearly established but includes an expansion of activated T lymphocytes in the tumour itself and its microenvironment [13]. This excessive tumour infiltration by lymphocytes could be amplified by an increased bulk of activated lymphocytes resulting from the boosting effect of the vaccination itself (Fig. 1). We have recently shown that it may be possible to identify patients under CPIs at risk for this THP by discriminating germinal genetic profiles [14]. This could serve as a tool for screening CPI-treated patients scheduled to receive COVID-19 vaccination.
The articles by Waissengrin et al. [4] and Au et al. [5] express more or less strong messages of caution and point to the need to gain a deeper knowledge of the reciprocal interaction between COVID-19 vaccination and CPI cancer treatment by investigating large cohorts of patients. To this end, along with the classical parameters of patient follow-up, more specific immunology-based investigations should be conducted to examine all the aspects of the long-term immune response. This is the main objective of the recently launched VOICE trial [15]. This prospective, multicentric trial aims to closely examine on a long-term basis whether immunotherapy and chemotherapy, alone or combined, influence COVID-19 vaccination in treated patients. Study parameters include the antibody response, the SARS-CoV-2-specific T cell response and the functional and phenotypical characterisation of the cellular immune response. This type of long-term follow-up is particularly necessary when considering current developments in CPI treatment and the increasing role played by their adjuvant setting. The immunotherapeutic management of malignant melanoma [16] and lung cancer [17] are clear illustrations of these current developments in CPI treatment. The association of chemotherapy and targeted therapies with CPI treatment in most therapeutic situations also generates potential difficulties in data interpretation, further increasing the need for multi-cohort studies. The ESMO recently emphasised the importance of monitoring COVID-19 vaccine effects in cancer patients through specific studies and registries [18]. In France, the ANRS S0001S COV-POPART cohort study was recently launched (NCT04824651). It monitors 8650 vaccinated subjects with various pathologies including cancer to evaluate their relative capacity to produce antibodies against SARS-CoV- 2 (the study includes a control group of 1850 subjects without the targeted pathologies). Concerning cancer treatment and more particularly early clinical trials, an international group of experts recently recommended that trials on anti-cancer drugs with unknown safety profiles should be avoided until 2 to 4 weeks after the second dose of the COVID-19 vaccine [19]. More generally, the reciprocal interaction between COVID-19 vaccination and cancer treatments should be examined in greater depth.
In spite of a complex and still evolving SARS-COV2, COVID-19 vaccination is increasingly combined with CPI treatments in cancer patients and is likely to impact every treatment. At first sight, this combination should boost the immunological stimulation with potential reciprocal benefits. However, the clinical picture described in this article tempers this judgement. Its aim is to deliver a message of caution and raise the awareness of caregivers and prescribers to the particular attention that should be paid to patients at risk.
Acknowledgements
We would like to thank the UCA Office of International Scientific Visibility for comments on the English version of the manuscript. Funding is acknowledged from the French Government (Agence Nationale de Recherche, ANR) through the ‘Investments for the Future’ LABEX SIGNALIFE (ANR-11-LABX-0028-01 and IDEX UCAJedi ANR-15-IDEX-01) and [AD-ME project R19162DD]; CANC’AIR Genexposomic project, Canceropole PACA; DREAL PACA, ARS PACA, Région Sud, INSERM cancer; INCA Plan Cancer; Children Medical Safety Research Institute (CMSRI, Vaccinophagy project R17033DJA).
Author contributions
PB, BM, PH and GM participated in writing the article. PB, BM and GM participated in the preparation of the figure. All authors read and approved the final manuscript.
Competing interests
PB and BM declare no financial ties with any organisation that might have an interest in the submitted work over the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work. GM has received honoraria from MERCK and Bristol Myers Squibb (BMS) for lectures specifically on the covered subject. PH has received honoraria from AstraZeneca, BMS, MSD, Lilly, Bayer, Pfizer, Thermo Fisher, Roche, Illumina, Novartis, Biocartis and Abbvie for lectures, consulting fees and membership on their advisory board
Ethics approval and consent to participate
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Desai A, Gainor JF, Hegde A, Schram AM, Curigliano G, Pal S, et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18:313–9. doi: 10.1038/s41571-021-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korompoki E, Gavriatopoulou M, Kontoyiannis DP. COVID-19 vaccines in patients with cancer—a welcome addition, but there is need for optimization. JAMA Oncol. 2021;7:1113. doi: 10.1001/jamaoncol.2021.1218. [DOI] [PubMed] [Google Scholar]
- 3.Corti C, Curigliano G. Commentary: SARS-CoV-2 vaccines and cancer patients. Ann Oncol. 2021;32:569–71. doi: 10.1016/j.annonc.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;2045:581–3. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Au L, Fendler A, Shepherd STC, Rzeniewicz K, Cerrone M, Byrne F, et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med. 2021;27:1362–6. doi: 10.1038/s41591-021-01387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang CK, Kim HR, Song KH, Keam B, Choi SJ, Choe PG, et al. Cell-mediated immunogenicity of influenza vaccination in patients with cancer receiving immune checkpoint inhibitors. J Infect Dis. 2020;222:1902–9. doi: 10.1093/infdis/jiaa291. [DOI] [PubMed] [Google Scholar]
- 7.Valachis A, Rosén C, Koliadi A, Digkas E, Gustavsson A, Nearchou A, et al. Improved survival without increased toxicity with influenza vaccination in cancer patients treated with checkpoint inhibitors. Oncoimmunology. 2021;10:e1886725. doi: 10.1080/2162402X.2021.1886725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P-F, Chen Y, Song S-Y, Wang T-J, Ji W-J, Li S-W, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharm. 2017;8:730. doi: 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldini C, Martin Romano P, Voisin AL, Danlos FX, Champiat S, Laghouati S, et al. Impact of aging on immune-related adverse events generated by anti–programmed death (ligand)PD-(L)1 therapies. Eur J Cancer. 2020;129:71–9. doi: 10.1016/j.ejca.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510–9. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosomi K, Kunisawa J. Impact of the intestinal environment on the immune responses to vaccination. Vaccine. 2020;38:6959–65. doi: 10.1016/j.vaccine.2020.08.079. [DOI] [PubMed] [Google Scholar]
- 12.Hwang JK, Zhang T, Wang AZ, Li Z. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol. 2021;14:1–11. doi: 10.1186/s13045-021-01046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–8. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 14.Refae S, Gal J, Brest P, Giacchero D, Borchiellini D, Ebran N, et al. Hyperprogression under immune checkpoint inhibitor: a potential role for germinal immunogenetics. Sci Rep. 2020;10:1–8. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Veldt AAM, Oosting SF, Dingemans AMC, Fehrmann RSN, GeurtsvanKessel C, Jalving M, et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med. 2021;27:568–9. doi: 10.1038/s41591-021-01240-w. [DOI] [PubMed] [Google Scholar]
- 16.Indini A, Grossi F. Adjuvant pembrolizumab for melanoma: update from the EORTC 1325-MG/KEYNOTE-054 trial. Lancet Oncol. 2021;22:573–5. doi: 10.1016/S1470-2045(21)00125-X. [DOI] [PubMed] [Google Scholar]
- 17.Chaft JE, Rimner A, Weder W, Azzoli CG, Kris MG, Cascone T. Evolution of systemic therapy for stages I–III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 2021;18:547–57. doi: 10.1038/s41571-021-00501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garassino MC, Vyas M, de Vries EGE, Kanesvaran R, Giuliani R, Peters S. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: vaccinate. monitor. educate. Ann Oncol. 2021;32:579–81. doi: 10.1016/j.annonc.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yap TA, Siu LL, Calvo E, Lolkema MP, LoRusso PM, Soria JC, et al. SARS-CoV-2 vaccination and phase 1 cancer clinical trials. Lancet Oncol. 2021;22:298–301. doi: 10.1016/S1470-2045(21)00017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]