Abstract
SARS-CoV-2 vaccines protect against symptomatic and severe COVID-19. The BNT162b2/Pfizer and mRNA-1273/Moderna vaccines represent new vaccine technology relying on administration of mRNA encoding SARS-CoV-2 viral spike protein encased in lipid nanoparticles. The vaccines are administered as two doses into muscle, which elicits a strong response, typically within 14 days after the second dose. Neuromuscular diseases are characterized by the progressive loss of muscle and are often treated with chronic glucocorticoid steroids, both of which may contribute to a blunted immune response to vaccination. Here, we measured IgG antibody content and neutralizing antibody response after mRNA COVID-19 vaccination in non-ambulatory neuromuscular disease patients. After two doses of mRNA COVID-19 vaccine, median anti-receptor binding domain IgG and percent surrogate viral neutralization in non-ambulatory neuromuscular disease samples were significantly elevated similar to healthy vaccinated controls. As in healthy controls, COVID-19 vaccines produce greater antibody levels compared to those with a history of outpatient COVID-19 infection. This data documents that non-ambulatory neuromuscular disease patients respond well to two doses of mRNA COVID-19 vaccine despite low muscle mass and even chronic steroid use.
Keywords: COVID-19, SARS-CoV-2, Serological testing, IgG, ELISA, Dried blood spots, Neuromuscular, Muscular dystrophy, Receptor binding domain
1. Introduction
In the US, approximately 300,000 people live with neuromuscular disease (NMD). The mRNA vaccines, BNT162b2/Pfizer and mRNA-1273/Moderna, rely on intramuscular administration of modified RNA encapsulated in lipid nanoparticles to elicit an immune response and protection against symptomatic SARS-CoV-2 infection. NMD is often associated with profoundly degenerative and atrophied muscle, raising the question whether this degenerative substrate responds to mRNA vaccines. Additionally, some NMDs are routinely treated with glucocorticoid steroids, which can suppress immune response to vaccination. We evaluated IgG antibody response after COVID-19 vaccination in non-ambulatory NMD patients since patients at this disease stage have little normal muscle. We measured total receptor binding domain (RBD) IgG and performance in a surrogate virus neutralization test since these assays are highly predictive of COVID-19 protection [1].
2. Methods
All research activities were implemented under protocols approved by the institutional review board at Northwestern University (#STU00212457 and #STU00212472).
Participants provided e-consent and completed an online questionnaire regarding health, COVID-19 viral status, and COVID-19 vaccination status. Participants were mailed a dried blood spot (DBS) kit, instructed to self-collect DBS 14 days after receiving vaccine dose two and return by mail. Results were compared to a similarly conducted community-based survey, Screening for Coronavirus Antibodies in Neighborhoods (SCAN), which measured antibody response after COVID-19 viral infection (COVID-19+) or after administration of vaccine dose two [2,3].
The anti-RBD (receptor binding domain) IgG enzyme-linked immunosorbent assay (ELISA) protocol was performed as described [4,5]. Anti-RBD IgG concentration was calculated from the 4PL regression of the CR3022 calibration curve [4,5]. The surrogate virus neutralization protocol was performed as described [6]. Percent surrogate neutralization = 100 × 1 - (sample mean fluorescence intensity (MFI) /negative control MFI). Samples were evaluated in duplicate and reported as the average.
3. Results
Fourteen participants (13 advanced non-ambulatory NMD patients and one ambulatory carrier) were monitored for IgG response after vaccination. Results were compared to healthy community-derived controls. As a third comparator, IgG levels were measured in unvaccinated non-NMD participants who had a history of outpatient COVID-19 infection. Table 1 describes the NMD cohort; 8 of 14 were taking chronic steroids in a wide range of doses and frequency, and none reported previous COVID-19 infection. Table 2 describes the IgG and surrogate neutralization response to vaccination in the NMD cohort.
Table 1.
Neuromuscular patient characteristics.
| NMD type | Steroid use | Ambulatory | Age (decade) | Sex |
|---|---|---|---|---|
| DMD | yes (daily) | no | 20s | male |
| DMD carrier | no | yes | 60s | female |
| Calpain LGMD | yes (weekly) | no | 60s | female |
| DMD | yes (daily) | no | 20s | male |
| DMD | no | no | 30s | male |
| DMD | yes | no | 20s | male |
| DMD | yes | no | 20s | male |
| DMD | yes | no | 20s | male |
| DMD | yes | no | 20s | male |
| SMA | no | no | 30s | male |
| DMD | no | no | 20s | male |
| BMD | no | no | 50s | male |
| Bethlem/Ullrich | no | no | 30s | male |
| Sarcoglycan LGMD | yes (weekly) | no | 20s | female |
BMD, Becker Muscular Dystrophy; DMD, Duchenne muscular dystrophy; LGMD, limb girdle muscular dystrophy; SMA, spinal muscular atrophy.
Table 2.
IgG and surrogate neutralization response to vaccination in NMD patients.
| Vaccine | Days post dose 2 | Anti-RBD IgG µg/ml | % neutralization spike |
|---|---|---|---|
| Moderna | 19 | 17.6 | 96.3 |
| Moderna | 19 | 52.1 | 99.4 |
| Moderna | 21 | 6.6 | 55.4 |
| Moderna | 14 | 19.4 | 89.8 |
| Moderna | 12 | 35.8 | 99.8 |
| Moderna | 14 | 16.4 | 99.7 |
| Moderna | 14 | 65.3 | 63.8 |
| Moderna | 13 | 73.8 | 99.8 |
| Moderna | 94 | 26.4 | NA |
| Moderna | 14 | 63.8 | 60 |
| Pfizer | 15 | 64.7 | 94.5 |
| Pfizer | 94 | 9.8 | 94.8 |
| Pfizer | 21 | 11.3 | 98.8 |
| Pfizer | 23 | 18.6 | 95.3 |
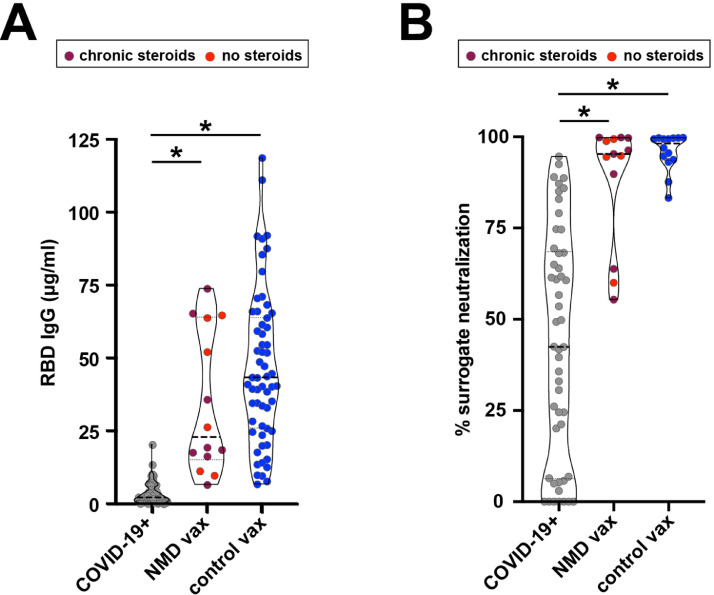
The median anti-RBD IgG concentration was 11-fold higher (p < 0.001) in the NMD group (22.9 µg/ml) compared to COVID-19+ individuals (2.1 µg/ml) and was not statistically different than vaccinated controls (43.3 µg/ml; p > 0.99) (Fig. 1 A). Median IgG levels in NMD patients taking chronic steroids (19.0 µg/ml) was 9-fold higher (p < 0.001) than COVID-19+ individuals (2.1 µg/ml). Moreover, the median percent surrogate neutralization in the NMD group was significantly higher than COVID-19+ individuals (95.3% vs 42.4%; p < 0.001) and similar to the vaccinated controls (95.3% vs 98.2%; p > 0.99) (Fig. 1 B). Percent surrogate neutralization was similar in NMD participants regardless of steroid usage (chronic steroids 95.8%; none 94.8%; p > 0.99). The self-report questionnaire indicated an average of two of seven symptoms after vaccination (pain/swelling at the injection site, fever, chills, fatigue, headache, muscle/joint pain, vomiting), similar to what was self-reported in the community acquired vaccinated control group.
Fig. 1.
Anti-receptor binding domain (RBD) IgG and percent surrogate neutralization after natural infection and vaccination. (A) Median anti-RBD IgG levels collected from neuromuscular (NMD) participants (22.9 µg/ml; n = 14) was significantly higher than individuals who were unvaccinated but had outpatient COVID-19 infection (COVID-19+) (2.1 µg/ml; n = 88), and not statistically different from community acquired vaccinated controls (vax) (43.3 µg/ml; n = 59). Kruskal-Wallis test was used to evaluate statistical significance. * P < 0.001. (B) Using an in vitro surrogate neutralization assay, median percent surrogate neutralization of Wuhan spike-ACE2 receptor binding was increased in vaccinated NMD participants (95.3%; n = 13) compared to community COVID-19+ samples (42.4%; n = 50), and not statistically different than vaccinated controls (98.2%; n = 14). Kruskal-Wallis tests were used to evaluate statistical significance. * P < 0.001. NMD patients on glucocorticoid steroids are indicated in maroon color.
4. Discussion
Advanced NMD patients have little intact muscle mass, yet these individuals responded well to two doses of mRNA vaccines. Although longitudinal health data is not yet available, the near-term vaccine response suggests a comparable response to that seen in healthy community controls and supports it is reasonable to expect similar protection. The presence of a normal immune response to mRNA vaccination indicates the non-muscle components as being more critical for immune response to these novel vaccines.
The study is limited by its small size and self-report nature of vaccination and health history. Although immunosuppression, including glucocorticoid use is known to lower the humoral vaccine response [7], in our small study we did not observe chronic glucocorticoid steroid use as strongly interfering with vaccine response. The number of participants taking glucocorticoids, combined with the wide range of dose and dosing schedules, does not allow us to draw any conclusions regarding the amount of steroid use and vaccine response. It is possible that those taking chronic daily steroids may have lower vaccine response, but further study is needed to confirm this.
Declaration of Competing Interest
Thomas McDade has a financial interest in EnMed Microanalytics, a company that specializes in laboratory testing of dried blood spot samples. All other authors declare no conflicts of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Supported by the National Science Foundation 2035114, NIH 3UL1TR001422–06S4, Northwestern University Office of Research, and a generous gift from Dr. Andrew Senyei and Noni Senyei. The funding sources had no role in the study design, data collection, analysis, interpretation, or writing of the report.
References
- 1.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 2.Demonbreun A.R., McDade T.W., Pesce L., Vaught L.A., Reiser N.L., Bogdanovic E., et al. Patterns and persistence of SARS-CoV-2 IgG antibodies in Chicago to monitor COVID-19 exposure. JCI Insight. 2021;6 doi: 10.1172/jci.insight.146148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demonbreun A.R., Sancilio A., Velez M.P., Ryan D.T., Saber R., Vaught L.A., et al. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDade T.W., McNally E.M., Zelikovich A.S., D'Aquila R., Mustanski B., Miller A., et al. High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0237833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sancilio A.E., D'Aquila R.T., McNally E.M., Velez M.P., Ison M.G., Demonbreun A.R., et al. A surrogate virus neutralization test to quantify antibody-mediated inhibition of SARS-CoV-2 in finger stick dried blood spot samples. Sci Rep. 2021;11:15321. doi: 10.1038/s41598-021-94653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deepak P., Kim W., Paley M.A., Yang M., Carvidi A.B., Demissie E.G., et al. Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2: a Prospective Cohort Study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]