Abstract
Objective:
To investigate the isolated and combined effects of compression and hypoxia on the osteoclastogenesis induced by periodontal ligament cells (PDLCs).
Materials and Methods:
A periodontal ligament tissue model (PDLtm) was established by 3-D culturing human PDLCs on a thin sheet of poly lactic-co-glycolic acid scaffold. The PDLtm was treated with hypoxia and/or compression for 6, 24, or 72 hours. After that, a real-time polymerase chain reaction was used for gene expression analysis. The conditioned media were used for the coculture of osteoblast and osteoclast (OC) precursors; tartrate-resistant acid phosphatase staining was done to examine OC formation.
Results:
Either compression or hypoxia alone significantly up-regulated the gene expression of pro-osteoclastogenic cytokines in the PDLtm and enhanced osteoclastogenesis in the cocultures, and the combination of the two had significantly stronger effects than either stimulation alone. In addition, comparing the two stimulants, we found that the osteoclastogenic property of the PDLCs peaked earlier (at 6 hours) in the compression group than in the hypoxia group (at 24 hours).
Conclusions:
Both compressive force and hypoxia may take part in initiating osteoclastogenesis in orthodontic tooth movement and may have combinatory effects, which could update our concepts of the mechanisms involved in the initiation of bone resorption on the pressure side of the tooth in question.
Keywords: Hypoxia, Compressive force, Periodontal ligament cell, Osteoclastogenesis
INTRODUCTION
Orthodontic tooth movement(OTM) is the consequence of pathological and physiological periodontal tissue remodeling in response to external applied loading.1 Though investigated widely, the mechanism of OTM remains to be understood. When a tooth is subjected to orthodontic force, numerous changes occur in the alveolar bone and periodontium, among which mechanical force and hypoxia have been the most studied and described to regulate bone remodeling.1,2
The role of mechanical compression in promoting periodontal ligament (PDL)-induced osteoclastogenesis has been reported by numerous studies.3–6 Briefly, when subjected to a compressive force, the periodontal ligament cells (PDLCs) elicit osteoclast (OC) formation through regulating the expression of related agents, including receptor activator for nuclear factor-κ B ligand (RANKL) expression and osteoprotegerin (OPG).6,7
When orthodontic force is applied, the PDL vascular system is gradually occluded, resulting in circulatory disturbances and leading to ischemia and local hypoxia on the compressive side.8 Low O2 environment has been reported to activate hypoxia inducible factor 1α (HIF-1α) and regulate bone resorption through enhancement of OC differentiation and activity.9,10 Recently, HIF-1α was found to up-regulate RANKL expression in PDLCs.11 Moreover, treatment of the inhibitor of HIF-1α suppressed the rate of tooth movement.12 These findings illustrate that oxygen tension might be involved in osteoclastogenesis during OTM.
Present evidence suggests that both mechanical force and hypoxia exist simultaneously on the pressure side and play important roles in OTM bone remodeling. However, no studies have yet reported the combinatory effects of mechanical loading and hypoxia on OC formation, as well as the differences in regulating patterns on the osteoclastogenic capacity of PDLCs. Therefore, in this study, we employed a 3-D PDLtm and a coculture system of osteoblast (OB) and osteoclast precursor (pre-OC) to investigate the issue.
MATERIALS AND METHODS
Synthesis of Scaffold
The poly lactic-co-glycolic acid (PLGA) scaffold was prepared following the detailed method reported in our previous studies.5,13
3D Culture of PDLCs
The teeth for human PDLC culture were collected from five young (10–14-year-old) donors whose noncarious and periodontally healthy teeth had been extracted for orthodontic reasons. Informed consent was obtained from all donors. The PDL tissue was separated from the middle third of the root and digested by α-minimum essential medium (α-MEM; Gibco, Grand Island, NY) containing collagenase type I (Gibco) for 1.5 hours at 37°C and then centrifuged. Subsequently, the tissue precipitate was resuspended by α-MEM supplemented with fetal bovine serum (10%; Hyclone, Auckland, New Zealand), penicillin (100 U/mL; Beyotime, Jiangsu, China), and streptomycin (100 mg/mL, Beyotime) and transferred to culture flasks (Corningware, Corning, NY) for PDLC culture. Cells of fourth–sixth passage were used for the experiment.13
The PDLtm was established by dripping the suspension of PDLCs (1 × 105 cells in approximately 2 mL medium)5 onto the scaffolds, which were placed on a six-well plate. To exclude the confounding effects from the 2-D cultured cells attached to the bottom of the well, the PDLtms were transferred to another six-well plate 24 hours later.5
Microscopic Observation
Four days after establishing the PDLtm, we performed two microscopies to histologically and morphologically observe the PLGA/PDLC constructs. The PDLtm was stained by 0.01% acridine orange in the dark, then observed using an inverted fluorescence microscope (Leica, Mannheim, Germany). In addition, after fixation with 2.5% glutaraldehyde solution, dehydration with gradient ethanol, and treatment with hexamethyldisilazane, the PDLtm was observed by scanning electron microscopy (SEM) (Inspect-FEI, Hillsboro, Ore).
Treatment of Hypoxia and Static Compression
The PDLtms were assigned to four groups (compression alone, hypoxia alone, hypoxia + compression, and control). To simulate the compressed PDL under orthodontic force, a modified weight method was used as previously described.5,13 Briefly, a cover glass was placed over each PDLtm, then a glass bottle with lead granules having a total weight of 100 g was placed on the cover glass to produce a mechanical force of 25 g/cm2. To induce hypoxia, the PDLtm was cultured in an incubator with an atmosphere of 2% O2. The PDLtm with only the cover glass and incubated in a normoxic environment served as the control. Three replicates were set for each group.
Real-Time PCR
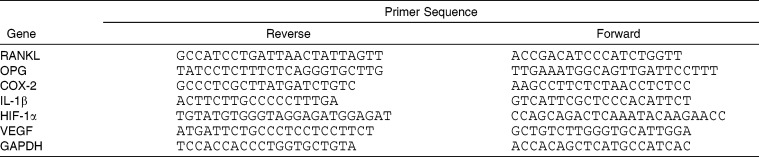
After applying interventions for 6, 24, and 72 hours,5 we evaluated the mRNA expression of RANKL, OPG, cyclooxygenase 2 (COX-2), interleukin-1 beta (IL-1β), hypoxia-inducible factor 1-alpha (HIF-1α), and vascular endothelial growth factor (VEGF) using a real-time polymerase chain reaction (PCR). Briefly, the PDLtm was washed twice using PBS and dissolved by TRIzol reagent (Invitrogen, Carlsbad, NM). Then the total RNA was extracted according to the manufacturer's instructions. The A260/A280 ratio of collected RNA was over 1.90, guaranteeing the high purity of the samples. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control. Sequences of the involved primers are shown in Table 1.
Table 1.
Primer Used in Real-Time PCR Analysis
Coculture and Tartrate-Resistant Acid Phosphatase Staining
The conditioned media from the intervened PDLtm of each stimulation and duration group were collected and stored at −20°C before being used for the coculture of OB and pre-OC.14
OBs and pre-OCs were derived from the rat as previously described.14,15 OBs of second passage were seeded to the 48-well plate, approximately 1 × 104 per well, and cultured using complete media with an additional 1 µM of prostaglandin E2 (PGE2; Sigma) and 10 nM of 1,25(OH)2D3 (Sigma) for 48 hours. After that, 1 × 105 pre-OCs were seeded to each well using the above media. After another 48 hours, the original media were removed carefully to avoid disturbing the confluent layer of pre-OCs, and 400 µL of conditioned media with the same dosage of PGE2 and 1,25(OH)2D3 was added. The conditioned media were refreshed every 2 days.
After 10 days, tartrate-resistant acid phosphatase (TRAP) staining was performed, employing an acid phosphatase kit (Sigma), to detect for mature OCs in the cocultures. Any multinucleated TRAP+ cells were counted as OCs.
The experimental protocol was approved by the Sichuan University Institutional Review Board.
Statistical Analysis
All data were expressed as means ± SD. One-way ANOVA was used for data analysis based on SPSS version 13.0 (SPSS Inc, Chicago, Ill). P < .05 was considered statistically significant.
RESULTS
Histological and Morphological Observations
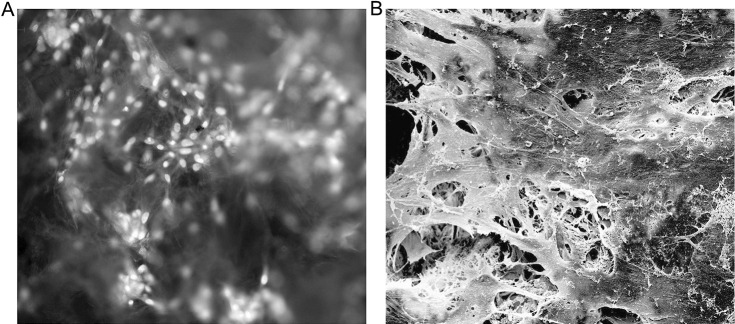
Four days after PDLC seeding, the PDLtm was observed under acridine orange staining to find spindle-shaped PDLCs having green-yellow nuclei within orange-red cytoplasm growing densely inside the PLGA scaffolds (Figure 1A). Under SEM, it was observed that the PDLCs, the synthesized extracellular matrix, and the scaffolds had been integrated into the network (Figure 1B), demonstrating the growth and function of PDLCs in the scaffolds.
Figure 1.
Microscopic observations of PDLtm. (A) Scaffolded, spindle-shaped PDLCs growing densely, with green-yellow nuclei and orange-red cytoplasm, under fluorescence inverted microscope after arcidine orange staining (×200). (B) Integration of PDLCs, secreted extracellular matrix, and PLGA scaffolds observed under SEM (×1200).
Compression/Hypoxia Regulates Osteoclastogenesis Inducers
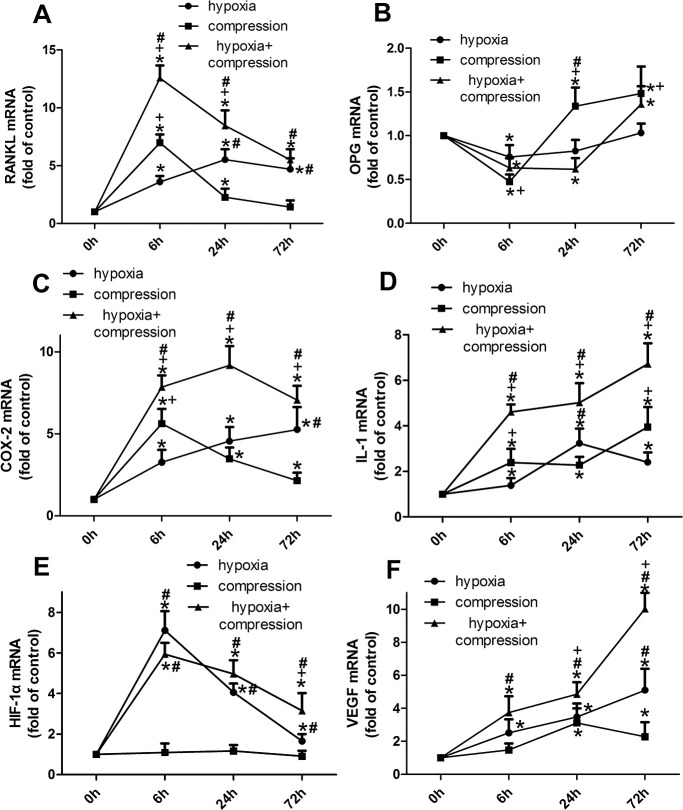
Generally, either compression or hypoxia was found to enhance the expression of osteoclastogenesis promoters and inhibit OPG expression, while the regulation pattern was somehow different (Figure 2).
Figure 2.
The time-course gene expression of RANKL (A), OPG (B), COX-2 (C), IL-1β (D), HIF-1α (E) and VEGF (F) in PDLCs. Generally, both hypoxia and compression enhanced the mRNA expression of RANKL, COX-2, IL-1β, VEGF and reduced OPG expression in a different manner; while HIF-1α was only regulated by hypoxia. *P < 0.05 vs the control group; +P < 0.05 vs the hypoxia group; #P < 0.05 vs the compression group.
Compared with the compressive force, hypoxic treatment improved the RANKL expression at lower magnitudes in 6 hours, but at higher magnitudes, in 24 and 72 hours. Moreover, no difference in RANKL was found between the compression group and the control group at 72 hours (Figure 2A). In the compressed PDLCs, OPG expression was reduced at 6 hours while elevated at 24 and 72 hours. However, a significant change of OPGs in the PDLCs under hypoxia was detected only at 6 hours (Figure 2B).
Either compression or hypoxia enhanced the expression of COX-2, IL-1β, and VEGF, which were further up-regulated in the hypoxia + compression group (Figure 2C, D, F). In contrast, HIF-1α was regulated only by hypoxia but not by compression (Figure 2E).
Compression/Hypoxia Enhances Osteoclast Formation
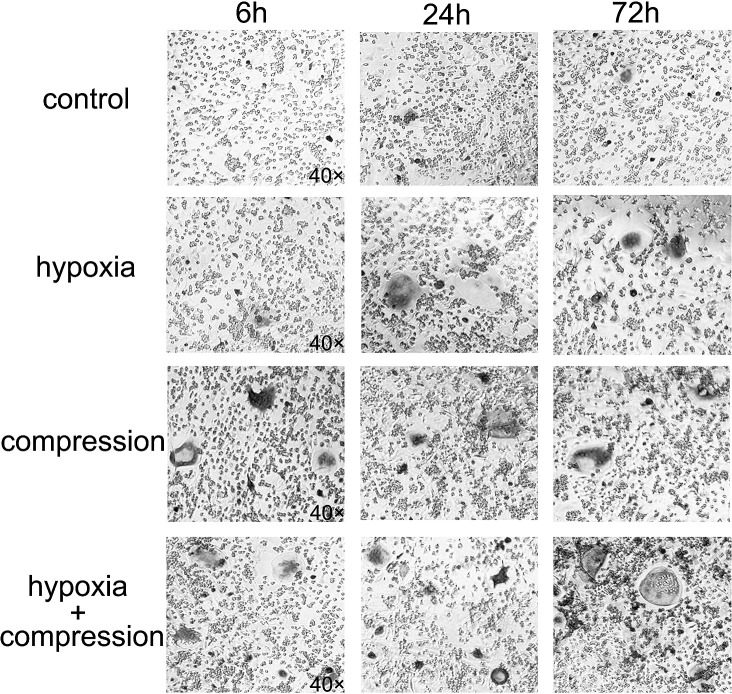
The number of OCs detected in the cocultures was enhanced significantly using conditioned media from the PDLtm subjected to compression or hypoxia alone. The number of mature OCs induced by the 6-hour compression group was larger than that of the 6-hour hypoxia group. Furthermore, OC formation was further enhanced by the combination of compression and hypoxia compared with isolated interventions (Figures 4, 5).
Figure 4.
TRAP staining of the coculture systems after a 10-day culture with conditioned media from PDLCs subjected to hypoxia and/or compression in a period of 6, 24, and 72 hours (×40).
Figure 5.
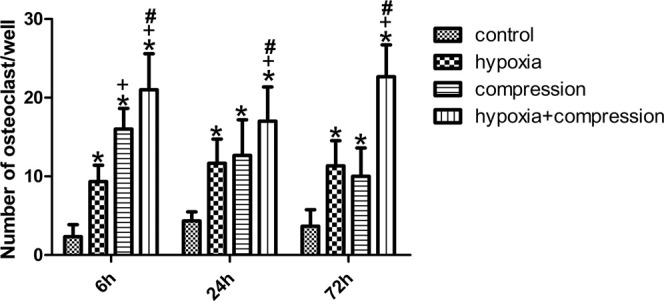
Number of OCs in the coculture systems. Either hypoxia or compression enhanced OC formation significantly. The number of OCs induced by 6-hour compression was higher than that of 6-hour hypoxia, while no difference was detected at 24 or 72 hours. Moreover, synergetic effects on OC formation were observed in the 72-hour group. *P < .05 vs control group; +P < .05 vs hypoxia group; #P < .05 vs compression group.
DISCUSSION
Periodontal tissue is essential to bone remodeling in orthodontic treatment.1 The in vivo environment on the pressure side during OTM features both compression and hypoxia, each of which have been reported to induce osteoclastogenesis.1,2 However, until now, most studies have focused on only a single factor (compression or hypoxia), while the interplay between them—and the potential differences in their effect patterns—remain little understood. In the present study, we employed a 3-D PDLtm and a coculture of OBs and pre-OCs to explore the isolated and combined effects of compression and hypoxia on the osteoclastogenic properties of PDLCs. We found that both compressive force and hypoxic treatment promoted OC formation by augmenting the osteoclastogenesis inducers of the PDLCs, although hypoxia behaved in a slower but more lasting manner compared with mechanical loadings. Moreover, the combinatory effects on osteoclastogenesis from hypoxia and compression were observed.
Hypoxia has long been recognized as a regulator of bone remodeling.9 Under hypoxic conditions, the hypoxia inducible factor 1 (HIF-1), a transcription factor, is activated to regulate its target genes because of the up-regulation of HIF-1α and its subsequent transfer to nuclei.16 The present study demonstrated that the RANKL/OPG ratio and HIF-1α expression of PDLCs are enhanced by hypoxic treatment, leading to osteoclastogenesis (Figures 2, 4, 5). Consistent with our results, HIF-1 was recently found to directly enhance RANKL expression in PDLCs, which might promote OC differentiation.11 Moreover, our group previously found that osteogenesis of PDLCs was improved when exposed to hypoxia.17,18 Taken together, these findings suggest that hypoxia accelerates alveolar bone remodeling by promoting osteogenic differentiation of PDLCs as well as improving the RANKL/OPG ratio, which leads to enhanced osteoclastogenesis.
VEGF acts as a key mediator in bone remodeling by regulating OB differentiation,19 angiogenesis,20 OC differentiation,10,21 and pre-OC recruitment.22 Our group previously reported the augmentation of VEGF expression in 2-D-cultured PDLCs under hypoxia.17 In the present study, hypoxic treatment and mechanical compression of 3-D-cultured PDLCs significantly increased VEGF expression (Figure 2F). These results may provide evidence for the involvement of VEGF in alveolar bone remodeling during OTM.
Previous studies have demonstrated that either hypoxia or compression elevates the reactive oxygen species expression that activates NF-κB migration to nuclei and further increases proinflammatory factors in the PDLCs such as COX-2 and IL-1.12,23 These results are further verified by our study (Figures 2C, 2D). Interestingly, these proinflammatory factors have also been found to enhance RANKL expression in PDLCs, OBs, and stromal cells, and to promote pre-OC recruitment.4,24–28 Based on these results, we concluded that the mechanism of osteoclastogenesis regulated by hypoxia and compression overlap in the NF-κB pathway (Figure 3).
Figure 3.
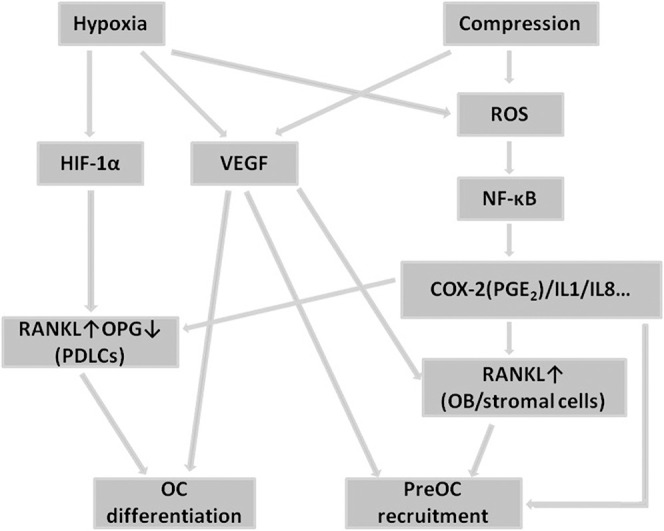
Pathways involved in the regulation of osteoclastogenesis from PDLCs under hypoxia and compression.
Similar to our previous findings,5 expression of RANKL in the compressed PDLCs peaked at 6 hours and was significantly higher than that of the PDLCs under hypoxia, while no significant difference was detected between the compressed PDLCs and the control group at 72 hours (Figure 2A). In contrast, OC formation induced by the conditioned media from the PDLCs subjected to 72-hour compression was significantly higher than that of the control group (Figure 5). This difference might be caused by the up-regulation of VEGF in that it could partially act as a macrophage colony-stimulating factor,21 and the RANKL expression in OBs induced by COX-2(PGE2)/IL-1.26,27 However, compression and hypoxia act in a magnitude-dependent manner,29,30 while the adopted interventions in the present study (compression of 25 g/cm2 and 2% O2) may not be proportional to the in vivo environment, which should be further clarified.
The osteoclastogenic capacity of the PDLCs induced by 6-hour compression was higher than that of the 6-hour hypoxia group. However, though not significant, compression for 72 hours elicited fewer OCs than that of the hypoxia group (Figure 5). This suggests that hypoxia exerts slower but more durable effects on the osteoclastogenic capacity of the PDLCs. Moreover, this characteristic may imply that hypoxia or compression plays the major role in the different stages of the OTM bioprocess.
In the present study, we found that either hypoxia or compression promoted PDL-induced osteoclastogenesis independently, and they also showed combinatory effects on this bioprocess (Figures 4, 5), providing evidence for the potential interplay of these two factors on alveolar bone remodeling during OTM. Moreover, our group had previously reported that though static compression rather than cyclic force was applied to the PDLCs, cytoskeleton-related genes showed significant changes even 72 hours after force application.13 This observation proves the continuous effects from static compression on the PDLCs. Therefore, it would be more reasonable to involve both hypoxia and compression when exploring the bone remodeling process on the pressure side regulated by the PDLCs in OTM rather than investigate a single factor.
In this study, to mimic the compressive side of the periodontium in vivo during OTM, a 3-D PDLtm and a coculture system of OBs and pre-OCs were involved. Under external force, the PDL vessels on the pressure side become occluded, leading to hypoxia, while little deformation is produced in the alveolar bone. Therefore, the PDLCs on the pressure side are exposed to low ambient oxygen tension while the OBs and pre-OCs are mainly in a normoxic environment, which is similar to the interventions in this study. Our group also tested the elastic modulus of PDLtm and found that its deformation rate under compression of 25 g/cm2 was only 6% (not published), suggesting that the media exchange would hardly be disturbed by simple compression, which guaranteed the constant O2 tension in the compression group's PDLtm.
Previous studies provide robust evidence for the interaction between human- and rodent-derived proteins. Human-derived OPG was reported to induce reduction in bone resorption and augment bone mineral density in rodents through binding with RANKL.31 Moreover, coculture of human PDLCs and mouse spleen cells also induced mature osteoclasts.32 In our study, the conditioned media from the intervening human PDLCs were used to coculture OBs and pre-OCs derived from the rat and successfully produce mature OCs. It further strengthened the previously reported crosstalk between proteins derived from humans and those derived from rodents.
CONCLUSIONS
The present findings, through an in vitro PDL tissue model, suggest that
Both compressive force and hypoxia take part in the initiation of osteoclastogenesis in orthodontic tooth movement and they may have combinatory effects.
Hypoxia acts in a slower but more durable manner.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China 11372202 and 31201087.
REFERENCES
- 1.Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niklas A, Proff P, Gosau M, Romer P. The role of hypoxia in orthodontic tooth movement. Int J Dent. 2013;2013:1–7. doi: 10.1155/2013/841840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakao K, Goto T, Gunjigake KK, Konoo T, Kobayashi S, Yamaguchi K. Intermittent force induces high RANKL expression in human periodontal ligament cells. J Dent Res. 2007;86:623–628. doi: 10.1177/154405910708600708. [DOI] [PubMed] [Google Scholar]
- 4.Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002;17:210–220. doi: 10.1359/jbmr.2002.17.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zheng W, Liu JS, Wang J, Yang P, Li ML, et al. Expression of osteoclastogenesis inducers in a tissue model of periodontal ligament under compression. J Dent Res. 2011;90:115–120. doi: 10.1177/0022034510385237. [DOI] [PubMed] [Google Scholar]
- 6.Kanzaki H, Chiba M, Shimizu Y, Mitani H. Dual regulation of osteoclast differentiation by periodontal ligament cells through RANKL stimulation and OPG inhibition. J Dent Res. 2001;80:887–891. doi: 10.1177/00220345010800030801. [DOI] [PubMed] [Google Scholar]
- 7.Kook YA, Lee SK, Son DH, Kim Y, Kang KH, Cho JH, et al. Effects of substance P on osteoblastic differentiation and heme oxygenase-1 in human periodontal ligament cells. Cell Biol Int. 2009;33:424–428. doi: 10.1016/j.cellbi.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Arai C, Nomura Y, Ishikawa M, Noda K, Choi JW, Yashiro Y, et al. HSPA1A is upregulated in periodontal ligament at early stage of tooth movement in rats. Histochem Cell Biol. 2010;134:337–343. doi: 10.1007/s00418-010-0737-3. [DOI] [PubMed] [Google Scholar]
- 9.Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8. doi: 10.1002/jcp.10321. [DOI] [PubMed] [Google Scholar]
- 10.Knowles HJ, Athanasou NA. Hypoxia-inducible factor is expressed in giant cell tumour of bone and mediates paracrine effects of hypoxia on monocyte-osteoclast differentiation via induction of VEGF. J Pathol. 2008;215:56–66. doi: 10.1002/path.2319. [DOI] [PubMed] [Google Scholar]
- 11.Park HJ, Baek KH, Lee HL, Kwon A, Hwang HR, Qadir AS, et al. Hypoxia inducible factor-1alpha directly induces the expression of receptor activator of nuclear factor-kappaB ligand in periodontal ligament fibroblasts. Mol Cells. 2011;31:573–578. doi: 10.1007/s10059-011-1055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae HS, Park HJ, Hwang HR, Kwon A, Lim WH, Yi WJ, et al. The effect of antioxidants on the production of pro-inflammatory cytokines and orthodontic tooth movement. Mol Cells. 2011;32:189–196. doi: 10.1007/s10059-011-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Li M, Tan L, Huang S, Zhao L, Tang T, et al. Analysis of time-course gene expression profiles of a periodontal ligament tissue model under compression. Arch Oral Biol. 2013;58:511–522. doi: 10.1016/j.archoralbio.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Itzstein C, van't Hof RJ. Osteoclast formation in mouse co-cultures. Methods Mol Biol. 2012;816:177–186. doi: 10.1007/978-1-61779-415-5_12. [DOI] [PubMed] [Google Scholar]
- 15.Orriss IR, Taylor SE, Arnett TR. Rat osteoblast cultures. Methods Mol Biol. 2012;816:31–41. doi: 10.1007/978-1-61779-415-5_3. [DOI] [PubMed] [Google Scholar]
- 16.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors—similar but not identical. Mol Cells. 2010;29:435–442. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Yang Y, Yang P, Gu Y, Zhao Z, Tan L, et al. The osteogenic differentiation of PDLSCs is mediated through MEK/ERK and p38 MAPK signalling under hypoxia. Arch Oral Biol. 2013;58:1357–1368. doi: 10.1016/j.archoralbio.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Cao H, Yang Y, Zhou Y, Gu Y, Zhao X, et al. Effects of vascular endothelial cells on osteogenic differentiation of noncontact co-cultured periodontal ligament stem cells under hypoxia. J Periodontal Res. 2013;48:52–65. doi: 10.1111/j.1600-0765.2012.01503.x. [DOI] [PubMed] [Google Scholar]
- 19.Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 20.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 21.Niida S, Kaku M, Amano H, Yoshida H, Kataoka H, Nishikawa S, et al. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. J Exp Med. 1999;190:293–298. doi: 10.1084/jem.190.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151:879–889. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima H, Jimi E, Okamoto F, Motokawa W, Okabe K. IL-1-induced receptor activator of NF-kappa B ligand in human periodontal ligament cells involves ERK-dependent PGE2 production. Bone. 2005;36:267–275. doi: 10.1016/j.bone.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Nukaga J, Kobayashi M, Shinki T, Song H, Takada T, Takiguchi T, et al. Regulatory effects of interleukin-1beta and prostaglandin E2 on expression of receptor activator of nuclear factor-kappaB ligand in human periodontal ligament cells. J Periodontol. 2004;75:249–259. doi: 10.1902/jop.2004.75.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Pilbeam CC, Pan L, Breyer RM, Raisz LG. Effects of prostaglandin E2 on gene expression in primary osteoblastic cells from prostaglandin receptor knockout mice. Bone. 2002;30:567–573. doi: 10.1016/s8756-3282(02)00683-x. [DOI] [PubMed] [Google Scholar]
- 27.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin J, McLeod KJ, Titus L, Nanes MS, Catherwood BD, Rubin CT. Formation of osteoclast-like cells is suppressed by low frequency, low intensity electric fields. J Orthop Res. 1996;14:7–15. doi: 10.1002/jor.1100140104. [DOI] [PubMed] [Google Scholar]
- 29.Bell EL, Klimova TA, Eisenbart J, Schumacker PT, Chandel NS. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007;27:5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitsuhashi M, Yamaguchi M, Kojima T, Nakajima R, Kasai K. Effects of HSP70 on the compression force-induced TNF-alpha and RANKL expression in human periodontal ligament cells. Inflamm Res. 2011;60:187–194. doi: 10.1007/s00011-010-0253-x. [DOI] [PubMed] [Google Scholar]
- 31.Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 32.Fukushima H, Jimi E, Kajiya H, Motokawa W, Okabe K. Parathyroid-hormone-related protein induces expression of receptor activator of NF-{kappa}B ligand in human periodontal ligament cells via a cAMP/protein kinase A-independent pathway. J Dent Res. 2005;84:329–334. doi: 10.1177/154405910508400407. [DOI] [PubMed] [Google Scholar]