Abstract
Objectives:
To investigate the effects of application of vibratory stimuli on interleukin (IL)–1β secretion during maxillary canine distalization.
Materials and Methods:
Split-mouth design study in 15 subjects (mean age, 22.9 years; range 19–25 years) whose bilateral maxillary first premolars were extracted with subsequent canine distalization. On the experimental side, light force (60 g) was applied to the canine for 3 months in combination with vibratory stimuli provided using an electric toothbrush 15 minutes a day for 2 months; only orthodontic force was applied to the contralateral control canine. Gingival crevicular fluid (GCF) was collected from the mesial and distal sides of each canine at each monthly appointment. IL-1β levels were analyzed using an enzyme-linked immunosorbent assay. Canine movement was measured monthly.
Results:
Overall, enhanced IL-1β secretion was observed at the pressure sites of experimental canines compared to control canines (mean, 0.64 ± 0.33 pg/µL vs 0.10 ± 0.11 pg/µL, respectively, P < .001). The accumulative amount of tooth movement was greater for the experimental canine than for the control canine (mean, 2.85 ± 0.17 mm vs 1.77 ± 0.11 mm, respectively, P < .001).
Conclusions:
This study demonstrates that, in combination with light orthodontic force, application of vibratory stimuli using an electric toothbrush enhanced the secretion of IL-1β in GCF and accelerated orthodontic tooth movement.
Keywords: Accelerated tooth movement, Bone remodeling, Cytokines, Light force, Tooth vibration
INTRODUCTION
Recently, there has been increasing interest in the application of vibratory stimuli, with the aim of speeding up the rate of orthodontic tooth movement by accelerating periodontal and alveolar bone remodeling. Nishimura et al.1 showed vibratory stimulation could accelerate the rate of tooth movement, with no collateral damage to periodontal tissue, in rats. The same study revealed that receptor activator of the NF kappa-B and ligand (RANK/RANKL) signaling pathway, which contributes to osteoclast formation, was activated in response to vibration. In vitro, weak intermittent vibratory forces can induce expression of RANKL in human periodontal ligament stem cells under optimal compression forces.2 A study3 in humans showed vibratory stimuli provided no clinical advantage for early resolution of crowding or alleviation of pain during initial alignment. In contrast, a number of studies4,5 have reported that short durations of low-magnitude, high-frequency resonance vibration combined with orthodontic force can increase the rate of orthodontic tooth movement without additional tissue damage in humans. One of these studies4 claimed a commercial vibratory device could enhance the rate of tooth movement by 2–3 mm/month4 with no significant change in root length and with clinically significant patient acceptance and compliance.5
As tooth movement occurs via bone resorption and formation in response to compression and tension,6 it would be interesting to investigate how vibratory stimuli increase orthodontic tooth movement and affect signaling pathways related to osteoblast and osteoclast differentiation. Pressure in the periodontal ligament (PDL) induced by orthodontic forces induces vascular changes that lead to activation of cellular signaling pathways and release of proinflammatory molecules such as interleukin (IL)–1β.7 IL-1β is secreted by osteoclasts as an immediate response to mechanical stress during the initial stage of orthodontic treatment and at the later stages by macrophages, which accumulate in compressed areas. The survival, fusion, and activation of osteoclasts correlates with the IL-1β level, which also determines the amount of tooth movement, as IL-1β regulates alveolar bone remodeling.8 However, studies on the response of mediators of bone remodeling to the combination of vibratory stimuli and orthodontic force are still lacking; such research may help to explain how vibratory stimuli affects tooth movement. Analysis of gingival crevicular fluid (GCF) is a useful, noninvasive method for investigating changes in the levels of signal molecules, especially in vivo studies.9 The aims of this clinical study were to investigate the levels of IL-1β in GCF after the application of vibratory stimuli combined with orthodontic force.
MATERIALS AND METHODS
Subjects and Treatment
This study was approved by the ethics committee of the Faculty of Dentistry at Prince of Songkla University (0521.1.03/600). Fifteen patients (11 females, 4 males) aged 19–25 years were randomly selected from the Orthodontic Clinic, Dental Hospital, Faculty of Dentistry, Prince of Songkla University; all patients had been indicated for maxillary first premolar extraction and bilateral maxillary canine distalization and demonstrated good oral hygiene, with probing depth values ≤3 mm. This study was a split-mouth design; the experimental side was allocated by randomization. All patients received repeated oral hygiene instructions appropriate for orthodontic patients.
Each patient was fitted with Roth's prescription preadjusted edgewise brackets (3M Gemini brackets; 3M Unitek Corporation, Monrovia, Calif) with 0.022-inch slots on the maxillary canines and posterior teeth 1 month after first premolar extraction. The stainless-steel wire segments were engaged passively to the buccal tubes bonded to the maxillary first and second molars and brackets on the second premolars and left in situ for 1 month before commencing canine retraction.
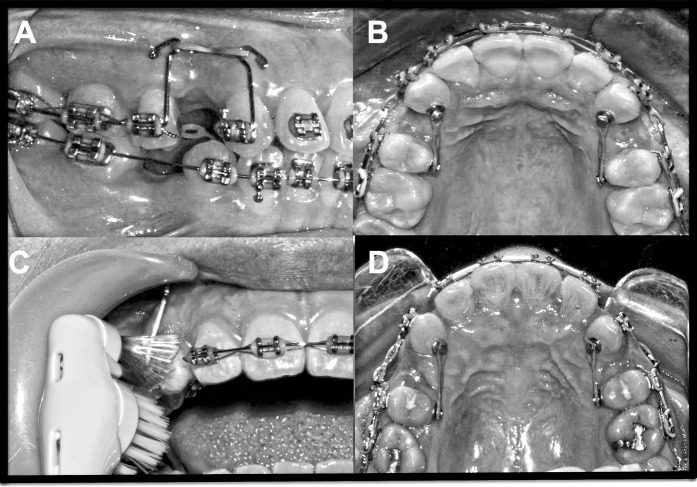
The mechanism for canine distalization consisted of a power arm fabricated from 0.021 × 0.025-inch stainless-steel archwire attached to the mesial end of each canine bracket, with the height of the hook as previously described.10 Retraction force was applied on the buccal and palatal side by attaching an elastomeric chain (Alastik™; 3M Unitek Corporation). Both the labial and palatal chains were adjusted to generate an approximately equal net force of 60 g, as determined using a force gauge (Figure 1). The same force was reapplied at the start of the second and third months (labeled T2 and T3).
Figure 1.
(A, B) Illustration of the mechanics employed for canine distalization; 60 g force was applied to both the control and experimental canines. (C) Application of vibratory stimuli to the canine on the experimental side using an electric toothbrush. (D) Images of the experimental (left) and control (right) sides in a representative case at T3.
Vibratory Stimuli
After the first month of retraction (T1), the right or left canine was randomly selected (by the trial supervisor) as the experimental tooth for additional stimulation with a battery-powered Colgate® Motion-Multi Action electric toothbrush with a rotating and vibrating (125 Hz) head. The patients were instructed not to clean their teeth with the electric toothbrush; rather, they were instructed to hold the toothbrush to apply mechanical vibration on the mesio-labial surface of the experimental canine for a minimum of 5 minutes three times a day for 2 months. A normal orthodontic cleaning routine was recommended for the entire mouth. The participants were requested to report the duration of use and were scheduled to visit once a month, during which time the elastic chains were replaced and batteries were provided for the electric toothbrush.
GCF Collection and Gingival Assessment
The plaque index11 and gingival index12 were recorded before collection of GCF.13 GCF was collected from the distal and mesial sides of the maxillary canine of each quadrant. The site was isolated using a lip retractor, any supragingival plaque was removed without invasion of the gingival margin using a cotton bud, the cervical area was gently dried using an air syringe, and GCF was collected using a number 30 standardized sterile absorbent paper point (Hygenic®; Coltene/Whaledent Inc, Langena, Germany), as previously described.13 GCF was collected five times from each individual: before bracket placement (baseline), before starting canine retraction (T0), after canine retraction for 1 month (T1), after canine retraction for 2 months with and without vibration (T2), and after canine retraction for 3 months with and without vibration (T3).
The paper points were transferred to sterile tubes and the volume of GCF was determined immediately by weighing, based on the difference in the weight of the paper point before and after GCF collection and assuming a GCF density value of 1. The GCF absorbed on each paper point was diluted with 250 µL of sterile phosphate-buffered saline (pH 7.4), centrifuged (13,000 g at 4°C for 15 minutes), and stored at −80°C until analysis.14
Analysis of IL-1β Levels
GCF samples were assayed in triplicate to determine the concentration of IL-1β using a commercially available IL-1β enzyme-linked immunosorbent assay kit (Cayman Chemical, Ann Arbor, Mich, USA.). The IL-1β concentration (pg/µL) was calculated by dividing the amount of IL-1β by the GCF volume for each sample.14
Measurement of the Amount of Tooth Movement
A series of models from each subject were used to assess the amount of canine movement relative to the stable landmark of the ipsilateral median end of the third rugae. Each initial model was used for making the palatal plug, with reference wires pointing at the mesial contact of the canines.15 The plug was then transferred to the consecutive models to measure displacement of the mesial contact of the canines relative to the reference wires. One investigator blinded to the experiment measured all models with a digital caliper (Mitutoyo, Mitutoyo Corporation, Kawasaki, Japan) to an accuracy of 0.01 mm.
Statistical Analysis
The amount of tooth movement for all samples was measured twice by the responsible investigator at a 4-week interval. Paired t-tests and Dahlberg's formula were used to assess intraobserver accuracy and reliability. Data analysis was carried out using R software (The Comprehensive R Archive Network, www.r-project.org). The Wilcoxon signed rank test was used to assess the differences in GCF volume between the experimental and control sites at each time point and to compare the levels of IL-1β and amount of tooth movement between the experimental and control sites and also between the compression and tension sites of each group. P < .05 was considered statistically significant.
RESULTS
Plaque accumulation was minimal, and no signs or symptoms of periodontal destruction were observed in any subject throughout the study. No subject reported discomfort as a result of using the electric toothbrush. Dahlberg's error was less than 0.5, indicating good intraobserver reliability, and paired t-tests indicated the two series of replicate measurements by the same investigator were not significantly different (P > .05).
GCF Volume
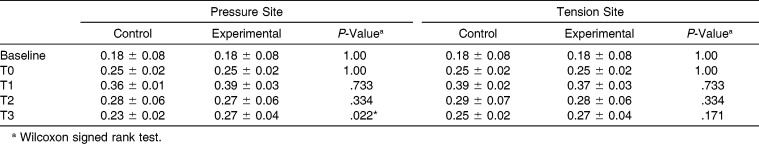
The patterns of fluctuation in the volumes of GCF collected were similar at the tension and pressure sites of control and experimental teeth. The lowest volumes were collected at baseline, then the volumes increased after bracketing (T0) and continued to increase and peaked at approximately two times the initial volume after force had been applied (T1). After T1, the volumes began to reduce toward the levels at T0; the volumes obtained at T2 and T3 were similar to the volumes collected at T0. The volumes were not significantly different between control and experimental teeth, except at the pressure site after vibration for 2 months (T3; P = .022; Table 1).
Table 1.
Means (± standard deviation [SD]) GCF Volumes (µL) at the Pressure and Tension Sites of the Control and Experimental Sides (µL)
IL-1β Concentration
At the pressure site of control teeth, the IL-1β level increased after 1 month of canine distalization (T1) compared to baseline and then subsequently decreased toward the level at T0. However, after 1 month of vibration, the IL-1β level for experimental teeth did not reduce at T2 and remained similar to the levels observed at T1 and then elevated markedly after vibration had been applied for another month (T3), to approximately three times the level observed at T1 and T2. Furthermore, the IL-1β level at the pressure site was significantly higher for experimental teeth than for control teeth (T2; P = .001, T3; P < .001, respectively; Figure 2A).
Figure 2.
Mean (± standard deviation [SD]) IL-1β concentrations at the pressure sites (A) and tension sites (B) of the control and experimental sides (* Significant difference; Wilcoxon signed rank test).
Conversely, no fluctuations in the IL-1β level were observed at the tension site of control teeth, nor were there any significant differences in the level of IL-1β between baseline and T1 at the tension site of experimental teeth. However, the levels of IL-1β at the tension site of experimental teeth increased significantly after vibratory stimulation had been applied for 1 month (T2) and 2 months (T3; Figure 2B). A significant difference between the levels of IL-1β at the tension sites of control and experimental teeth was only observed at T3.
In addition, the IL-1β levels of experimental teeth were significantly higher at the pressure site than at the tension site after retraction (T1; P < .001) and after 1 month of vibratory stimuli (T2; P = .023, T3; P = .001; Figure 3A). For control teeth, the IL-1β levels were significantly higher at the pressure site than at the tension site after retraction (T1; P < .001; Figure 3B).
Figure 3.
Mean (± standard deviation [SD]) IL-1β concentrations of the control side (A) and the experimental side (B) at the pressure sites and tension sites (* Significant difference; Wilcoxon signed rank test).
Amount of Tooth Movement
During the first month of canine distalization (T0-T1), the amount of canine movement was equal for control and experimental teeth. The amount of canine movement was higher for experimental teeth at T2 and T3 compared to control teeth at T2 and T3, respectively (both P < .001; Figure 4). After the first month of retraction in combination with vibratory stimulation (T2) the amount of movement for experimental teeth had doubled. In the second month of retraction with vibration (T2-T3) the amount of movement for experimental teeth reduced when compared to T2-T1 but remained higher than the amount of tooth movement for control teeth.
Figure 4.
![Figure 4. Mean (± standard deviation [SD]) amount of canine movement on the control and experimental sides (* Significant difference; Wilcoxon signed rank test).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/63c3/8603964/5dbd0c21ef0f/i0003-3219-86-1-74-f04.jpg)
Mean (± standard deviation [SD]) amount of canine movement on the control and experimental sides (* Significant difference; Wilcoxon signed rank test).
DISCUSSION
IL-1β is directly involved in bone resorption, as it induces expression of RANKL in osteoblasts and human PDL cells and also directly stimulates osteoclast precursor differentiation.8,16 Studies17–19 in humans have demonstrated that the level of IL-1β in GCF elevates significantly during orthodontic tooth movement.
Vibration has been used to increase the rate of orthodontic tooth movement by accelerating periodontal and alveolar bone remodeling. Here, we investigated the effect of vibratory stimuli provided by an electric toothbrush on the level of IL-1β in GCF. The GCF volumes at each time point were not significantly different between the experimental and control sites, in agreement with the findings of Drummond et al.,20 who showed that GCF volume is not a reliable biomarker of tissue remodeling during orthodontic treatment. However, in this study, the levels of IL-1β in GCF increased after application of vibratory stimuli combined with orthodontic force, even though the GCF volumes did not vary significantly between control and experimental teeth at any time point. Therefore, vibratory stimuli can alter the concentration of GCF components.
The greater amount of movement observed at the experimental site could be due to the effects of increased IL-1β secretion as a result of vibratory stimuli. Application of vibratory stimuli for 2 months markedly elevated the average IL-1β level by approximately threefold compared to orthodontic force alone, whereas the IL-1β levels for the control teeth decreased during the second and third months of retraction (T2 and T3). These observations indicate the presence of a biological reaction to additional stimuli in addition to the application of conventional orthodontic force and suggest a temporary limitation of the tissues to adapt to further movement. Moreover, the cyclical nature of tooth movement reflects periods of periodontal ligament and alveolar bone remodeling.21 Nevertheless, the deceleration in the reduction in the IL-1β level observed for the control teeth suggests that restoration of the IL-1β levels in response to vibration may be due to a lack of force consistency.22
Interestingly, the control canine could be moved with 60 g force, and even by the degrading force of the chain elastic. This indicates that very light force can effectively move a free-standing tooth when applied with the same mechanics described in a previous study;10 the Regional Accelerated Phenomenon at the extraction site may also affect canine movement.23 Unexpected distal tipping of both experimental and control teeth was observed; however, the extent of tipping was low. Therefore, the increased levels of IL-1β secretion on the tension sides of the moving canine at T2 and T3 could be due to bone resorption activity in some areas of the tension side. However, the levels of IL-1β remained significantly higher on the pressure side than on the tension side at T2 and T3 (P = .023 and P = .001, respectively).
Although elevated levels of IL-1β have been related to the severity of periodontal disease, the increased concentrations of IL-1β observed in this study at T2 and T3 (0.18 pg/µL and 0.64 pg/µL, respectively) are markedly below those of patients with gingivitis and periodontitis.24 Therefore, the increased IL-1β levels measured in this study may still be within the limits of an acceptable physiological response. Nevertheless, further increased accumulation of IL-1β may occur after continuous application of mechanical vibration over the course of more than 2 months of canine retraction, and this possibility should be carefully considered before vibratory stimuli is adopted as a routine intervention in clinical practice. Most subjects in this study used the electric toothbrush as instructed, and all subjects reported that the electric toothbrush was comfortable and practical to use. Therefore, the electric toothbrush may have potential as a simple method of applying vibratory stimuli to accelerate tooth movement and reduce overall treatment time.
GCF was analyzed in this study to evaluate tissue responses without damaging the periodontium;9 however, this clinical study did not address the complex cell-to-cell interactions that occur in the periodontium in response to vibratory stimuli in combination with orthodontic force. Nevertheless, this study suggests vibratory stimuli could be a fruitful area for further research on accelerated tooth movement. Before clinical application of vibratory stimuli can be recommended, the pathways of mechanotransduction should be clarified in order to understand the biological events that occur in response to vibratory stimuli and to define the optimal period for vibratory stimulation during orthodontic treatment. In the meantime, a long-term clinical study with a larger sample size is warranted.
CONCLUSIONS
Production of IL-1β during orthodontic tooth movement can be measured by GCF analysis.
In combination with orthodontic force, vibratory stimuli enhanced secretion of IL-1β in GCF and appeared to increase bone resorption activity and accelerate tooth movement.
An electric toothbrush can be used to provide vibratory stimuli, and no pathological effects were noted after application of such vibratory stimuli to a single tooth for a short period of time.
ACKNOWLEDGMENTS
We would like to thank Dr Keith Godfrey for critical comments, Miss Parichart Dumthongsuk for statistical advice, and the Graduate School, Prince of Songkla University, for grant support.
REFERENCES
- 1.Nishimura M, Chiba M, Ohashi T, et al. Periodontal tissue activation by vibration: intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am J Orthod Dentofacial Orthop. 2008;133:572–583. doi: 10.1016/j.ajodo.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Li J, Zhang L, et al. Effects of mechanical vibration on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Arch Oral Biol. 2012;57:1395–1407. doi: 10.1016/j.archoralbio.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Miles P, Smith H, Weyant R, Rinchuse DJ. The effect of a vibration appliance on tooth movement and patient discomfort: a prospective randomized clinical trial. Aust Orthod J. 2012;28:213–218. [PubMed] [Google Scholar]
- 4.Kau CH, Nguyen JT, English JD. The clinical evaluation of a novel cyclical force generating device in orthodontics. Orthod Pract. 2010;1:1–4. [Google Scholar]
- 5.Kau Chung H. A radiographic analysis of tooth morphology following the use of a novel cyclical force device in orthodontics. Head Face Med. 2011;7:1–5. doi: 10.1186/1746-160X-7-14. doi:10.1186/1746-160X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka M, Ejiri S, Toyooka E, Kohno S, Ozawa H. Effects of ovariectomy on trabecular structures of rat alveolar bone. J Periodont Res. 2002;37:161–165. doi: 10.1034/j.1600-0765.2002.01601.x. [DOI] [PubMed] [Google Scholar]
- 7.Davidovitch Z, Nicolay OF, Ngan PW, Shanfeld JL. Neurotransmitters, cytokines, and the control of alveolar bone remodeling in orthodontics. Dent Clin North Am. 1988;32:411–435. [PubMed] [Google Scholar]
- 8.Teixeira CC, Khoo E, Tran J, et al. Cytokine expression and accelerated tooth movement. J Dent Res. 2010;89:1135–1141. doi: 10.1177/0022034510373764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grieve WG, Johnson GK, Moore RN, Reinhardt RA. Prostaglandin E (PGE) and interleukin-1b (IL-1b) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1994;105:369–374. doi: 10.1016/s0889-5406(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 10.Leethanakul C, Kanokkulchai S, Pongpanich S, Leepong N, Charoemratrote C. Interseptal bone reduction on the rate of maxillary canine retraction. Angle Orthod. 2014;84:839–845. doi: 10.2319/100613-737.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Leary TJ, Drake R, Naylor JE. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 13.Leethanakul C, Kittichaikarn C, Charoemratrote C, Jitpukdeebodintra S. Effects of continuous and interrupted orthodontic force on interleukin1-beta and interleukin-8 secretion in human gingival crevicular fluid. J Oral Biosci. 2008;50:230–238. [Google Scholar]
- 14.Paolantonio M, Di Placido G, Tumini V, Di Stilio M, Contento A, Spoto G. Aspartate aminotransferase activity in crevicular fluid from dental implants. J Periodontol. 2000;71:1151–1157. doi: 10.1902/jop.2000.71.7.1151. [DOI] [PubMed] [Google Scholar]
- 15.Limpanichkul W, Godfrey K, Srisuk N, Rattanayatikul C. Effect of low-level laser therapy on the rate of orthodontic tooth movement. Orthod Craniofac Res. 2006;9:38–43. doi: 10.1111/j.1601-6343.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- 16.Saito M, Saito S, Ngan PW, Shanfeld J, Davidovitch Z. Interleukin 1 beta and prostaglandin E are involved in the response of periodontal cells to mechanical stress in vivo and in vitro. Am J Orthod Dentofacial Orthop. 1991;99:226–240. doi: 10.1016/0889-5406(91)70005-H. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki LR, Gibson CS, Crouch LD, Marx DB, Pandey JP, Nickel JC. Speed of tooth movement is related to stress and IL-1 gene polymorphisms. Am J Orthod Dentofacial Orthop. 2006;130:698.e1–698.e9. doi: 10.1016/j.ajodo.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Ren Y, Hazemeijer H, de Haan B, Qu N, de Vos P. Cytokine profiles in crevicular fluid during orthodontic tooth movement of short and long durations. J Periodontol. 2007;78:453–458. doi: 10.1902/jop.2007.060261. [DOI] [PubMed] [Google Scholar]
- 19.Ren Y, Vissink A. Cytokines in crevicular fluid and orthodontic tooth movement. Eur J Oral Sci. 2008;116:89–97. doi: 10.1111/j.1600-0722.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 20.Drummond S, Canavarro C, Perinetti G, Teles R, Capelli J., Jr The monitoring of gingival crevicular fluid volume during orthodontic treatment: a longitudinal randomized split-mouth study. Eur J Orthod. 2012;34:109–113. doi: 10.1093/ejo/cjq172. [DOI] [PubMed] [Google Scholar]
- 21.Proffit WR, Fields HW, Sarver DM. Contemporary Orthodontics 4th ed. St Louis, Mo: Mosby; 2007. pp. 331–342. [Google Scholar]
- 22.Lee KJ, Park YC, Yu HS, Choi SH, Yoo YJ. Effects of continuous and interrupted orthodontic force on interleukin-1beta and prostaglandin E2 production in gingival crevicular fluid. Am J Orthod Dentofacial Orthop. 2004;125:168–177. doi: 10.1016/j.ajodo.2003.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Yaffe A, Fine N, Binderman I. Regional accelerated phenomenon in the mandible following mucoperiosteal flap surgery. J Periodontol. 1994;65:79–83. doi: 10.1902/jop.1994.65.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Figueredo CM, Ribeiro MS, Fischer RG, Gustafsson A. Increased interleukin-1beta concentration in gingival crevicular fluid as a characteristic of periodontitis. J Periodontol. 1999;70:1457–1463. doi: 10.1902/jop.1999.70.12.1457. [DOI] [PubMed] [Google Scholar]



![Figure 2. Mean (± standard deviation [SD]) IL-1β concentrations at the pressure sites (A) and tension sites (B) of the control and experimental sides (* Significant difference; Wilcoxon signed rank test).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/63c3/8603964/b39cab179b94/i0003-3219-86-1-74-f02.jpg)
![Figure 3. Mean (± standard deviation [SD]) IL-1β concentrations of the control side (A) and the experimental side (B) at the pressure sites and tension sites (* Significant difference; Wilcoxon signed rank test).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/63c3/8603964/6b363f4fd5b2/i0003-3219-86-1-74-f03.jpg)