Summary:
Aplasia Cutis Congenita (ACC) in the scalp is a rare congenital malformation. The treatment for ACC with large defects involving the scalp, bone, and the dura is challenging. Local debridement of necrotic tissue is important to prevent lethal complications such as infection and meningitis. However, debridement has the risk of damaging the sagittal sinus or the dura. Recent developments in ultra-high-frequency ultrasound(US) systems provide frequencies of 70 MHz and capability resolution as fine as 30 μm, which could allow precise imaging of small and thin anatomical structures. The study aimed to describe the methods of precise evaluation of the defect in the scalp and safe debridement using ultra-high-frequency US. This is the first report on direct observation of a newborn’s brain using ultra-high-frequency US. The boy was delivered spontaneously with a large defect of the scalp and bone. After 14 days, due to signs of infection, local debridement was performed carefully under ultra-high-frequency US-based evaluation. The dura, the sagittal sinus, and the small anatomical structures such as arachnoid granulations could be observed. Because the brain herniation gradually aggravated, dural reconstruction using fascia lata and scalp reconstruction using transposition flap was performed. Finally, good skin coverage over the defects was obtained. This method minimizes the risk of damaging the sagittal sinus and the brain parenchyma, which may cause fatal complications. Although further clinical investigations will be required to confirm its efficacy, ultra-high-frequency US has the potential to be a useful device for ACC treatment.
INTRODUCTION
Aplasia Cutis Congenita (ACC) in the scalp is a rare congenital malformation that was first described by Campbell.1 ACC may be associated with other malformations, including genetic disorder such as Adams-Oliver syndrome.2 ACC commonly includes a localized absence of skin on the scalp. Bone defects are present in about 20% of the cases, and dura defects are also found much less frequently.3–5 ACC may result in lethal complications such as sagittal sinus hemorrhage, infection, and meningitis.
The management of ACC includes surgical and conservative treatment. Some articles recommended moist dressings and early skin coverage with surgical procedures because lethal hemorrhage from the sagittal sinus may occur.3,5,6 The other studies reported that conservative treatment is the preferred initial treatment.7,8 The treatment for ACC with large defects involving the scalp, bone, and the dura is still controversial. Local debridement of necrotic tissue is important to prevent lethal complications such as infection and meningitis. However, debridement has the risk of damaging the sagittal sinus or the dura.
Recent developments in ultra-high-frequency ultrasound (US) systems provide frequencies of 70 MHz and capability resolution as fine as 30 μm, which could allow more precise imaging of small and thin anatomical structures than conventional US.9–12
Here, we report the case of a successful treatment for ACC with Adams-Oliver syndrome where a precise evaluation of the defect in the scalp and safe debridement were achieved using ultra-high-frequency US.
PATIENT AND METHODS
The boy was delivered spontaneously with an 8 × 5 cm round defect of the scalp and bone covered with necrotic tissue (Fig. 1). Bilateral hypoplasia and partial syndactyly of the toes indicated the presence of Adams-Oliver syndrome. Initially, the defects were covered with a dressing to keep the wound moist to avoid infection. After 14 days, however, elevated leukocyte counts and C-reactive protein indicated bacterial infection. Then, local debridement was performed carefully under ultra-high-frequency US-based evaluation. Ultra-high-frequency US was performed with Vevo MD ultrasound device (FUJIFILM VisualSonics, Amsterdam, the Netherlands) using a 70-MHz linear array transducer. The presence of the dura and the depth of the brain parenchyma could be evaluated using ultra-high-frequency US through the extensive defects that involve the skull (Figs. 2, 3). The sagittal sinus could also be observed with the US, and the surgeon could take care not to damage it during debridement (Fig. 3). The defect of dura could be observed and brain herniation was expected to occur (Figs. 3, 4). Because the brain herniation gradually aggravated with signs of infection, reconstruction using scalp transposition flap was planned at the age of 17 weeks (See figure 1, Supplemental Digital Content 1, which displays a: the preoperative view. The brain herniation gradually aggravated. b: Intraoperative view after the resection of the protruded brain. c: The dura was closed using the fascia lata. d: The scalp transposition flap was elevated and transferred to the defect. The donor site was covered with a full-thickness skin graft from the thigh. http://links.lww.com/PRSGO/B809.)
Fig. 1.
The infant was born with an 8 × 5 cm round defect of the scalp and bone covered with necrotic tissue.
Fig. 2.
The view of the defect at the age of 8 weeks. Blue box: the site of the sagittal sinus. The US findings are shown in Figure 3A. Green box: the area of defect of the dura. The US findings are shown in Figure 3B. Yellow arrow: protruded brain.
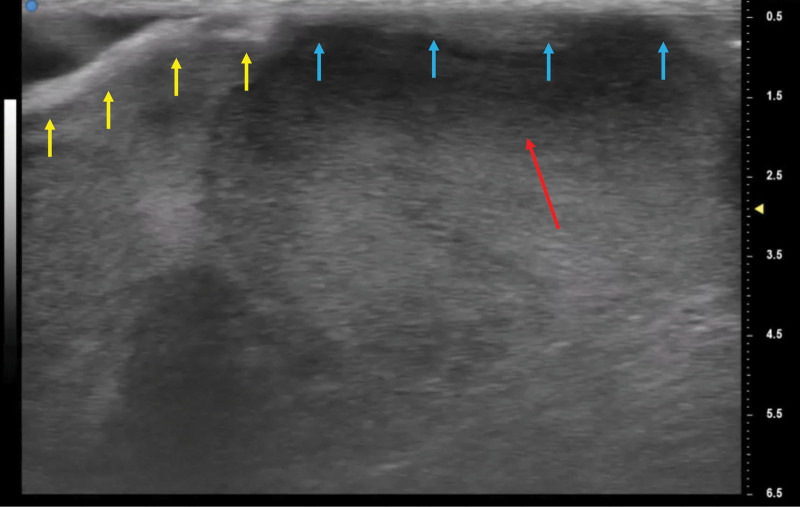
Fig. 3.
A, The US finding of blue box area of Figure 2. Ultra-high-frequency US showed a clear image of the dura (yellow arrow), sagittal sinus (blue arrow), subarachnoid space (orange arrow), arachnoid granulations (green arrow), pia mater (pink arrow), and brain parenchyma (red arrow). B, The ultra-high-frequency US finding of green box area of Figure 2. The brain is herniated from the defect of the dura. Yellow arrow: the dura; red arrow: brain parenchyma.
Fig. 4.
The defect of dura could be observed, and brain herniation was expected to occur. Yellow arrow: the dura; blue arrow: the area of the defect of the dura; red arrow: brain parenchyma.
The neurosurgeon resected the protruded brain, and the dura was closed using the fascia lata (SDC 1, http://links.lww.com/PRSGO/B809). The scalp transposition flap was elevated and transferred to the defect. The donor site was covered with a full-thickness skin graft from the thigh (SDC 1, http://links.lww.com/PRSGO/B809). Although partial flap necrosis occurred, the flap and skin graft healed well, providing good skin coverage over the defects. The infant was in stable condition over a follow-up of 2 months. (See figure 2, Supplemental Digital Content 2, which displays the postoperative view at 2 months after the surgery. http://links.lww.com/PRSGO/B810.)
DISCUSSION
This is the first report to show the feasibility of using ultra-high-frequency US to visualize the superficial brain structure of the ACC patient. The high-resolution imaging of the superficial brain structure enables safe debridement and evaluation of the scalp defect for treatment of ACC.
The treatment for the ACC involving the skull and dura is challenging. The goal of ACC treatment is to achieve complete closure of the defect avoiding fatal complications such as hemorrhages and infection. 4There is no consensus about the treatment for ACC with a bony defect.4 Although some reports recommend conservative treatment,13–15 there are also reports of deaths due to massive hemorrhage from the sagittal sinus in the patients with ACC that was being treated conservatively.16,17 A report described brain herniation after disruption of the leptomeningeal membrane in the Adams-Oliver syndrome.18 In this case, we initially performed conservative treatment. After signs of infection were observed, safe debridement was performed using ultra-high-frequency US. We confirmed the location and depth of the sagittal sinus, assessed the risk of hemorrhage, and confirmed the defect of the dura (Fig. 3). When the area around the defect of the dura became epithelialized, dural reconstruction using a fascia lata and local flap closure was performed and successfully closed.
Ultra-high-frequency US is a novel device that was originally developed for experimental studies using small animals.19,20 In recent years, this novel device has been approved for use in humans.21,22 With a discrimination power of 30 µm, ultra-high-frequency US could permit precise detection and enhanced visualization of superficial or small-size anatomical structures.23 Our experience has shown that it is possible to visualize the surface layers of the brain using this device in the ACC patient. We could observe not only the dura and the sagittal sinus, but also the small anatomical structures such as arachnoid, subarachnoid space, arachnoid trabecula, arachnoid granulations, superior cerebral artery and vein, and pia mater. The visualization of these small anatomical structures may lead to the elucidation of diseases caused by these structures. This technique could also be applied to the evaluation of meningocele.
The use of ultra-high-frequency US for ACC patients confers several advantages. First, we can know the exact location and depth of the sagittal sinus. This method is advantageous in that it minimizes the risk of damaging the sagittal sinus, which may cause fatal complications. Second, because this device makes it possible to visualize the dura, we can evaluate the presence of a dural defect. This reduces the risk of damage to the brain parenchyma and also allows us to assess possible areas of brain herniation. One of the disadvantages of ultra-high-frequency US is the limited reach of US. The deepest layer from which the device can obtain clear images is 10 mm from the superficial surface. However, this reach of US is enough for the evaluation of ACC.
Although further clinical investigations will be required to confirm its efficacy, ultra-high-frequency US has the potential to be a useful device for ACC treatment.
Supplementary Material
Footnotes
Published online 14 October 2021.
Disclosure: The authors have no financial interest in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Campbell W. Case of congenital ulcer on the cranium of a fetus, terminating in fatal hemorrhage, on 18th day after birth. J Med Sci (Edinburgh) 1826. 1826;(2):82–84. [Google Scholar]
- 2.Schnabl SM, Horch RE, Ganslandt O, et al. Aplasia cutis congenital—plastic reconstruction of three scalp and skull defects with two opposed scalp rotation flaps and split thickness skin grafting. Neuropediatrics. 2009;40:134–136. [DOI] [PubMed] [Google Scholar]
- 3.Bajpai M, Pal K. Aplasia cutis cerebri with partial acrania—total reconstruction in a severe case and review of the literature. J Pediatr Surg. 2003;38:e4. [DOI] [PubMed] [Google Scholar]
- 4.Ribuffo D, Costantini M, Gullo P, et al. Aplasia cutis congenita of the scalp, the skull, and the dura. Scand J Plast Reconstr Surg Hand Surg. 2003;37:176–180. [DOI] [PubMed] [Google Scholar]
- 5.Theile RJ, Lanigan MW, McDermant GR. Reconstruction of aplasia cutis congenita of the scalp by split rib cranioplasty and a free latissimus dorsi muscle flap in a nine month old infant. Br J Plast Surg. 1995;48:507–510. [DOI] [PubMed] [Google Scholar]
- 6.Verhelle NA, Heymans O, Deleuze JP, et al. Abdominal aplasia cutis congenita: case report and review of the literature. J Pediatr Surg. 2004;39:237–239. [DOI] [PubMed] [Google Scholar]
- 7.Santos de Oliveira R, Barros Jucá CE, Lopes Lins-Neto A, et al. Aplasia cutis congenita of the scalp: is there a better treatment strategy? Childs Nerv Syst. 2006;22:1072–1079. [DOI] [PubMed] [Google Scholar]
- 8.Rhee ST, Colville C, Buchman SR, et al. Complete osseous regeneration of a large skull defect in a patient with cutis aplasia: a conservative approach. J Craniofac Surg. 2002;13:497–500. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi A, Giacalone G, Yamamoto T, et al. Ultra high-frequency ultrasonographic imaging with 70 MHz scanner for visualization of the lymphatic vessels. Plast Reconstr Surg Glob Open. 2019;7:e2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimatsu H, Karakawa R, Fuse Y, Okada A, Hayashi A, Yano T. Use of preoperative high-resolution ultrasound system to facilitate elevation of the superficial circumflex iliac artery perforator flap [published online ahead of print April 14, 2021]. J Reconstr Microsurg. [DOI] [PubMed] [Google Scholar]
- 11.Visconti G, Bianchi A, Hayashi A, et al. Thin and superthin perforator flap elevation based on preoperative planning with ultrahigh-frequency ultrasound. Arch Plast Surg. 2020;47:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimatsu H, Hayashi A, Yamamoto T, et al. Visualization of the “intradermal plexus” using ultrasonography in the dermis flap: a step beyond perforator flaps. Plast Reconstr Surg Glob Open. 2019;7:e2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muakkassa KF, King RB, Stark DB. Nonsurgical approach to congenital scalp and skull defects. J Neurosurg. 1982;56:711–715. [DOI] [PubMed] [Google Scholar]
- 14.Six EG, Kelly DL, Jr. Conservative management of aplasia cutis congenita: case report. Neurosurgery. 1981;8:233–235. [DOI] [PubMed] [Google Scholar]
- 15.Wexler A, Harris M, Lesavoy M. Conservative treatment of cutis aplasia. Plast Reconstr Surg. 1990;86:1066–1071. [DOI] [PubMed] [Google Scholar]
- 16.Glasson DW, Duncan GM. Aplasia cutis congenita of the scalp: delayed closure complicated by massive hemorrhage. Plast Reconstr Surg. 1985;75:423–425. [DOI] [PubMed] [Google Scholar]
- 17.Ross DA, Laurie SW, Coombs CJ, et al. Aplasia cutis congenita: failed conservative treatment. Plast Reconstr Surg. 1995;95:124–129. [DOI] [PubMed] [Google Scholar]
- 18.Tröbs RB, Barenberg K, Hemminghaus M, et al. Herniation of the brain after conservative treatment of a large congenital skull defect in an infant with Adams-Oliver syndrome. J Pediatr Surg. 2010;45:2064–2067. [DOI] [PubMed] [Google Scholar]
- 19.Gan LM, Grönros J, Hägg U, et al. Non-invasive real-time imaging of atherosclerosis in mice using ultrasound biomicroscopy. Atherosclerosis. 2007;190:313–320. [DOI] [PubMed] [Google Scholar]
- 20.Grassi R, Cavaliere C, Cozzolino S, et al. Small animal imaging facility: new perspectives for the radiologist. Radiol Med. 2009;114:152–167. [DOI] [PubMed] [Google Scholar]
- 21.Belfiore MP, Berritto D, Iacobellis F, et al. A longitudinal study on BIO14.6 hamsters with dilated cardiomyopathy: micro-echocardiographic evaluation. Cardiovasc Ultrasound. 2011;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osika W, Dangardt F, Montgomery SM, et al. Sex dif`ferences in peripheral artery intima, media and intima media thickness in children and adolescents. Atherosclerosis. 2009;203:172–177. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi A, Visconti G, Hayashi A, et al. Ultra-High frequency ultrasound imaging of lymphatic channels correlates with their histological features: a step forward in lymphatic surgery. J Plast Reconstr Aesthet Surg. 2020;73:1622–1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.