Summary
New knowledge about the neural aspects of cough has revealed a complex network of pathways that initiate cough. The effect of inflammation on cough neural processing occurs at multiple peripheral and central sites within the nervous system. Evidence exists that direct or indirect neuroimmune interaction induces a complex response, which can be altered by mediators released by the sensory or parasympathetic neurons and vice versa. The aim of this study was to clarify changes of cough reflex sensitivity – the activity of airway afferent nerve endings - in asthmatic children. 25 children with asthma and 15 controls were submitted to cough reflex sensitivity measurement - capsaicin aerosol in doubling concentrations (from 0.61 to 1250 μmol/l) was inhaled by a single breath method. Concentrations of capsaicin causing two (C2) and five coughs (C5) were reported. Asthmatic children’ (11 boys and 14 girls, mean age 9 ± 1 yrs) cough reflex sensitivity (geometric mean, with the 95 % CI) for C2 was 4.25 (2.25–8.03) μmol/l vs. control C2 (6 boys and 9 girls, mean age 8 ± 1 yrs) was 10.61 (5.28–21.32) μmol/l (p=0.024). Asthmatic children’ C5 was 100.27 (49.30–203.93) μmol/l vs. control C5 56.53 (19.69–162.35) μmol/l (p=0.348). There was a statistically significant decrease of C2 (cough threshold) in the asthmatic patients relative to controls (p-value for the two-sample t-test of log(C2) for the one-sided alternative, p-value = 0.024). The 95 % confidence interval for the difference of the mean C2 in asthma vs. control, [1.004, 6.207]. For C5, the difference was not statistically significant (p-value = 0.348). There was a statistically significant decrease of cough reflex sensitivity (the activity of airway afferent nerve endings) - C2 value in the asthmatic children relative to controls.
Keywords: Cough, Asthma, Cough sensitivity, Children
Introduction
The neurophysiology of cough has been changed and new clinical etiologies have been presented during the last few years. Specifically, cough hypersensitivity in adults and protracted bacterial bronchitis (PBB) in children have been increasingly investigated, and differences between chronic cough in children and adults have been widely reported. In young children, postinfectious cough, bronchiectasis, airway malacia, PBB, and asthma appear to be the main causes of cough; however, by adolescence, the causes of cough are more likely to become those common in adults, namely, gastroesophageal reflux, asthma, and upper airway syndrome. These differences are attributed to changes in various characteristics of the respiratory tract, immune system, and nervous system between children and adults (Kantar and Seminara 2019). Cough is a troublesome and often refractory symptom of asthma, which is associated with poor control of the disease. The pathogenesis of asthmatic cough has mainly been attributed to bronchoconstriction, but recent evidence indicates that cough reflex hypersensitivity or neuronal dysfunction is a feature of asthma, even in those with mild stable disease (Niimi et al. 2019). The aim of this study was to clarify changes of cough reflex sensitivity (CRS) – the activity of airway afferent nerve endings - in asthmatic children.
Methods
Children were referred to National Institute of Pediatric Tuberculosis and Respiratory Diseases, Dolny Smokovec, Slovak Republic by their pediatric pulmonologist. Asthma was defined by a complaint of wheezing, cough, dyspnea or chest tightness at rest or on exercise and a positive response to exercise challenge. Children had no respiratory symptoms for at least 4 weeks and baseline FEV1 was larger than 80 %. Bronchodilator treatment was discontinued 72 hours before being examined.
All subjects underwent personal and family history taking, physical examination and initial screening of their basic lung functions measured by spirometry before and after capsaicin challenge (KoKo DigiDoser-Spirometer; nSpire health Inc., Louisville, CO, USA).
This prospective clinical study was approved by the institutional Research Ethics Committee and was performed according to the Declaration of Helsinki. Each parent of the observed child was properly informed about the study, about the cough reflex sensitivity and was asked to sign an informed consent.
CRS was assessed using capsaicin cough challenge, performed in agreement with the ERS guidelines (Morice et al. 2007) with modification for pediatric use (Varechova et al. 2008) (we used a compressed air-driven nebuliser (model 646; DeVilbiss Health Care, Inc., Somerset, PA, USA) controlled by a dosimeter (KoKo DigiDoser-Spirometer; nSpire health Inc., Louisville, CO, USA) with an inspiratory flow regulator valve added (RIFR; nSpire health Inc., Louisville, CO, USA) to assign identical inspiratory flow rate during capsaicin inhalations in all subjects. Each subject inhaled saline randomly interposed among 12 inhalations of incremental capsaicin aerosol concentrations (0.61–1250 μmol/l). Each administration of saline and capsaicin aerosol was performed at 1 min intervals with the inhalation time set at 400 msec. The number of coughs within 30 s after aerosol administration was counted by two independent observers. The end-point of cough challenge was the inhalation of capsaicin concentration that provoked at least 5 coughs (C5) or when the maximum concentration of capsaicin (1250 μmol/l) was achieved. The concentration of capsaicin causing at least two coughs was assigned as C2 and concentration of capsaicin causing at least 5 coughs was assigned as C5. For children that did not cough at any concentration of capsaicin, CRS value was assigned 1250 μmol/l.
Statistical analysis
Obtained parameters of CRS were statistically evaluated. The results were evaluated separately for each individual and subsequently for the group as a whole. The results were expressed as median values, the level of statistical significance was determined as P<0.05 and P<0.01. Wilcoxon test with continuity correction was used. The level of statistical significance was determined as P<0.05). Software used: R Core Team (2015), R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, URL - https://www.R-project.org/, R version 3.2.3, 2015-12-10).
Results
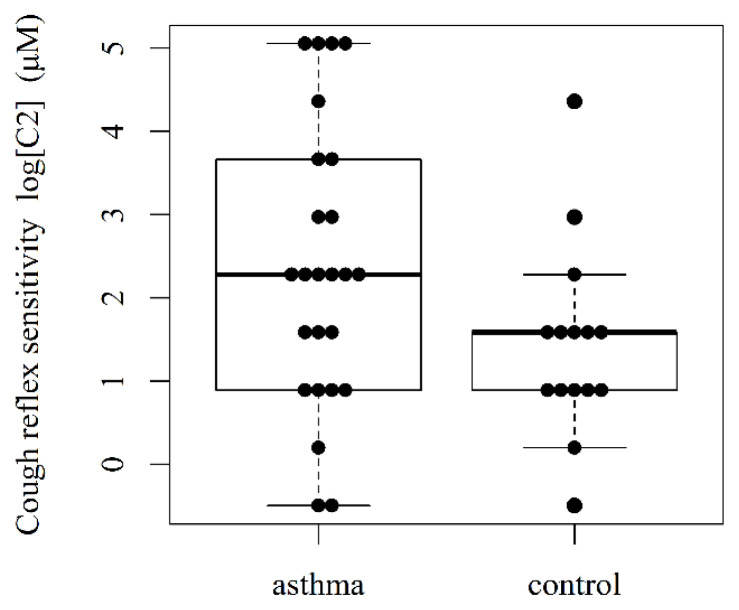
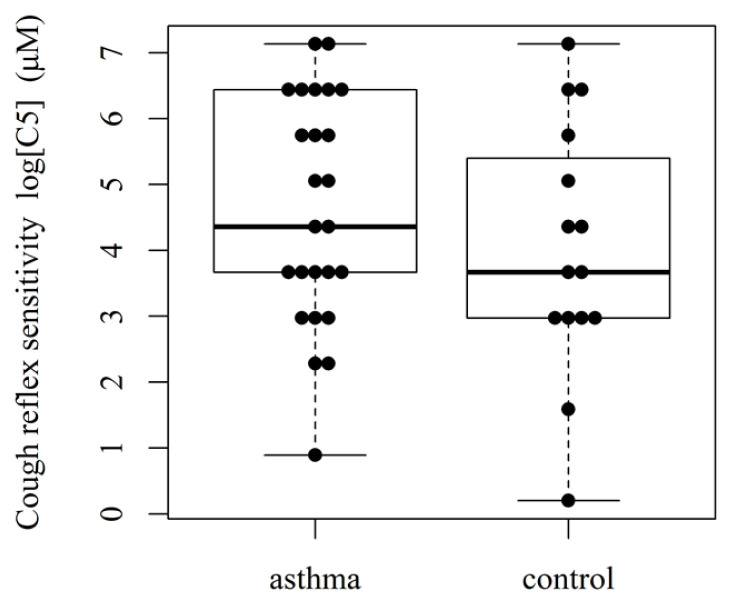
Twenty-five asthmatic children’ (11 boys and 14 girls, mean age 9 ± 1 yrs) and 15 controls (6 boys and 9 girls, mean age 8 ± 1 yrs; presumed unconfirmed diagnosis of asthma) were prospectively recruited into the study. Concentrations of capsaicin causing two (C2) and five coughs (C5) were reported. Cough reflex sensitivity (geometric mean, with the 95 % CI) for C2 was 4.25 (2.25–8.03) μmol/l vs. control C2 was 10.61 (5.28–21.32) μmol/l (p=0.024) (Fig 1). Asthmatic children’ C5 was 100.27 (49.30–203.93) μmol/l vs. control C5 56.53 (19.69–162.35) μmol/l (p=0.348) (Fig. 2). There was a statistically significant decrease of C2 value (cough threshold) in the asthmatic patients relative to controls (p-value for the two-sample t-test of log(C2) for the one-sided alternative, p-value = 0.024). The 95 % confidence interval for the difference of the mean C2 in asthma vs. control, [1.004, 6.207]. For C5, the difference was not statistically significant (p-value = 0.348).
Fig. 1.
Cough reflex sensitivity (C2) in asthmatic children
Fig. 2.
Cough reflex sensitivity (C5) in asthmatic children
Discussion
Our objective in this study was to characterize the change of cough reflex sensitivity – the activity of afferent nerve endings in asthmatic children compared to controls.
New knowledge about the neural aspects of cough has revealed a complex network of pathways that initiate cough. The effect of inflammation on cough neural processing occurs at multiple peripheral and central sites within the nervous system. Evidence exists that direct or indirect neuroimmune interaction induces a complex response, which can be altered by mediators released by the sensory or parasympathetic neurons and vice versa. During childhood, the respiratory tract and the nervous system undergo a series of anatomical and physiological maturation processes that produce the cough neural circuits (Kantar and Seminara 2019).
Recently, a new paradigm, “cough hypersensitivity syndrome,” was proposed (Morice et al. 2012). The main mechanism of cough hypersensitivity syndrome has been suggested to be dysregulated sensory neural pathways and central processing in cough reflex regulation (Chung et al. 2013, Niimi and Chung 2015, Driessen et al. 2017). However, direct evidence for neural dysfunction is lacking because, except for peripheral lung tissues, human neural tissues are very difficult to obtain. Thus, at present, cough hypersensitivity syndrome is still a conceptual entity. However, accumulating evidence supports the notion that neuropathology is the key pathophysiology underlying this syndrome (Song and Morice 2017).
Several peripheral inputs, such as viruses, allergens, and irritants, can induce phenotypic switches in sensory neurons and up-regulate host cough responses. Nociceptive C-fibre neurons, but not Aδ-neurons, typically express TRPV1 and produce neuropeptides, such as substance P or calcitonin gene-related peptide (CGRP); thus, the expression of TRPV1 has been used as a marker for a phenotypic switch in lung sensory neurons. These neuronal phenotypic switches are significantly correlated with cough responses to tussigens, such as capsaicin and citric acid, and have also been associated with increased expression of neurotrophic factor receptors in the neurons (Zaccone et al. 2016). Similar phenotypic changes in sensory neurons and heightened cough responses were observed in guinea pig models of allergic airway inflammation (Undem and Taylor-Clark 2014). These phenotypic changes in peripheral sensory neurons may, in turn, provoke inflammatory responses from immune cells, so-called neurogenic inflammation. Released neuropeptides, such as substance P and CGRP, may induce local vascular dilatation (McCormack et al. 1989), chemotaxis (Numao and Agrawal 1992), immune cell activation, and promote type 2 helper T-cell polarization (Mikami et al. 2011). Such neuro-immune interactions, particularly in cases of repeated sensory inputs (e.g. viruses or allergens), would lead to a vicious cycle of hypersensitive responses (Song and Chang 2015). There is also progress in the identification of various signalling pathways (Hellebrandová et al. 2016) involved in the sensitization of the TRP channels by pro-inflammatory agents (Kádková et al. 2017).
In children with asthma, CRS is heightened in acute severe asthma in the subgroup of children who have cough as a significant symptom with their asthma episodes (Chang et al. 1997). In our study, there was a statistically significant increase of cough reflex sensitivity in the asthmatic patients relative to controls (C2 threshold decreased).
The cough reflex is a vital protective mechanism against aspiration. It relies on a complex vagally mediated neuronal pathway which is still only partially understood. In some disease states (bronchial asthma in our study), both acutely as in a viral respiratory tract infection and chronically, a state of hypersensitivity is created. Our understanding of the mechanism of cough hypersensitivity and the realization that this represents a distinct clinical condition has advanced the field enormously over the past decades.
Acknowledgements
This work was supported by the project of Ministry of Health of the Slovak Republic - 2018/12-UKMT-8
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- CHANG AB, PHELAN PD, ROBERTSON CF. Cough receptor sensitivity in children with acute and non-acute asthma. Thorax. 1997;52:770–774. doi: 10.1136/thx.52.9.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG KF, MCGARVEY L, MAZZONE SB. Chronic cough as a neuropathic disorder. Lancet Respir Med. 2013;1:414–422. doi: 10.1016/S2213-2600(13)70043-2. [DOI] [PubMed] [Google Scholar]
- DRIESSEN AK, McGOVERN AE, NARULA M, YANG SK, KELLER JA, FARRELL MJ, MAZZONE SB. Central mechanisms of airway sensation and cough hypersensitivity. Pulm Pharmacol Ther. 2017;47:9–15. doi: 10.1016/j.pupt.2017.01.010. [DOI] [PubMed] [Google Scholar]
- HELLEBRANDOVÁ L, CHLUMSKÝ J, VOSTATEK P, NOVÁK D, RÝZNAROVÁ Z, BUNC V. Airflow limitation is accompanied by diaphragm dysfunction. Physiol Res. 2016;65:469–479. doi: 10.33549/physiolres.933064. [DOI] [PubMed] [Google Scholar]
- KÁDKOVÁ A, SYNYTSYA V, KRUSEK J, ZÍMOVÁ L, VLACHOVÁ V. Molecular basis of TRPA1 regulation in nociceptive neurons. A review. Physiol Res. 2017;66:425–439. doi: 10.33549/physiolres.933553. [DOI] [PubMed] [Google Scholar]
- KANTAR A, SEMINARA M. Why chronic cough in children is different. Pulm Pharmacol Ther. 2019;56:51–55. doi: 10.1016/j.pupt.2019.03.001. [DOI] [PubMed] [Google Scholar]
- McCORMACK DG, MAK JC, COUPE MO, BARNES PJ. Calcitonin gene-related peptide vasodilation of human pulmonary vessels. J Appl Physiol. 1989;67:1265–1270. doi: 10.1152/jappl.1989.67.3.1265. [DOI] [PubMed] [Google Scholar]
- MIKAMI N, MATSUSHITA H, KATO T, KAWASAKI R, SAWAZAKI T, KISHIMOTO T, OGITANI Y, WATANABE K, MIYAGI Y, SUEDA K, FUKADA S, YAMAMOTO H, TSUJIKAWA K. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J Immunol. 2011;86:6886–6893. doi: 10.4049/jimmunol.1100028. [DOI] [PubMed] [Google Scholar]
- MORICE AH, FONTANA GA, BELVISI MG, BIRRING SS, CHUNG KF, DICPINIGAITIS PV, KASTELIK JA, MCGARVEY LP, SMITH JA, TATAR M, WIDDICOMBE J. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29:1256–1276. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- MORICE AH, McGARVEY LP, DICPINIGAITIS PV. Cough hypersensitivity syndrome is an important clinical concept: a pro/con debate. Lung. 2012;190:3–9. doi: 10.1007/s00408-011-9351-y. [DOI] [PubMed] [Google Scholar]
- NIIMI A, CHUNG KF. Evidence for neuropathic processes in chronic cough. Pulm Pharmacol Ther. 2015;35:100–104. doi: 10.1016/j.pupt.2015.10.004. [DOI] [PubMed] [Google Scholar]
- NIIMI A, FUKUMITSU K, TAKEDA N, KANEMITSU Y. Interfering with airway nerves in cough associated with asthma. Pulm Pharmacol Ther. 2019;59:101854. doi: 10.1016/j.pupt.2019.101854. [DOI] [PubMed] [Google Scholar]
- NUMAO T, AGRAWAL DK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol. 1992;149:3309–3315. [PubMed] [Google Scholar]
- SONG WJ, CHANG YS. Cough hypersensitivity as a neuro-immune interaction. Clin Transl Allergy. 2015;5:24. doi: 10.1186/s13601-015-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG WJ, MORICE AH. Cough hypersensitivity syndrome: a few more steps forward. Allergy Asthma Immunol Res. 2017;9:394–402. doi: 10.4168/aair.2017.9.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDEM BJ, TAYLOR-CLARK T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014;133:1521–1534. doi: 10.1016/j.jaci.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARECHOVA S, PLEVKOVA J, HANACEK J, TATAR M. Role of gender and pubertal stage on cough sensitivity in childhood and adolescence. J Physiol Pharmacol. 2008;59:719–726. [PubMed] [Google Scholar]
- ZACCONE EJ, LIEU T, MUROI Y, POTENZIERI C, UNDEM BE, GAO P, HAN L, CANNING BJ, UNDEM BJ. Parainfluenza 3-induced cough hypersensitivity in the guinea pig airways. PLoS One. 2016;11:e0155526. doi: 10.1371/journal.pone.0155526. [DOI] [PMC free article] [PubMed] [Google Scholar]