Summary
Since the recognition of angiotensin-converting enzyme inhibitors (ACEIs)-induced cough, drug has been considered as a potential cause of chronic cough. This review presents recent knowledge on drug-induced coughs in patients with chronic cough. The focus is placed on ACEIs, for which there are a multitude of studies documenting their associations with cough. Additional drugs are discussed for which there are reports of cough as a side effect of treatment, and the potential mechanisms of these effects are discussed.
Keywords: Cough, Drug, Medication, Angiotensin-converting enzyme inhibitors
Introduction
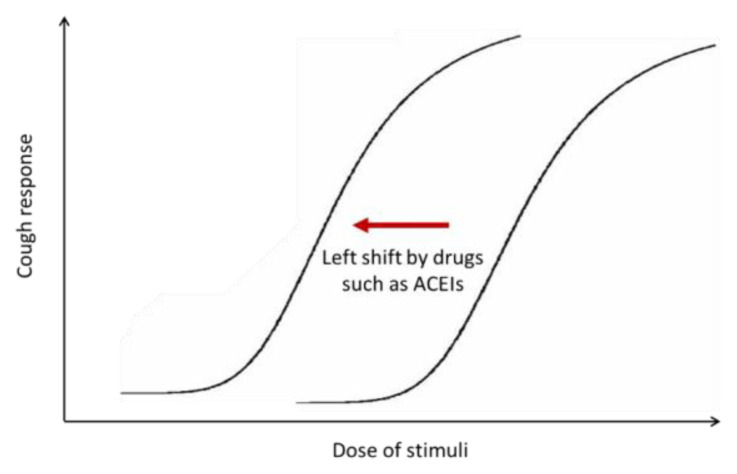
Chronic cough is a multi-factorial disease with a neurological basis in adults (Morice et al. 2014a, Song and Morice 2017). The recognition of angiotensin-converting enzyme inhibitors (ACEIs)-induced coughs (Sesoko and Kaneko 1985, Semple and Herd 1986, Morice et al. 1987) has not only led to the clinical recommendation that drug should be considered as a potential cause of cough (Irwin et al. 2018, Song et al. 2018, Morice et al. 2019), but also has provided an important clue to the notion that cough reflex hypersensitivity commonly underlies chronic cough in adults (Morice et al. 2014a) (Fig. 1). Left-shift in dose-response curve in capsaicin inhalation challenge was a proof for cough reflex hypersensitivity induced by ACEIs (Morice et al. 1987). Also, given the fact that ACEI-induced cough occurs in only about 10 % of patients taking the medicine (Matchar et al. 2008), the drug is likely to be a trigger in susceptible individuals, rather than be a direct cause of cough.
Fig. 1.
Schematic presentation of drug-induced cough reflex hypersensitivity
While causative potential of ACEIs in the pathogenesis of cough was consistently demonstrated in clinical trials (Dicpinigaitis 2006), it is unclear if any drugs other than ACEIs warrant further clinical attention in patients presenting with cough. In the literature, several drugs such as sitagliptin, topiramate, or methotrexate were reported to have pro-tussive potentials in some patients. Given rapid global population ageing (Song et al. 2019), it is supposed to be increasingly important to consider drug-induced cough in the clinics. This review aims to summarize recent knowledge on coughs induced by systemic medications, including the epidemiology and potential mechanisms.
Angiotensin-converting enzyme inhibitors
Since the first introduction of captopril in the late 1970s, ACEIs have been widely utilized for patients with hypertension, coronary diseases, heart failure, or chronic kidney diseases (Edwards and Padfield 1985, Li et al. 2014, Bertrand 2004, Jafar et al. 2001). They are usually well tolerated, but may cause side effects; cough is one of the common adverse reactions potentially leading to the drug discontinuation or more anti-tussive uses (Dicpinigaitis 2006, Matchar et al. 2008, Vegter et al. 2013).
Clinical manifestation of ACEI-induced cough
ACEI-induced cough is typically a persistent dry cough, combined with a tickle in the throat (Dicpinigaitis 2006). ACEI-induced cough may occur within a few hours of the first dose as well as weeks or months later, and is more frequent in female patients and nonsmokers (Os et al. 1994, Yilmaz 2019). In the observational study of Japanese patients with hypertension, cough frequently occurred within the first month and mostly within 6 months since the drug initiation (Sato and Fukuda 2015). In the French pharmacovigilance database analysis, the mean duration of onset was 156.8 days (95 % confidence interval [95 % CI]: 84.9 to 241.7 days) (Humbert et al. 2017). Patients who develop ACEI-induced cough or angioedema cannot usually tolerate another medication in the same class, which would act via a similar mechanism. The only effective management of ACEI-induced cough is discontinuation of the ACEI, with improvement typically observed within 1 to 4 weeks, but some cases have been reported to last up to 3 months (Dicpinigaitis 2006).
Epidemiology of ACEI-induced cough
The incidence of coughing related to ACEIs was reported to vary widely among studies of patients taking an ACEI, ranging from 0 % to 37 % (mean, 10 %) (Yusuf et al. 1991, Bart et al. 1999, Matchar et al. 2008, Brugts et al. 2014, Sato and Fukuda 2015). These variations may originate from heterogeneity in study population and design, duration of observation, or measurement of cough. However, importantly, patients taking placebos in randomized controlled trials (RCTs) may also experience coughing, and thus the relative risk (RR), rather than absolute risk, would be more appropriate in understanding the epidemiology of ACEI-induced cough. In a recent meta-analysis of RCTs reporting cough (as a side effect) in patients taking ACEI or placebo due to cardiovascular diseases, only a portion (37 %) of the incidences comprising an overall rate of ACEI-induced cough (13.5 %) were caused by the ACEI after correcting for cases of cough in the placebo groups (Vukadinovic et al. 2019). The findings suggested that a substantial proportion of cough in patients taking ACEIs may be potentially unrelated to the drug intake. Indeed, there have been cases of spontaneous resolution of cough while maintaining ACEI treatment as well as nonrecurrence of cough after the ACEI is re-administered (Reisin and Schneeweiss 1992, Sato and Fukuda 2015). These findings remind us that re-exposure to ACEI should be considered before complete withdrawal, particularly in patients likely to benefit from the therapy. Meanwhile, interestingly, the proportion of cough caused by the ACEIs (after correction for placebos) varied substantially among patients with different underlying conditions, showing the highest rate in patients with hypertension (85 %) but lower rates in those with coronary disease (42 %) or heart failure (29 %) (Vukadinovic et al. 2019). These findings suggest that host factors are likely to be important in the pathogenesis of ACEI-induced cough.
The frequency of cough on ACEI is consistently higher in female patients (Os et al. 1994, Visser et al. 1995, Kim et al. 2000, Morimoto et al. 2004, Brugts et al. 2014, Sato and Fukuda 2015, Alharbi et al. 2017). In a pooled analysis of three RCTs comparing perindopril and placebo in 27,492 patients with vascular diseases, older age (≥65 years) and female gender were positively associated with risk of ACEI-induced cough, with adjusted odds ratio (OR) of 1.53 (95 % CI: 1.35 to 1.73) and 1.92 (95 % CI: 1.68 to 2.18), respectively (Brugts et al. 2014). In a prospective observational study of 176 outpatients taking perindopril or imidapril for hypertension in Japan (average observation period: 18 months), the incidence of cough was 19.9 % and two thirds of them were females (Sato and Fukuda 2015). Among the 446 ACEI-induced cough cases registered in the French pharmacovigilance database, the incidence in women (mean age, 64 years) was twice that in men, and the mean duration of onset was 156.8 days (Humbert et al. 2017). Meanwhile, in a pooled of analysis of individual patient data from 6 placebo-controlled trials of children with hypertension on ACEIs, the risk of cough in ACEI treatment group was not different with that in placebo group (3.2 % vs. 2.5 %), and the female predominance in the ACEI group was not statistically significant (4.8 % vs. 2.2 %, p=0.13) (Baker-Smith et al. 2010). The age and sex characteristics seen in patients with ACEI-induced cough are in line with those seen in most patients attending specialist clinics with chronic cough (Morice et al., 2014b). These similarities support the notion that ACEI is a trigger factor to enhance cough reflex and thus provoke cough in susceptible individuals.
The risk of ACEI-induced cough is reported to be higher in East Asian patients (McDowell et al. 2006). In a meta-analysis of possible ethnic differences in risks of adverse reactions to cardiovascular drugs, the RR of cough from ACEI was 2.7 (95 % CI: 1.6 to 4.5) in East Asians than in whites, whereas it did not differ between black and non-black patients (RR: 1.1, 95 % CI: 0.54 to 2.27) (McDowell et al. 2006). However, the ethnic difference was not confirmed in a pooled analysis of individual patient data from 3 RCTs comparing perindopril and placebo (Brugts et al. 2014).
Mechanisms of ACEI-induced cough
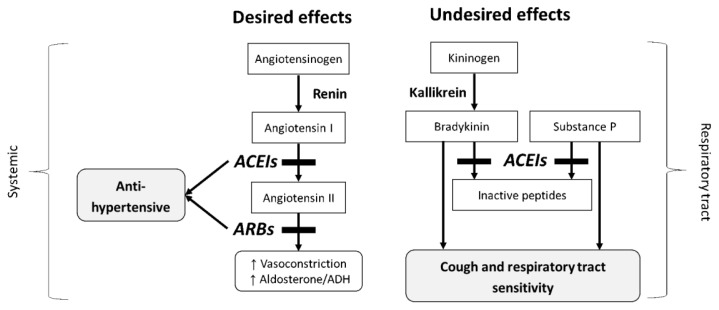
ACEI-induced cough appears only to occur in susceptible individuals, regardless of the dose of drug, though the individual susceptibility is not yet fully understood. However, the development of cough may be attributable to the accumulation of bradykinin or substance P, which are degraded by ACE in the upper and lower respiratory tracts (Dykewicz 2004, Morice et al. 1987) (Fig. 2). Bradykinin, converted from kininogen by kallikrein, has a short half-life as a result of rapid degradation by ACE (Packard et al. 2002). ACEIs suppress this degradation, resulting in an increase in bradykinin concentrations (Packard et al. 2002, Dykewicz 2004). In guinea pigs, inhaled bradykinin sensitizes cough reflex (El-Hashim and Amine 2005) and also triggers cough responses (Hewitt et al. 2016). Bradykinin also activates phospholipase A2, which leads to the generation of arachidonic acid derivatives such as leukotrienes, histamines, and prostaglandin I2 and E2, which may cause cough, bronchospasm, and nasal discharge (Trifilieff et al. 1993, Packard et al. 2002, Dykewicz 2004). Substance P pathways involving neurokinin-1 receptor may have important implications in the pathogenesis of ACEI-induced cough. Substance P is degraded by ACE, and has the potential to mediate cough induced by cigarette smoke or allergen challenge (Ujiie et al. 1993, Sekizawa et al. 1995). In a recent pilot study, a neurokinin-1 receptor antagonist, orvepitant, significantly improved cough outcomes in patients with chronic refractory cough (Smith et al. 2019).
Fig. 2.
Effects of angiotensin converting enzyme inhibitors on blood pressure and cough. Angiotensin-converting enzyme (ACE) mediates the conversion of angiotensin I to angiotensin II, but also it is involved in the degradation of bradykinin and substance P; thus, the inhibition of ACE may lead to undesired respiratory tract sensitivity including cough.
Studies have reported there is likely individual genetic susceptibility to ACEI-induced cough, including polymorphisms in genes encoding the bradykinin receptor, ACE (insertion/deletion), and aminopeptidase P, which is involved in bradykinin degradation (Kaufman et al. 1989, Kaufman et al. 1992, O’Connell et al. 1994, Ravid et al. 1994, Mukae et al. 2000, Lee and Tsai 2001, Ignjatovic et al. 2002, Nikpoor et al. 2005, Dicpinigaitis 2006), suggesting the pathophysiologic mechanisms are likely involve ACE-bradykinin pathways. Studies identified a genetic variant of the bradykinin B2 receptor promoter in patients with ACEI-induced cough, suggesting that the transcription of this receptor contributes to ACEI-induced cough (Mukae et al. 2000, Mukae et al. 2002). However, relationships between ACEI-induced cough and genetic polymorphisms in ACE or bradykinin receptors were controversial (Woo et al. 2009). In patients with non-productive chronic cough, neurokinin-2 receptor polymorphisms were significantly associated with enhanced capsaicin sensitivity (Park et al. 2006). Meta-analyses indicated that ACEI-induced cough is associated with the insertion/deletion polymorphisms of the ACE gene (Li et al. 2012) and single-nucleotide polymorphisms in RBFOX3, GABRG2, SH2B1, and MBOAT1, for which the functional roles were not identified (Mahmoudpour et al. 2017). A genome-wide association study of a Swedish population found near-significant associations between genes outside the bradykinin pathway and ACEI-induced cough, indicating that additional pathways might also contribute (Hallberg et al. 2017).
As some ACEIs may cross the blood-brain barrier (Fazal et al. 2017), it would be interesting to see whether the cough is related to central or peripheral activity of the drugs. However, in our recent systematic reviews of RCTs of patients with cardiovascular disease (unpublished data), the RRs of ACEI-induced cough (vs. placebo) did not appear to differ by the blood-brain barrier permeability of ACEIs (centrally acting ACEIs, RR: 2.56, 95 % CI: 1.78–3.68, vs. peripherally acting ACEIs, RR: 2.18, 95 % CI: 1.78–2.68), implying that the site of drug action on the cough reflex is likely to in the periphery.
Angiotensin II receptor blockers
Patients in whom ACEIs are discontinued because of cough may be given an angiotensin II receptor blockers (ARBs). In patients with hypertension, the incidence of withdrawal due to adverse effects was lower for ARBs compared with ACEIs (RR: 0.83, 95 % CI: 0.74 to 0.93), which was largely attributable to cough (Li et al. 2014). In patients with intolerance to ACEIs, ARBs were well tolerated with a significantly fewer risk of cough (RR: 0.37, 95 % CI: 0.28 to 0.48), and the incidence of cough on ARBs was comparable to that of placebos (RR: 1.01, 95 % CI: 0.74 to 1.39) (Caldeira et al. 2012). These findings confirmed that the risk of cough is not elevated in most patients taking ARBs. However, there is a case report that cough occurred 3 days after initiating an ARB and disappeared 1 week after the drug was changed to an ACEI (Dashti-Khavidaki et al. 2008).
Sitagliptin
Sitagliptin is a highly selective oral dipeptidyl peptidase-4 (DPP IV) inhibitor that inhibits the breakdown of incretins such as glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide (Kim et al. 2014). In a placebo-controlled trial assessing the efficacy and tolerability of sitagliptin in patients with type 2 diabetes mellitus who were inadequately controlled on metformin alone, the incidence of cough as a side effect was reported to be higher in the sitagliptin group compared with the placebo group (14/464 vs. 4/237) (Charbonnel et al. 2006). A case series described 15 patients intolerant to sitagliptin, where 13 of them reported cough with other respiratory symptoms such as rhinorrhea or dyspnea/wheeze. All of them had underlying allergic rhinitis, and the frequency was significantly higher than that in sitagliptin-tolerant patients (6 of 18, p<0.001). Sitagliptin was re-administered to 5 intolerant patients, and cough recurred in 4 of them (Baraniuk and Jamieson 2010). However, the risk of cough was not evident in any other RCTs or pooled analyses comparing sitagliptin and placebo (Williams-Herman et al. 2010, Engel et al. 2013, Arjona Ferreira et al. 2013, Ahren et al. 2014). Placebo-controlled trials with other DPP IV inhibitors, including linagliptin, saxagliptin and vildagliptin, also did not report any increased risk of cough (Cai et al. 2012, Lehrke et al. 2014, Doucet et al. 2011). Mechanistically, inhibition of DPP IV pathways may aggravate allergic inflammation (Yan et al. 2012). DPP IV is expressed in bronchial epithelial cells, which is up-regulated by interleukin-13 stimulation (Shiobara et al. 2016). These findings collectively suggest the risk of sitagliptin-induced cough is not clear, but might be potentially attributed to host-drug interactions, such as allergic comorbidity, which warrants further investigation for clinical impact.
Calcium channel blockers
Calcium channel blockers may not have direct pro-tussive effects, but potentially trigger cough in certain individuals, possibly by attenuating the lower esophageal sphincter and reducing esophageal clearance (Medford 2012). Cough in these patients may represent a symptom of reflux, even without dyspepsia, and this reflux cough may be aggravated after meals and when in a stooping posture (Morice et al. 2006). In an observational study of 371 patients receiving calcium channel blockers for hypertension, 45.4 % of 130 patients with pre-existing gastrointestinal symptoms reported a worsening of reflux symptoms (Hughes et al. 2007). When reflux cough is suspected, it is recommended that the calcium channel blocker should be discontinued for up to 3 months to determine if the cough improves (Morice et al. 2006, Medford 2012).
Fentanyl
Fentanyl-induced cough is a common problem encountered in perioperative settings. Fentanyl is widely used to achieve analgesia and reduce anxiety with general anesthesia because it has a rapid onset and short duration, induces less histamine release, and has no negative inotropic effects (Tsou et al. 2002, Saleh et al. 2014). However, when administered intravenously as a bolus, 18–65 % of patients reportedly exhibit cough (Saleh et al. 2014). Several patho-mechanisms have been proposed to explain this. For example, fentanyl inhibits central sympathetic outflow to induce vagal predominance, which may lead to bronchoconstriction and cough (Agarwal et al. 2003, Kamei et al. 2013). In addition, fentanyl may induce a pulmonary chemoreflex, possibly by constricting tracheal smooth muscles and stimulating vagal C-fibers, irritant receptors, or pulmonary vessels of the upper airway mucosa (Bohrer et al. 1990). Intravenous fentanyl may also induce the release of histamine and neuropeptides by acting on prejunctional μ-opioid receptors (Ai et al. 2010).
A meta-analysis of 34 trials showed that lidocaine, ketamine, dexmedetomidine, a priming dose of fentanyl, propofol, dezocine, dexamethasone, dextromethorphan, and magnesium sulfate may help to reduce fentanyl-induced cough, whereas salbutamol, tramadol, midazolam, and atropine are likely to be ineffective (Shuying et al. 2016). There are no reports that transdermal administration of fentanyl via a patch causes coughing, although it has been reported to aggravate respiratory symptoms in asthmatics, which resolved 72 h after the patch was removed (Parmar 2009).
Latanoprost
Latanoprost is a prostaglandin F2-α analog ophthalmic solution, which is commonly used for the management of glaucoma (Klimko and Sharif 2019). In humans, prostaglandin F2-α receptors are present in the respiratory tracts and inhalation of prostaglandin F2-α significantly induced left-shifts in dose response curves in capsaicin cough challenge tests (Nichol et al. 1990, Stone, Barnes, and Fuller 1992). In a case report of 51-year-old woman, it was also demonstrated that even ophthalmic drops of latanoprost may up-regulate cough reflex and cause clinically troublesome cough (Fahim and Morice 2009). The prevalence of latanoprost-induced cough is unclear, but this case report highlights the importance of recognizing topical drug exposure as a potential cause of cough.
Miscellaneous
Topiramate
Topiramate is a new-generation antiepileptic drug widely used for migraine prophylaxis that increases the main inhibitory neurotransmitter in the brain, gamma aminobutyric acid (Tosun et al. 2012, Silberstein 2017). Since topiramate was approved by the United States Food and Drug Administration in 2004 for the prevention of migraines (Lainez et al. 2007), only two case reports on four patients so far have documented cough caused by topiramate (Maggioni et al. 2010, Tosun et al. 2012). To our knowledge, cough was not reported as a common issue in RCTs with topiramate. A case in one of the reports involved a dry cough that did not improve with antitussives, an inhaled glucocorticoid, or bronchodilators but only disappeared once topiramate was discontinued because of its sedative effect; however, the cough returned one day after the patient began retaking topiramate, but again disappeared over 3 weeks after the drug was discontinued (Tosun et al. 2012). In another report, 3 cases of topiramate-induced intractable coughing was described (Maggioni et al. 2010). In all these cases, cough developed soon after topiramate initiation, and resolved rapidly (within 1 week) after the drug discontinuation (Maggioni et al. 2010, Tosun et al. 2012).
Phenytoin
A case was reported of an immediate occurrence of nocturnal dry cough after oral phenytoin administration (Nascimento et al. 2016). In this case, the symptom lasted for 6 months and disappeared after phenytoin was discontinued. In another case report, intravenous administration of phenytoin immediately produced bronchospasm and cough, which have involved central sympathetic inhibition with vagal predominance (Dube and Rath 2012).
Methotrexate
Although pneumonitis is a well-known side effect of methotrexate, a case series documented ten patients who developed sustained nonproductive cough while receiving methotrexate, in the absence of dyspnea, impaired lung function, or evidence of lung parenchymal diseases in chest radiograph or lung biopsies (Schnabel et al. 1996). All ten patients were being treated with methotrexate for rheumatoid arthritis, and the coughs improved with symptomatic care with or without the discontinuation of methotrexate (Schnabel et al. 1996).
Mycophenolate mofetil
There is a report of five kidney transplant patients who developed cough after taking mycophenolate mofetil (Elli et al. 1998). Despite normal chest radiography, nonproductive cough occurred 36 to 84 days after these patients were administered the drug, and only discontinuation of mycophenolate mofetil reduced the cough symptoms (Elli et al. 1998).
Omeprazole
Omeprazole is one of widely used proton pump inhibitors to relieve peptic symptoms in patients with gastroesophageal acidic reflux diseases (Kahrilas et al. 2013). Omeprazole-induced cough is very rare, but two case reported has been documented so far (Howaizi and Delafosse 2003, Reiche et al. 2010).
Conclusions
In this review, we summarized drug-induced coughs and their potential mechanisms reported in the literature so far (Table 1). Cases of ACEI-induced cough have suggested that drug may act as a trigger for cough reflex up-regulation and have drawn attention to the thinking that cough hypersensitivity underlies chronic cough. Except for ACEIs, however most clinical evidence for other drugs is still limited to case reports and the mechanisms of associations are unclear. Some drugs including calcium channel blockers, sitagliptin, or latanoprost showed the potential to provoke clinical coughs in susceptible individuals with certain cough-related comorbidities, such as allergic inflammation or reflux, however, the prevalence and clinical impact is still unclear. A few case reports described patients with cough possibly related to topiramate, phenytoin, methotrexate, mycophenolate mofetil, or omeprazole. Recognition of drug-induced cough is clinically important, as its identification will help the resolution of cough without further needs for diagnostic or therapeutic efforts. Further attention should be given to any unexplained cough cases, particularly when patients are taking drugs with potentials to influence cough.
Table 1.
Drug-induced cough and possible mechanisms reported in the literature
| Drug | Route of administration | Clinical manifestation | Possible mechanisms |
|---|---|---|---|
| ACEI | Oral | Dry cough | Impaired degradation of bradykinin and substance P which mediated by ACE, causing enhanced cough reflex, accumulation of AA derivatives, nitric oxide production |
|
| |||
| Sitagliptin | Oral | Cough, rhinorrhea, dyspnea, wheeze | May aggravate underlying allergic conditions |
|
| |||
| Calcium channel blocker | Usually oral | Cough with or without reflux symptoms | May aggravate underlying reflux conditions |
|
| |||
| Fentanyl | IV | Cough, bronchoconstriction (usually in perioperative settings) | May inhibit central sympathetic tone and increase vagal tone |
|
| |||
| Latanoprost | Ophthalmic | Dry cough | Absorption of PGF2-α may enhance cough reflex in central nerve systems |
|
| |||
| Miscellaneous: | |||
| Topiramate | Oral | Dry cough | Unknown |
| Phenytoin | Oral, IV | Nocturnal dry cough | Unknown |
| Methotrexate | Oral | Dry cough | Unknown |
| Mycophenolate mofetil | Oral | Dry cough | Unknown |
| Omeprazole | Oral | Dry cough, worsened at night | Unknown |
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- ARB
Angiotensin II receptor blockers
- DPP IV
dipeptidyl peptidase-4
- AA
arachidonic acid
- PGF2-α
prostaglandin F2-α
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- AGARWAL A, AZIM A, AMBESH S, BOSE N, DHIRAJ S, SAHU D, SINGH U. Salbutamol, beclomethasone or sodium chromoglycate suppress coughing induced by iv fentanyl. Can J Anaesth. 2003;50:297–300. doi: 10.1007/BF03017801. [DOI] [PubMed] [Google Scholar]
- AHREN B, JOHNSON SL, STEWART M, CIRKEL DT, YANG F, PERRY C, FEINGLOS MN HARMONY STUDY GROUP. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–2148. doi: 10.2337/dc14-0024. [DOI] [PubMed] [Google Scholar]
- AI Q, HU Y, WANG Y, WU S, QIN Z, WANG J, WANG G, ZHANG J, AN M. Pentazocine pretreatment suppresses fentanyl-induced cough. Pharmacol Rep. 2010;62:747–750. doi: 10.1016/S1734-1140(10)70333-9. [DOI] [PubMed] [Google Scholar]
- ALHARBI FF, KHOLOD AAV, SOUVEREIN PC, MEYBOOM RH, DE GROOT MCH, DE BOER A, KLUNGEL OH. The impact of age and sex on the reporting of cough and angioedema with renin-angiotensin system inhibitors, a case/noncase study in VigiBase. Fundam Clin Pharmacol. 2017;31:676–684. doi: 10.1111/fcp.12313. [DOI] [PubMed] [Google Scholar]
- ARJONA FERREIRA JC, CORRY D, MOGENSEN CE, SLOAN L, XU L, GOLM GT, GONZALEZ EJ, DAVIES MJ, KAUFMAN KD, GOLDSTEIN BJ. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis. 2013;61:579–587. doi: 10.1053/j.ajkd.2012.11.043. [DOI] [PubMed] [Google Scholar]
- BAKER-SMITH CM, BENJAMIN DK, CALIFF RM, MURPHY MD, LI JS, SMITH PB. Cough in pediatric patients receiving angiotensin-converting enzyme inhibitor therapy or angiotensin receptor blocker therapy in randomized controlled trials. Clin Pharmacol Ther. 2010;87:668–671. doi: 10.1038/clpt.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARANIUK JN, JAMIESON MJ. Rhinorrhea, cough and fatigue in patients taking sitagliptin. Allergy Asthma Clin Immunol. 2010;6:8. doi: 10.1186/1710-1492-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BART BA, ERTL G, HELD P, KUCH J, MAGGIONI AP, McMURRAY J, MICHELSON EL, ROULEAU JL, WARNER L, STEVENSON K, SWEDBERG J, YOUNG B, YUSUF S, SELLERS MA, GRANGER CB, CALIFF RM, PFEFFER MA. Contemporary management of patients with left ventricular systolic dysfunction. Results from the Study of Patients Intolerant of Converting Enzyme Inhibitors (SPICE) Registry. Eur Heart J. 1999;20:1182–1190. doi: 10.1053/euhj.1998.1481. [DOI] [PubMed] [Google Scholar]
- BERTRAND ME. Provision of cardiovascular protection by ACE inhibitors: a review of recent trials. Curr Med Res Opin. 2004;20:1559–1569. doi: 10.1185/030079904X4185. [DOI] [PubMed] [Google Scholar]
- BOHRER HF, FLEISCHER F, WERNING P. Tussive effect of a fentanyl bolus administered through a central venous catheter. Anaesthesia. 1990;45:18–21. doi: 10.1111/j.1365-2044.1990.tb14496.x. [DOI] [PubMed] [Google Scholar]
- BRUGTS JJ, ARIMA H, REMME W, BERTRAND M, FERRARI R, FOX K, DINICOLANTONIO J, MACMAHON S, CHALMERS J, ZIJLSTRA F, CALISKAN K, SIMOONS ML, MOURAD JJ, BOERSMA E, AKKERHUIS KM. The incidence and clinical predictors of ACE-inhibitor induced dry cough by perindopril in 27,492 patients with vascular disease. Int J Cardiol. 2014;176:718–723. doi: 10.1016/j.ijcard.2014.07.108. [DOI] [PubMed] [Google Scholar]
- CAI LY, CAI Z, LU J, ZHANG Y, LIU P. The efficacy and safety of vildagliptin in patients with type 2 diabetes: a meta-analysis of randomized clinical trials. J Clin Pharm Ther. 2012;37:386–398. doi: 10.1111/j.1365-2710.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- CALDEIRA D, DAVID C, SAMPAIO C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2012;12:263–277. doi: 10.1007/BF03261835. [DOI] [PubMed] [Google Scholar]
- CHARBONNEL B, KARASIK A, LIU J, WU M, MEININGER G GROUP SITAGLIPTIN STUDY. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- DASHTI-KHAVIDAKI S, FAGHIHI T, AHMADI F, KHALILI H. Cough induced by losartan with resolution after substitution with enalapril. Clin Ther. 2008;30:548–551. doi: 10.1016/j.clinthera.2008.03.003. [DOI] [PubMed] [Google Scholar]
- DICPINIGAITIS PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:169–73. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- DOUCET J, CHACRA A, MAHEUX P, LU J, HARRIS S, OSENSTOCK R. Efficacy and safety of saxagliptin in older patients with type 2 diabetes mellitus. Curr Med Res Opin. 2011;27:863–869. doi: 10.1185/03007995.2011.554532. [DOI] [PubMed] [Google Scholar]
- DUBE SK, RATH GP. Cough and bronchospasm after rapid intravenous administration of phenytoin. J Neurosurg Anesthesiol. 2012;24:239–240. doi: 10.1097/ANA.0b013e318259b46a. [DOI] [PubMed] [Google Scholar]
- DYKEWICZ MS. Cough and angioedema from angiotensin-converting enzyme inhibitors: new insights into mechanisms and management. Curr Opin Allergy Clin Immunol. 2004;4:267–270. doi: 10.1097/01.all.0000136759.43571.7f. [DOI] [PubMed] [Google Scholar]
- EDWARDS CR, PADFIELD PL. Angiotensin-converting enzyme inhibitors: past, present, and bright future. Lancet. 1985;1:30–34. doi: 10.1016/S0140-6736(85)90975-4. [DOI] [PubMed] [Google Scholar]
- EL-HASHIM AZ, AMINES A. The role of substance P and bradykinin in the cough reflex and bronchoconstriction in guinea-pigs. Eur J Pharmacol. 2005;513:125–133. doi: 10.1016/j.ejphar.2005.02.007. [DOI] [PubMed] [Google Scholar]
- ELLI A, AROLDI A, MONTAGNINO G, TARANTINO A, PONTICELLI C. Mycophenolate mofetil and cough. Transplantation. 1998;66:409. doi: 10.1097/00007890-199808150-00026. [DOI] [PubMed] [Google Scholar]
- ENGEL SS, ROUND E, GOLM GT, KAUFMAN KD, GOLDSTEIN BJ. Safety and tolerability of sitagliptin in type 2 diabetes: pooled analysis of 25 clinical studies. Diabetes Ther. 2013;4:119–145. doi: 10.1007/s13300-013-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHIM A, MORICE AH. Heightened cough sensitivity secondary to latanoprost. Chest. 2009;136:1406–07. doi: 10.1378/chest.09-0070. [DOI] [PubMed] [Google Scholar]
- FAZAL K, PERERA G, KHONDOKER M, HOWARD R, STEWART R. Associations of centrally acting ACE inhibitors with cognitive decline and survival in Alzheimer’s disease. BJPsych open. 2017;3:158–164. doi: 10.1192/bjpo.bp.116.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLBERG P, PERSSON M, AXELSSON T, CAVALLI M, NORLING P, JOHANSSON JE, YUE YJ, MAGNUSSON PK, WADELIUS C, ERIKSSON N, WADELIUS M. Genetic variants associated with angiotensin-converting enzyme inhibitor-induced cough: a genome-wide association study in a Swedish population. Pharmacogenomics. 2017;18:201–213. doi: 10.2217/pgs-2016-0184. [DOI] [PubMed] [Google Scholar]
- HEWITT M, ADAMS G, MAZZONE SB, MORI N, YU L, CANNING BJ. Pharmacology of BRADYKININ-EVOKED COUGHING IN GUINEA PIGS. J PHARMACOL EXP THER. 2016;357:620–628. doi: 10.1124/jpet.115.230383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWAIZI M, DELAFOSSE C. Omeprazole-induced intractable cough. Ann Pharmacother. 2003;37:1607–1609. doi: 10.1345/aph.1D185. [DOI] [PubMed] [Google Scholar]
- HUGHES J, LOCKHART J, JOYCE A. Do calcium antagonists contribute to gastro-oesophageal reflux disease and concomitant noncardiac chest pain? Br J Clin Pharmacol. 2007;64:83–89. doi: 10.1111/j.1365-2125.2007.02851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMBERT X, ALEXANDRE J, SASSIER M, DEFAULT A, GOURAUD A, YELEHE-OKOUMA M, PUDDU PE, FEDRIZZI S. Long delay to onset of ACE inhibitors-induced cough: Reason of difficult diagnosis in primary care? Eur J Intern Med. 2017;37:50–51. doi: 10.1016/j.ejim.2016.10.006. [DOI] [PubMed] [Google Scholar]
- IGNJATOVIC T, TAN F, BROVKOVYCH V, SKIDGEL RA, ERDOS EG. Novel mode of action of angiotensin I converting enzyme inhibitors: direct activation of bradykinin B1 receptor. J Biol Chem. 2002;277:16847–16852. doi: 10.1074/jbc.M200355200. [DOI] [PubMed] [Google Scholar]
- IRWIN RS, FRENCH CL, CHANG AB, ALTMAN KW, ADAMS TM, AZOULAY E, BARKER AF, BIRRING SS, BLACKHALL F, BOLSER DC. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018;153:196–209. doi: 10.1016/j.chest.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAFAR TH, SCHMID CH, LANDA M, GIATRAS I, TOTO R, REMUZZI G, MASCHIO G, BRENNER BM, KAMPER A, ZUCCHELLI P, BECKER G, HIMMELMANN A, BANNISTER K, LANDAIS P, SHAHINFAR S, de JONG PE, de ZEEUW D, LAU J, LEVEY AS. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- KAHRILAS PJ, HOWDEN CW, HUGHES N, MOLLOY-BLAND M. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest. 2013;143:605–612. doi: 10.1378/chest.12-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMEI J, NAKANISHI Y, ASATO M, IKEDA H. Fentanyl enhances the excitability of rapidly adapting receptors to cause cough via the enhancement of histamine release in the airways. Cough. 2013;9:3. doi: 10.1186/1745-9974-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN J, CASANOVA JE, RIENDL P, SCHLUETER DP. Bronchial hyperreactivity and cough due to angiotensin-converting enzyme inhibitors. Chest. 1989;95:544–548. doi: 10.1378/chest.95.3.544. [DOI] [PubMed] [Google Scholar]
- KAUFMAN J, SCHMITT S, BARNARD J, BUSSE W. Angiotensin-converting enzyme inhibitors in patients with bronchial responsiveness and asthma. Chest. 1992;101:922–925. doi: 10.1378/chest.101.4.922. [DOI] [PubMed] [Google Scholar]
- KIM NH, YU T, LEE DH. The nonglycemic actions of dipeptidyl peptidase-4 inhibitors. Biomed Res Int. 2014;2014:368703. doi: 10.1155/2014/368703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM YS, PARK HS, SUNWOO S, BYEON JJ, SONG YM, SEO HG, KIM CH, CHEON KS, YOO SM, LEE JK GROUP KOREA POST-MARKETING SURVEILLANCE RESEARCH. Short-term safety and tolerability of antihypertensive agents in Korean patients: an observational study. Pharmacoepidemiol Drug Saf. 2000;9:603–609. doi: 10.1002/pds.554. [DOI] [PubMed] [Google Scholar]
- KLIMKO PG, SHARIF NA. Discovery, characterization and clinical utility of prostaglandin agonists for the treatment of glaucoma. Br J Pharmacol. 2019;176:1051–1058. doi: 10.1111/bph.14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAINEZ MJ, FREITAG FG, PFEIL J, ASCHER S, OLSON WH, SCHWALEN S. Time course of adverse events most commonly associated with topiramate for migraine prevention. Eur J Neurol. 2007;14:900–906. doi: 10.1111/j.1468-1331.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- LEE YJ, TSAI JC. Angiotensin-converting enzyme gene insertion/deletion, not bradykinin B2 receptor -58T/C gene polymorphism, associated with angiotensin-converting enzyme inhibitor-related cough in Chinese female patients with non-insulin-dependent diabetes mellitus. Metabolism. 2001;50:1346–1350. doi: 10.1053/meta.2001.27212. [DOI] [PubMed] [Google Scholar]
- LEHRKE M, MARX N, PATEL S, SECK T, CROWE S, CHENG K, von EYNATTEN M, JOHANSEN OE. Safety and tolerability of linagliptin in patients with Type 2 diabetes: A comprehensive pooled analysis of 22 placebo-controlled studies. Clin Ther. 2014;36:1130–1146. doi: 10.1016/j.clinthera.2014.06.008. [DOI] [PubMed] [Google Scholar]
- LI EC, HERAN BS, WRIGHT JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2014:CD009096. doi: 10.1002/14651858.CD009096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI YF, ZHU XM, LIU F, XIAO CS, BIAN YF, LI H, CAI J, LI RS, YANG XC. Angiotensin-converting enzyme (ACE) gene insertion/deletion polymorphism and ACE inhibitor-related cough: a meta-analysis. PLoS One. 2012;7:e37396. doi: 10.1371/journal.pone.0037396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGIONI F, MAMPRESO E, MAINARDI F, LISOTTO C, MALVINDI ML, ZANCHIN G. Topiramate-induced intractable cough during migraine prophylaxis. Headache. 2010;50:301–304. doi: 10.1111/j.1526-4610.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- MAHMOUDPOUR SH, VELUCHAMY A, SIDDIQUI MK, ASSELBERGS FW, SOUVEREIN PC, de KEYSER CE, HOFMAN A, LANG CC, DONEY AS, STRICKER BH, de BOER A, MAITLAND-van der ZEE AH, PALMER CN. Meta-analysis of genome-wide association studies on the intolerance of angiotensin-converting enzyme inhibitors. Pharmacogenet Genomics. 2017;27:112–119. doi: 10.1097/FPC.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATCHAR DB, McCRORY DC, ORLANDO LA, PATEL MR, PATEL UD, PATWARDHAN MB, POWERSB, SAMSA GP, GRAY RN. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148:16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- McDOWELL SE, COLEMAN JJ, FERNER RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ. 2006;332:1177–1181. doi: 10.1136/bmj.38803.528113.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDFORD AR. A 54 year-old man with a chronic cough – Chronic cough: don’t forget drug-induced causes. Prim Care Respir J. 2012;21:347–348. doi: 10.4104/pcrj.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORICE AH, JAKES AD, FARUQI S, BIRRING SS, MCGARVEY L, CANNING BJ, SMITH JA, PARKER SM, CHUNG KF, LAI K, PAVORD ID, VAN DEN BERG J, SONG WJ, MILLQVIST E, FARRELL MJ, MAZZONE SB, DICPINIGAITIS P REGISTRY CHRONIC COUGH. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014;44:1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
- MORICE AH, LOWRY R, BROWN MJ, HIGENBOTTAM T. Angiotensin-converting enzyme and the cough reflex. Lancet. 1987;2:1116–8. doi: 10.1016/S0140-6736(87)91547-9. [DOI] [PubMed] [Google Scholar]
- MORICE AH, McGARVEY L, PAVORD I. Recommendations for the management of cough in adults. Thorax. 2006;61(Suppl 1):i1–24. doi: 10.1136/thx.2006.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORICE AH, MILLQVIST E, BELVISI MG, BIEKSIENE K, BIRRING SS, CHUNG KF, DAL NEGRO RW, DICPINIGAITIS P, KANTAR A, MCGARVEY LP. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J. 2014;44:1132–1148. doi: 10.1183/09031936.00218613. [DOI] [PubMed] [Google Scholar]
- MORICE AH, MILLQVIST E, BIEKSIENE K, BIRRING SS, DICPINIGAITIS P, RIBAS CD, BOON MH, KANTAR A, LAI K, McGARVEY L, RIGAU D, SATIA I, SMITH J, SONG JW, TONIA T, van den BERG JWK, van MANEN MJG, ZACHARASIEWICZ A. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55 doi: 10.1183/13993003.01136-2019. pii:1901136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIMOTO T, GANDHI TK, FISKIO JM, SEGER AC, SO JW, COOK EF, FUKUI T, BATES DW. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- MUKAE S, AOKI S, ITOH S, IWATA T, UEDA H, KATAGIRI T. Bradykinin B2 receptor gene polymorphism is associated with angiotensin-converting enzyme inhibitor-related cough’. Hypertension. 2000;36:127–131. doi: 10.1161/01.HYP.36.1.127. [DOI] [PubMed] [Google Scholar]
- MUKAE S, ITOH S, AOKI S, IWATA T, NISHIO K, SATO R, KATAGIRI T. Association of polymorphisms of the renin-angiotensin system and bradykinin B2 receptor with ACE-inhibitor-related cough. J Hum Hypertens. 2002;16:857–863. doi: 10.1038/sj.jhh.1001486. [DOI] [PubMed] [Google Scholar]
- NASCIMENTO FA, TAKESHITA BT, KOWACS PA. Phenytoin-induced isolated chronic, nocturnal dry cough. Epilepsy Behav Case Rep. 2016;5:44–45. doi: 10.1016/j.ebcr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOL G, NIX A, BARNES PJ, CHUNG KF. Prostaglandin F2 alpha enhancement of capsaicin induced cough in man: modulation by beta 2 adrenergic and anticholinergic drugs. Thorax. 1990;45:694–698. doi: 10.1136/thx.45.9.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKPOOR B, DUAN QL, ROULEAU GA. Acute adverse reactions associated with angiotensin-converting enzyme inhibitors: genetic factors and therapeutic implications. Expert Opin Pharmacother. 2005;6:1851–1856. doi: 10.1517/14656566.6.11.1851. [DOI] [PubMed] [Google Scholar]
- O’CONNELL F, THOMAS WE, PRIDE NB, FULLER RW. Capsaicin cough sensitivity decreases with successful treatment of chronic cough. Am J Respir Crit Care Med. 1994;150:374–380. doi: 10.1164/ajrccm.150.2.8049818. [DOI] [PubMed] [Google Scholar]
- OS I, BRATLAND B, DAHLOF B, GISHOLT K, SYVERTSEN JO, TRETLI S. Female preponderance for lisinopril-induced cough in hypertension. Am J Hypertens. 1994;7:1012–1015. doi: 10.1093/ajh/7.11.1012. [DOI] [PubMed] [Google Scholar]
- PACKARD KA, WURDEMAN RL, AROUNI AJ. ACE inhibitor-induced bronchial reactivity in patients with respiratory dysfunction. Ann Pharmacother. 2002;36:1058–1067. doi: 10.1345/aph.1A332. [DOI] [PubMed] [Google Scholar]
- PARK HK, OH SY, KIM TB, BAHN JW, SHIN ES, LEE JE, OH HB, KIM YK, PARK T, CHO SH, MIN KU, KIM YY. Association of genetic variations in neurokinin-2 receptor with enhanced cough sensitivity to capsaicin in chronic cough. Thorax. 2006;61:1070–1075. doi: 10.1136/thx.2005.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARMAR MS. Exacerbation of asthma secondary to fentanyl transdermal patch. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.10.2008.1062. pii: bcr10.2008.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVID D, LISHNER M, LANG R, RAVID M. Angiotensin-converting enzyme inhibitors and cough: a prospective evaluation in hypertension and in congestive heart failure. J Clin Pharmacol. 1994;34:1116–1120. doi: 10.1002/j.1552-4604.1994.tb01989.x. [DOI] [PubMed] [Google Scholar]
- REICHE I, TROGER U, MARTENS-LOBENHOFFER J, KANDULSKI A, NEUMANN H, MALFERTHEINER P, BODE-BOGER SM. Omeprazole-induced cough in a patient with gastroesophageal reflux disease. Eur J Gastroenterol Hepatol. 2010;22:880–882. doi: 10.1097/MEG.0b013e3283320129. [DOI] [PubMed] [Google Scholar]
- REISIN L, SCHNEEWEISS A. Complete spontaneous remission of cough induced by ACE inhibitors during chronic therapy in hypertensive patients. J Hum Hypertens. 1992;6:333–335. [PubMed] [Google Scholar]
- SALEH AJ, ZHANG L, HADI SM, OUYANG W. A priming dose of intravenous ketamine-dexmedetomidine suppresses fentanyl-induced coughing: a double-blind, randomized, controlled study. Ups J Med Sci. 2014;119:333–337. doi: 10.3109/03009734.2014.968270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO A, FUKUDA S. A prospective study of frequency and characteristics of cough during ACE inhibitor treatment. Clin Exp Hypertens. 2015;37:563–568. doi: 10.3109/10641963.2015.1026040. [DOI] [PubMed] [Google Scholar]
- SCHNABEL AK, DALHOFF S, BAUERFEIND J, GROSS WL. Sustained cough in methotrexate therapy for rheumatoid arthritis. Clin Rheumatol. 1996;15:277–282. doi: 10.1007/BF02229707. [DOI] [PubMed] [Google Scholar]
- SEKIZAWA K, EBIHARA T, SASAKI H. Role of substance P in cough during bronchoconstriction in awake guinea pigs. Am J Respir Crit Care Med. 1995;151:815–821. doi: 10.1164/ajrccm.151.3.7533603. [DOI] [PubMed] [Google Scholar]
- SEMPLE PF, HERD GW. Cough and wheeze caused by inhibitors of angiotensin-converting enzyme. N Engl J Med. 1986;314:61. doi: 10.1056/NEJM198601023140119. [DOI] [PubMed] [Google Scholar]
- SESOKO S, KANEKO Y. Cough associated with the use of captopril. Arch Intern Med. 1985;145:1524–1524. doi: 10.1001/archinte.145.8.1524. [DOI] [PubMed] [Google Scholar]
- SHIOBARA T, CHIBANA K, WATANABE T, ARAI R, HORIGANE Y, NAKAMURA Y, HAYASHI Y, SHIMIZU Y, TAKEMASA A, ISHII Y. Dipeptidyl peptidase-4 is highly expressed in bronchial epithelial cells of untreated asthma and it increases cell proliferation along with fibronectin production in airway constitutive cells. Respir Res. 2016;17:28. doi: 10.1186/s12931-016-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUYING L, PING L, JUAN N, DONG L. Different interventions in preventing opioid-induced cough: a meta-analysis. J Clin Anesth. 2016;34:440–447. doi: 10.1016/j.jclinane.2016.05.034. [DOI] [PubMed] [Google Scholar]
- SILBERSTEIN SD. Topiramate in migraine prevention: a 2016 perspective. Headache. 2017;57:165–178. doi: 10.1111/head.12997. [DOI] [PubMed] [Google Scholar]
- SMITH J, ALLMAN D, BADRI H, MILLER R, MORRIS J, SATIA I, WOOD A, TROWER M. The neurokinin-1 receptor antagonist orvepitant is a novel antitussive therapy for chronic refractory cough: results from a Phase 2 Pilot Study (VOLCANO-1) Chest. 2020;157:111–118. doi: 10.1016/j.chest.2019.08.001. [DOI] [PubMed] [Google Scholar]
- SONG DJ, SONG WJ, KWON JW, KIMGW, KIM MA, KIM MY, KIM MH, KIM SH, KIM ST, KIM SH, KIM JK, KIM JH, KIM HJ, KIM HB, PARK KH, YOON JK, LEE BJ, LEE SE, LEE YM, LEE YJ, LIM KH, JEON YH, JO EJ, JEE YK, JIN HJ, CHOI SH, HUR GY, CHO SH, KIM SH, LIM DH. KAAACI Evidence-based clinical practice guidelines for chronic cough in adults and children in Korea. Allergy Asthma Immunol Res. 2018;10:591–613. doi: 10.4168/aair.2018.10.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG WJ, MORICE AH. Cough hypersensitivity syndrome: a few more steps forward. Allergy Asthma Immunol Res. 2017;9:394–402. doi: 10.4168/aair.2017.9.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG WJ, WON HK, AN J, KANG SY, JO EJ, CHANG YS, LEE BJ, CHO SH. Chronic cough in the elderly. Pulm Pharmacol Ther. 2019;56:63–68. doi: 10.1016/j.pupt.2019.03.010. [DOI] [PubMed] [Google Scholar]
- STONE RP, BARNES J, FULLER RW. Contrasting effects of prostaglandins E2 and F2 alpha on sensitivity of the human cough reflex. J Appl Physiol. 1992;73:649–653. doi: 10.1152/jappl.1992.73.2.649. [DOI] [PubMed] [Google Scholar]
- TOSUN E, TOPALOGLU O, AKKALYONCU B. As a rare cause of drug-induced cough: topiramate. Acta Neurol Belg. 2012;112:217–220. doi: 10.1007/s13760-012-0016-2. [DOI] [PubMed] [Google Scholar]
- TRIFILIEFF A, DA SILVA A, GIES JP. Kinins and respiratory tract diseases. Eur Respir J. 1993;6:576–587. [PubMed] [Google Scholar]
- TSOU CH, LUK HN, CHIANG SC, HSIN ST, WANG JH. Fentanyl-induced coughing and airway hyperresponsiveness. Acta Anaesthesiol Sin. 2002;40:165–172. [PubMed] [Google Scholar]
- UJIIE Y, SEKIZAWA K, AIKAWA T, SASAKI H. Evidence for substance P as an endogenous substance causing cough in guinea pigs. Am J Respir Crit Care Med. 1993;148:1628–1632. doi: 10.1164/ajrccm/148.6_Pt_1.1628. [DOI] [PubMed] [Google Scholar]
- VEGTER S, BOER P, VAN DIJK KW, VISSER S. The effects of antitussive treatment of ACE inhibitor-induced cough on therapy compliance: a prescription sequence symmetry analysis. Drug Safety. 2013;36:435–439. doi: 10.1007/s40264-013-0024-z. [DOI] [PubMed] [Google Scholar]
- VISSER LE, STRICKER BH, VELDEN JD, PAES AH, BAKKER A. Angiotensin converting enzyme inhibitor associated cough: a population-based case-control study. J Clin Epidemiol. 1995;48:851–857. doi: 10.1016/0895-4356(94)00231-E. [DOI] [PubMed] [Google Scholar]
- VUKADINOVIC D, VUKADINOVIC AN, LAVALL D, LAUFS U, WAGENPFEIL S, BOHM M. Rate of cough during treatment with angiotensin-converting enzyme inhibitors: a meta-analysis of randomized placebo-controlled trials. Clin Pharmacol Ther. 2019;105:652–660. doi: 10.1002/cpt.1018. [DOI] [PubMed] [Google Scholar]
- WILLIAMS-HERMAN D, ENGEL SS, ROUND E, JOHNSONJ, GOLM GT, MUSSER BJ, DAVIES MJ, KAUFMAN KD, GOLDSTEIN BJ. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord. 2010;10:7. doi: 10.1186/1472-6823-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOO SW, BANG S, CHUNG MW, JIN SK, KIMYS, LEE SH. Lack of association between ACE and bradykinin B2 receptor gene polymorphisms and ACE inhibitor-induced coughing in hypertensive Koreans. J Clin Pharm Ther. 2009;34:561–567. doi: 10.1111/j.1365-2710.2009.01028.x. [DOI] [PubMed] [Google Scholar]
- YUN S, GESSNER R, DIETEL C, SCHMIEDEK U, FAN H. Enhanced ovalbumin-induced airway inflammation in CD26−/− mice. Eur J Immunol. 2012;42:533–540. doi: 10.1002/eji.201041038. [DOI] [PubMed] [Google Scholar]
- YILMAZ I. Angiotensin-converting enzyme inhibitors induce cough. Turk Thorac J. 2019;20:36–42. doi: 10.5152/TurkThoracJ.2018.18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUSUF S, PITT B, DAVIS CE, HOOD WB, COHN JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]