Abstract
PURPOSE:
α-Pyrrolidinovalerophenone (α-PVP) is a second-generation synthetic cathinone which acts as an inhibitor at the dopamine and norepinephrine transporters in the brain. These novel studies determined the pharmacokinetics (PK) of α-PVP in rats and then evaluated the effects of an α-PVP vaccine on the PK profile.
METHODS:
Adult male Sprague-Dawley rats were randomly divided into treatment groups (n = 24/group) in which the vaccinated rats received an initial and two booster immunizations of the α-PVP vaccine at 0, 3, and 9 wks. Control rats received saline injections. α-PVP (0.56, 1, 3 mg/kg, sc) was then administered to both groups between 11–12 weeks and serum samples were collected for determination of α-PVP serum concentrations by LC-MS/MS (n=6 rats/treatment/time). At 13 weeks, brain, heart and kidney concentrations of α-PVP were determined by LC-MS/MS after administration of 1 mg/kg α-PVP (n=4–5 rats/treatment/time).
RESULTS:
PK values in control rats showed dose-dependent increases in maximum serum concentrations (Cmax) and area under the curve (AUCinf) values with an elimination half-life (t1/2) of approximately 2.1 h. α-PVP exhibited linear PK profile in control rats. Vaccinated rats had significantly (p<0.05) higher serum Cmax and AUCinf values than controls, and significantly reduced total body clearance, volume of distribution and t1/2 values. Vaccinated rats had significantly lower α-PVP concentrations in the brain, heart, and kidney in comparison to control rats at early time points.
CONCLUSION:
Vaccination with the novel α-PVP vaccine significantly altered serum PK leading to a time-dependent reduction in brain, kidney and heart concentrations of α-PVP compared to controls.
INTRODUCTION
α-Pyrrolidinovalerophenone (α-PVP) is a second-generation synthetic cathinone that is classified as a Schedule 1 compound in the United States. α-PVP is one of the most potent synthetic cathinones that inhibits dopamine and norepinephrine reuptake in the brain with little effect on the serotonin transporter (1–4). In behavioral assays with rodents, α-PVP causes increased hyperlocomotion, substitutes for other psychostimulants, and is self-administered (3,5–8). Metabolism of α-PVP using in vitro human liver microsomes yields six potential phase 1 metabolites with α-PVP lactam and β-hydroxy α-PVP as the most commonly formed metabolites (2,9,10).
With a paucity of medications for the treatment of substance use disorders (SUD), vaccines continue to be developed and tested as a potential treatment for cocaine, methamphetamine, nicotine and opioid SUD (11–15). Vaccines for drugs of abuse function by stimulating in vivo production of polyclonal antibodies against the drug of abuse which bind the drug with high affinity (16,17) in serum and thereby limit its distribution into organs such as the brain (18,19). Recently, there was a report of a vaccine for α-PVP that was found to lower wheel running activity and alter iv self-administration of drug patterns in male rats, but this study by Nguyen et al. did not evaluate how the vaccine altered the PK properties of α-PVP (20).
Understanding drug PK aids in the development of vaccine treatments since the antibodies act as PK antagonists, which reduce the volume of distribution, reduce total body clearance of the drug, and under optimal conditions reduce the amount and rate of entry of drugs of abuse into organs like the brain (15). We previously reported the development of a bi-specific vaccine for α-PVP and its analog 3,4-methylenedioxypyrovaleone (MDPV) that produces high affinity antibodies for both drugs of abuse (21). Our prior study only evaluated PK changes of two doses (0.56 and 3 mg/kg) at two time points (30 and 120 min) in serum, brain, kidney and heart in control and vaccinated rats.
The purpose of the current studies was to determine the PK profile of α-PVP after sc dosing and to expand on the more limited PK studies of α-PVP after subcutaneous (sc) administration in rats (21). With these new data, we gained further insights into the pharmacokinetics of α-PVP and how the bi-specific vaccine-produced changes in α-PVP half-life, volume of distribution and clearance.
METHODS
Chemicals and reagents
Racemic α-pyrrolidinovalerophenone HCl was obtained from the National Institute on Drug Abuse drug supply program (Research Triangle Institute, Research Triangle Park, NC). All α-PVP concentrations were calculated as the free base. For the vaccine, Sigma Adjuvant System (SAS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Keyhole limpet hemocyanin (ICKLH; Biosyn Corp, Carlsbad, CA), consisting of two stable subunit monomers with masses of ~360 and ~390 kDa, was used as the antigenic carrier protein for conjugation to an α-PVP hapten. This conjugate vaccine (α-PVP-ICKLH) led to the development of a bi-specific vaccine that cross reacted with α-PVP and MDPV (21). In this report the vaccine will be called an α-PVP vaccine. All other chemicals were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA) or Sigma-Aldrich Chemical Company (St. Louis, MO) unless otherwise noted.
Animals and immunization
Male Sprague-Dawley rats (8 weeks old) were purchased from Charles River (Kingston, NY). Rats were housed three per cage and food restricted to 20 g per day to maintain a stable body weight (300–350 g). Rats were divided randomly into two groups (n=24/group), phosphate buffered saline (PBS pH = 7.35) control or vaccine treatment. Rats assigned to the vaccine treatment (n=24) were injected in hind limbs (sc, 150 μl/limb, 300 μl total) with the α-PVP vaccine consisting of 100 μg of α-PVP-ICKLH in 150 μl of PBS mixed with 150 μl of Sigma Adjuvant System. Control rats were injected with 300 μl of PBS. Rats received a booster vaccination or PBS injection at 3 and 9 weeks after initial vaccine or PBS injections. Optimal scheduling of immunization was based on previous experiments in our laboratory in mice with a methamphetamine-conjugate vaccine and in rats during the development of the bispecific vaccine (21,22). All procedures were conducted in accordance with the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences and the Guide for Care and Use of Laboratory Animals (National Research Council, 2011).
Pharmacokinetic studies
Study design for the PK experiments was based on results from our previous publication of the design, synthesis and biological evaluation of this vaccine (21). In those studies, a functional titer of the rat’s antiserum was measured using radioligand binding at weeks 5, 9, 11, and 16 for α-PVP and MDPV. The results showed that the drug binding for both drugs was highest and essentially the same at weeks 11 and 16 with a very low variance. Thus, we reasoned the PK experiments for α-PVP should be conducted during this time period.
At 11 weeks after initial immunization, control and α-PVP vaccine treated rats were randomly assigned within treatments into three dosing groups (n=8/group/treatment) for use in α-PVP PK experiments followed by tissue distribution studies using the same rats. Randomization was determined using a random number generator to assign three rats within a cage to one of the three α-PVP doses (0.56, 1, 3 mg/kg, sc) for blood collections. Group assignments were only used to determine during which α-PVP dose each rat would have blood samples collected. All rats were administered ascending doses of α-PVP (0.56, 1, 3 mg/kg, sc) with each dose separated by 48–72 h.
For collecting blood via tail vein by venous puncture, tails were thoroughly cleaned and sampled starting at the tip of the tail and moving towards the base for additional sampling to preserve the vein and avoid contamination. The blood samples (50–150 μl) were collected at 7 – 8 time points while rats were restrained. These time points were 5 m, 30 m, 2, 3, 4, 6, and 8 h for the 0.56 mg/kg group with an additional 10 h sample for the 1 and 3 mg/kg α-PVP group and an additional 12 h sample for the 3 mg/kg α-PVP group. The total blood volume collected during each experiment was <1% of rat’s total body weight. Blood samples were maintained at 4°C to allow for clotting, centrifuged 7 min at 21,000 rcf at 4 °C for serum collection and stored at −80°C. A blood sample at 48–72 h after each dose was collected to ensure no α-PVP was detectable in the serum.
Two weeks after the administration of the final α-PVP dose (3 mg/kg) from the initial serum PK studies, rats were administered 1 mg/kg of α-PVP (sc) for tissue distribution studies. The rats were sacrificed at 30 m, 2, 4, 6, and 8 h after drug administration (n=4–5 rats/treatment). Blood, brain, heart, and kidney were collected from each rat. Organs were quickly weighed, placed in liquid nitrogen, and then stored at −80°C. Blood samples were kept on ice, allowed to clot for >1 h, and centrifuged at 21,000 rcf. Serum was collected and stored at −80°C.
LC-MS/MS (liquid chromatography-mass spectrometer/mass spectrometer) analysis of α-PVP concentrations
Racemic α-PVP serum and tissue concentrations were determined at the McWhorter School of Pharmacy, Pharmaceutical Sciences Research Institute at Samford University, (Birmingham, AL). The chromatography system consisted of a HPLC (high performance liquid chromatography; Shimadzu, Columbia, MD) equipped with dual HPLC pumps, an autosampler and an in-line degasser. Detection was performed using an Applied BioSystems 4000 QTRAP (Applied BioSystems, Forest City, CA) LC-MS/MS. Mass calibration, data acquisition and quantitation were performed using Applied BioSystems software (V1.6.2). A Phenomenex Luna Omega Polar C18 100 × 2.1 mm 5 μm analytical column (Torrance, CA) was used with a gradient of distilled water with 0.1% formic acid and acetonitrile with 0.1% formic acid at a flow rate of 0.4 mL/min.
Rat serum (20 μl) proteins were precipitated with ice cold acetonitrile (n=6/group/treatment). Based on a power analysis, only 6 of the 8 rats/group/treatment were analyzed for the initial PK study with the additional 2 rats serving as supporting subjects, if needed, and to complete the numbers for the tissue distribution study. After centrifugation for 5 min at 21,000 x g, the supernatant was analyzed by LC-MS/MS in the positive ion mode with 10 ng/mLof α-pyrrolidinopentiophenone-d8 hydrochloride (α-PVP-d8; Cayman Chemical, Ann Arbor, MI) as the internal standard. Based on the results from method validation the lower limit of quantitation (LLOQ) across the validated range was 0.5 ng/mL and the upper limit of quantitation (ULOQ) was 1000 ng/mL. The accuracy and precision of the method showed average values in the range of 85% to 115% and coefficients of variation of less than 15% for the quality control concentrations, respectively. Over a concentration range of 1–500 ng/mL, recovery of α-PVP averaged 91.7% and 101%, respectively. α-PVP response in the standards averaged 99.2% in extracted serum samples for at least 59 h at 4 °C. α-PVP was stable through three freeze thaw cycles. Methods were also developed and validated for determining concentrations of α-PVP in brain, heart and kidney. The calibration range for all tissue samples was 5, 50, and 500 ng/ml. Rat brains were homogenized with 4 volumes (1:5) of cold 5 mM ammonium acetate buffer (n=4–5 rats/treatment). Heart and kidney from the same rats were homogenized with 9 volumes (1:10) of ammonium acetate buffer. The drug was extracted, and drug concentrations were determined by LC-MS/MS as described for serum (see above).
PK data and statistical analysis
The PK parameters were determined using Phoenix WinNonLin (V8, Certara USA, Princeton, NJ). Noncompartmental analysis with extravascular administration by the sc route was chosen as the PK model. The best-fit line to each rats α-PVP log-linear concentration-time terminal elimination phase at each dose was selected based on WinNonLin best-fit line values and visual inspection. PK values for the terminal elimination half-life (t1/2), area under the α-PVP concentration time curve extrapolated to time infinity (AUCinf) and observed maximum concentration (Cmax) were determined. The estimated total body clearance (CL/F) and estimated volume of distribution (Vd/F) were also calculated for α-PVP in each rat. The CL/F and Vd/F values were considered estimates, since AUCinf was not determined after an iv dose in the current study to calculate actualbioavailability. These values for CL/F and Vd/F were used with the assumption that the dose was completely absorbed after sc dosing and that the fraction of the dose absorbed was 1.0 (100 % bioavailable).
Statistical comparisons of PK values were computed using GraphPad Prism (V7, San Diego, CA). Results are presented as mean ± SD except for t1/2 which are reported as harmonic mean ± a pseudo standard deviation as described by Lam et al. (23). PK parameters were compared using a two-way ANOVA. Statistical significance was declared at p<0.05.
RESULTS
Pharmacokinetic data for α-PVP in control and vaccinated animals
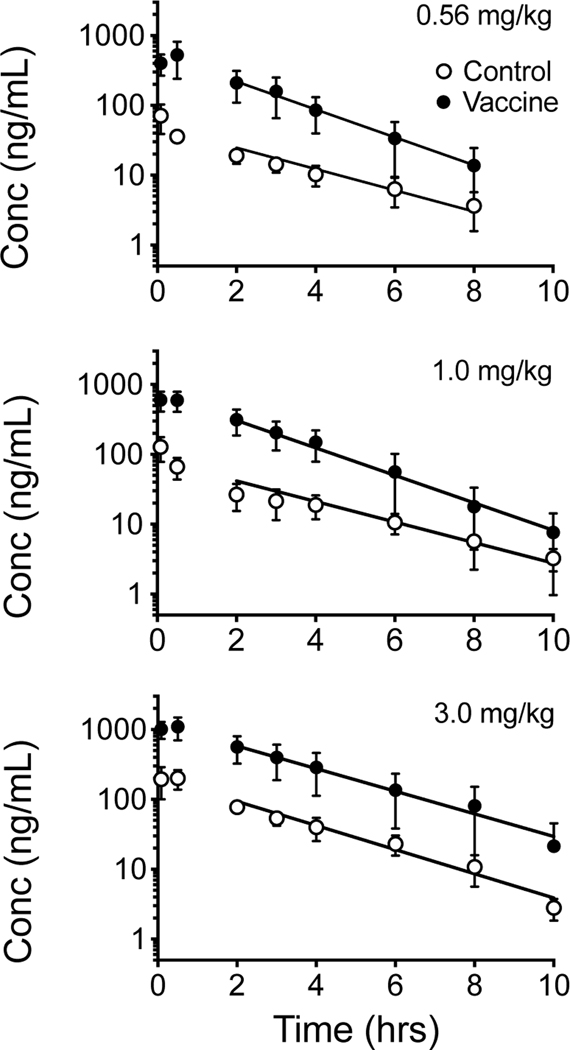
Figure 1 shows the average rat serum concentration versus time plots of α-PVP after sc administration of three different doses of α-PVP in non-vaccinated controls and vaccinated male SD rats (n=6/treatment). This analysis was for graphical presentation in this figure only. The actual PK parameters in Tables 1 and 2 were based on the average values calculated from each rat’s data set for each dose.
Figure 1.
Average serum concentration versus time curves of α-PVP after a sc dose of 0.56, 1.0 and 3.0 mg/kg α-PVP in non-vaccinated control and vaccinated male SD rats (n=6 per group). Symbols denote average observed concentration. The best-fit line for the terminal elimination phase on this plot is based on the average individual concentrations represented as mean ± SD. See Table 1 for statistical comparison of all pharmacokinetic values after sc dosing based on the noncompartmental PK analysis of individual rat data sets at each dose and each treatment.
Table 1.
Pharmacokinetic parameters for α-PVP after 0.56, 1, and 3 mg/kg sc administration of α-PVP in control and vaccinated rats reported as mean ± SD.
| Dose (mg/kg) | Treatment (N) | Half-life (h) | Cmax (ng/mL) | AUCinf (n•min/mL) | CL/F (mL/min/kg) | Vd/F (L/kg) |
|---|---|---|---|---|---|---|
| 0.56 | Control (6) | 1.97 ± 0.97 | 75 ± 24 | 7943 ± 1791 | 73 ± 14 | 14.1 ± 4.7 |
| Vaccine (6) | 1.35 ± 0.23* | 557 ± 273* | 75932 ±37671* | 9 ± 5* | 1.1 ± 5.1* | |
|
| ||||||
| 1.0 | Control (6) | 2.54 ± 0.58 | 135 ± 37 | 13634 ± 3327 | 77 ±17 | 17.9 ± 5.9 |
| Vaccine (6) | 1.33 ± 0.26* | 646 ± 181* | 100958 ±40732* | 11 ± 5* | 1.3 ± 0.3* | |
|
| ||||||
| 3.0 | Control (6) | 1.82 ± 0.36 | 291 ± 71 | 32712 ± 4501 | 94 ± 15 | 15.6 ± 4.0 |
| Vaccine (6) | 1.77 ± 0.55 | 1117 ± 354* | 208402 ±98795* | 18 ± 12* | 2.7 ± 1.0* | |
Asterisks indicate significant difference from the control rats at the same α-PVP dose (*p<0.05).
Table 2.
Pharmacokinetic parameters of α-PVP corrected for dose after 0.56, 1, and 3 mg/kg (sc) administration of α-PVP in control and vaccinated rats. The time to maximum concentration at all three doses occurred at either the 5 or 30 min blood collection time point in both the control and vaccinated rats (see Figure 1).
| Dose (mg/kig) | Treatment (N) | Cmax (ng/mL) | AUCinf (n•mm/mL) |
|---|---|---|---|
| 0.56 | Control (6) | 134 ± 42 | 14184±3198 |
| Vaccine (6) | 994 ± 487 | 135592±67270 | |
|
| |||
| 1.0 | Control (6) | 135 ± 37 | 13634±3327 |
| Vaccine (6) | 646 ± 181 | 100958 ±40732 | |
|
| |||
| 3.0 | Control (6) | 77 ± 24 | 10904± 1500 |
| Vaccine (6) | 372± 118 | 69467 ± 32932 | |
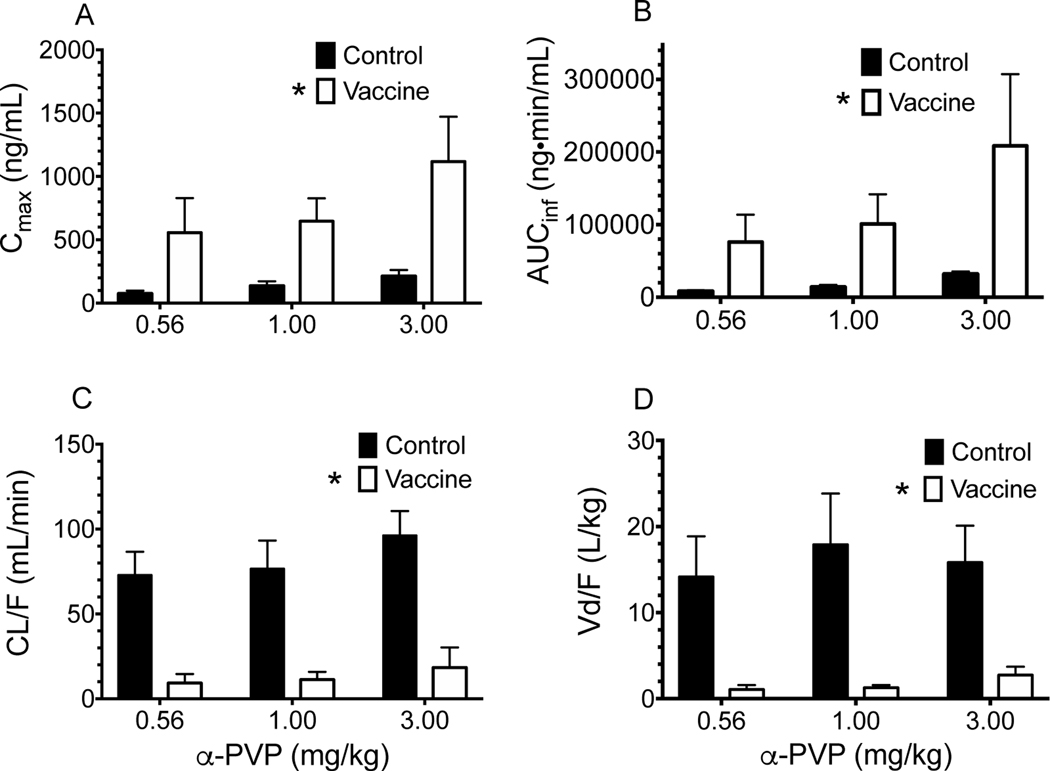
Table 1 summarizes the PK parameters of α-PVP calculated from the concentrations versus time data sets of individual rats. Table 2 shows the PK values for Cmax and AUCinf corrected for α-PVP dose. Vaccination with the α-PVPvaccine significantly altered pharmacokinetic parameters of α-PVP (Table 1 and Figure 2). There was a main effect of treatment on the CL/F, Vd/F, observed Cmax, and AUCinf values for α-PVP. CL/F and Vd/F values were significantly (p<0.05) decreased in comparison to control rats, while Cmax and AUCinf were significantly increased in vaccinated rats. There was a significant decrease in half-life in vaccinated rats at 0.56 and 1.0 mg/kg sc administration of α-PVP.
Figure 2.
Comparison of pharmacokinetic parameters in control and vaccinated rats at three different α-PVP doses. These plots were created from the data in Table 1 and 2. Asterisks indicate significant difference in main effect from non-vaccinated control rats (p<0.05).
Organ concentration-time profiles of α-PVP in control and vaccinated rats
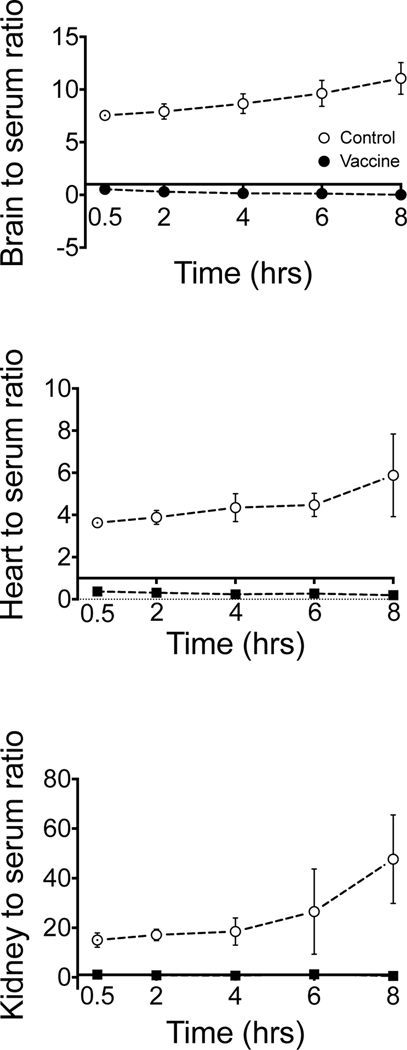
Following completion of individual rat serum PK studies (Figure 1 and Tables 1, 2, and 3), the same animals were used for studies of determination of organ concentrations of α-PVP at 1 mg/kg sc over time in control and vaccinated rats (n=4–5 rats/treatment/time point). There was a statistically significant (p <0.05) main effect of treatment for brain, kidney, and heart with vaccinated rats having lower concentrations of α-PVP than non-vaccinated rats (Figure 3 B-D). Population serum concentrations were significantly higher in vaccinated compared to non-vaccinated rats (Figure 3 A). Figure 4 compares the relative changes in PK parameters of α-PVP represented as a ratio of tissue to serum concentrations. The concentrations were derived from the data points used to plot Figure 3.
Figure 3.
Concentrations of α-PVP in serum, brain, kidney, and heart after 1 mg/kg α-PVP (sc) in control and vaccinated male SD rats (n=4–5 rats/treatment/time; mean ± SD). Please note that the y-axis on each plot is a different concentration range. Asterisks indicate significant difference from non-vaccinated control rats (p<0.05).
Figure 4.
Serum to tissue ratios over time in the brain, heart and kidney after 1 mg/kg α-PVP (sc) in control and vaccinated male SD rats (n=4–5 rats/treatment/time; mean ± SD). Please note that the y-axis on each plot is a different ratio range.
DISCUSSION
This study reports the pharmacokinetics of a-PVP across multiple doses in serum and tissue organs in rats, and the PK profile with and without vaccination with an a-PVP vaccine. Human users report orally administering 1–2 mg of a-PVP to induce psychoactive effects and 20–25 mg of a-PVP to achieve “strong” effects (24). This corresponds to a range of 0.014 –0.36 mg/kg of α-PVP dose in a 70 kg individual. Human blood samples of intoxicated a-PVP users that sought medical attention or had impaired driving range from 5 –90 ng/mL (25,26). Fatalities involving a-PVP use have blood concentrations ranging from 33 to > 20,000 ng/mL, with the large toxicity range attributable to the unknown time of dose administration (26–29). In the current study, the serum concentrations of a-PVP in control male SD rats after administration of 3 mg/kg reached an average observed Cmax value of 291 ng/mL (Table 1), which is high compared to self-reported values in humans, but much lower than values associated with a human fatality.
While the average terminal elimination half-life of a-PVP in control rats ranged from 1.82 to 2.54 h (average of range values = 2.18 h, Table 1), there was only a significant dose-dependent difference between t1/2 values in control rats after sc administration of 0.56 and 1 mg/kg a-PVP. In comparison to the a-PVP structural analog MDPV, the average t1/2 of a-PVP (2.1 h) is longer than the average t1/2 of racemic MDPV at 1.3 h after sc administration in SD male rats (30). This means the t1/2 of a-PVP is 62% longer than the t1/2 for MDPV after sc administration. If a difference of this magnitude was found between the t1/2 values for the two drugs in humans it could attribute to the lower doses of a-PVP self-administered in humans as compared with MDPV, despite similar affinities at norepinephrine and dopamine transporters and equipotency in in vivo assays (24).
Like MDPV, the data points from the a-PVP terminal elimination phase are linear on a log concentration-time curve (Figure 1). This finding suggests linear PK properties over the range of a-PVP doses studied. Also, a-PVP does not show dose-dependent differences in serum AUCinf (when corrected for each a-PVP dose), CL/F, or Vd/F values (Tables 1 and 2) in controls. A limitation to this second conclusion is the assumption that the bioavailability after sc dosing is approximately 100%. Nevertheless, these data suggest a-PVP has linear pharmacokinetics, which was also reported for sc administered MDPV by Horsley et al. (31) in male SD rats.
Active vaccination of the rats with the α-PVP vaccine significantly altered the serum pharmacokinetics of α-PVP (Figure 2). The apparent CL/F and Vd/F of α-PVP in vaccinated rats were significantly lower than controls, while the Cmax and AUCinf were significantly higher (Table 1, Figure 2). The t1/2 of α-PVP was significantly lower after 0.56 and 1 mg/kg α-PVP; however, there was no difference in t1/2 between control and vaccinated rats at 3 mg/kg α-PVP (Table 1). In our study, the value for Vd/F decreased more than the value for CL/F in vaccinated rats. This resulted in a shorter t1/2 compared to control rats, as predicted from the PK equation t1/2 = 0.693 × (Vd/CL). This finding is also reported in studies of phencyclidine PK changes after treating rats or dogs with an anti-phencyclidine monoclonal antibodies or polyclonal antibody, respectively. In both of these phencyclidine studies, the t1/2 of phencyclidine remained unchanged after antibody administration due to an equal order of magnitude decrease in Vd/F and CL/F(32,33). A similar equal order of magnitude change in CL/F and Vd/F was produced with the α-PVP vaccine, which led to minimal change in t1/2. Regardless, due to the reversible binding of α-PVP with the antibody, α-PVP having a t1/2 vastly shorter than that of the antibodies in vaccinated animals (i.e., hrs vs weeks, respectively). Due to this reversible binding, the unbound antibodies are regenerated to inhibit multiple self-administrations of drug (34).
Changes in the pharmacokinetic properties of α-PVP with this α-PVP vaccine successfully decreased the distribution of α-PVP into organs after sc administration of 1 mg/kg α-PVP (Figure 3). In the brain and kidney, the vaccine significantly decreased the concentration of α-PVP at 0.5 and 2 h. The heart concentrations were only decreased at 0.5 h. In a previous study from our group the duration of locomotor activity following a 1 mg/kg α-PVP dose averaged 191 min in the controls and 93 min in vaccinated rats (21). The total distance traveled was also significantly reduced in the vaccinated rats at this dose. While the rate of entry of α-PVP into the brain is unknown, MDPV is reported to reach maximum brain concentrations in ~ 0.5 h in male rats after sc administration (35). It is likely that the α-PVP equilibrates in the brain similarly to MDPV and that the α-PVP vaccine was able to blunt α-PVP entry for at least the first 2 h. This is important since the effects of the vaccine at a test dose of 3 mg/kg α-PVP sc failed to show significant reduction in brain concentration and failed to show effects on the total distance traveled (21).
An important observation was the very high values for the apparent Vd/F, which suggested extensive extravascular distribution. Average values for Vd/F ranged from about 14.1 to 17.9 L/kg (Table 1) after sc doses of 0.56 to 3.0 mg/kg, which aligns with the range of Vd/F values for MDPV (11.2 to 6.9 L/kg) after iv doses of 3 and 5.6 mg/kg (36). These very high values for the apparent volume of distribution suggest that the antibodies are under constant pressure to confine the α-PVP in the serum and extracellular fluid compartments of the body, where the antibodies are circulating. Indeed, this is supported by the serum to tissue ratios in Figure 4 which shows elevated ratios for control rats across time compared to vaccinated rats with ratios below 1. These data provide important insights into the time-dependence of antibody sequestering of α-PVP within the space that the antibody occupies. These observations in changing the volume of distribution of drug binding are not apparent in the plots of concentration over time (Figure 3).
In conclusion, male rats exhibited linear serum pharmacokinetics following sc doses of α-PVP. Active vaccination with the α-PVP vaccine significantly increased the concentration of α-PVP in the serum leading to substantial decreased values for CL/F and Vd/F of α-PVP. Significant differences in the pharmacokinetics of α-PVP between control and vaccinated rats begin to diminish at 3 mg/kg indicating the capacity of the anti-α-PVP antibodies is likely being approached. Nonetheless, the α-PVP vaccine treatment group showed significantly reduced α-PVP concentrations in brain, heart, and kidney tissues after 1 mg/kg of α-PVP. Considering that data from human use produces α-PVP concentrations similar to those reported in this study, it is possible that this experimental therapy could have a similar positive effect in humans (23–27).
ACKNOWLEDGEMENTS:
This research was supported by grants from the National Institute on Drug Abuse (R01 DA039195 to SMO and F31 DA046121 to SJM) and from the National Institute of Health (T32 GM106999). We thank Dr. Greg Gorman of the McWhorter School of Pharmacy at Samford University in Birmingham, AL for the determination of α-PVP concentrations in serum and tissue samples.
ABBREVIATIONS
- α-PVP
α-pyrrolidinovalerophenone
- AUCinf
area under the α-PVP concentration time curve extrapolated to time infinity
- CL/F
estimated total body clearance
- Cmax
maximum serum concentration
- ICKLH
keyhole limpet hemocyanin
- iv
intravenous
- LC-MS/MS
liquid chromatography-mass spectrometer/mass spectrometer
- MDPV
3,4-methylenedioxypyrovaleone
- PBS
phosphate buffered saline
- PK
pharmacokinetic
- SD
Sprague Dawley
- sc
subcutaneous
- t1/2
terminal elimination half-life
- Vd/F
estimated volume of distribution
Footnotes
CONFLICT OF INTEREST
No conflict of interest to report.
REFERENCES
- 1.Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: Para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol. Elsevier; 2015. March 1;25(3):365–76. doi: 10.1016/j.euroneuro.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 2.Zawilska JB, Wojcieszak J. α-Pyrrolidinophenones: a new wave of designer cathinones. Forensic Toxicol. Springer Japan; 2017. January 25;35(2):201–16. doi: 10.1007/s11419-016-0353-6 [DOI] [Google Scholar]
- 3.Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology. 2014. December;87:206–13. doi: 10.1016/j.neuropharm.2014.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, et al. Structure-Activity Relationships of Substituted Cathinones, with Transporter Binding, Uptake, and Release. J Pharmacol Exp Ther. American Society for Pharmacology and Experimental Therapeutics; 2017. January 1;360(1):33–47. doi: 10.1124/jpet.116.236349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl). Springer Berlin Heidelberg; 2015;232(16):3045–55. doi: 10.1007/s00213-015-3944-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatch MB, Dolan SB, Forster MJ. Comparative Behavioral Pharmacology of Three Pyrrolidine-Containing Synthetic Cathinone Derivatives. J Pharmacol Exp Ther. American Society for Pharmacology and Experimental Therapeutics; 2015. August 1;354(2):103–10. doi: 10.1124/jpet.115.223586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gannon BM, Baumann MH, Walther D, Jimenez-Morigosa C, Sulima A, Rice KC, et al. The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacol. 8 ed. Nature Publishing Group; 2018. November;43(12):2399–407. doi: 10.1038/s41386-018-0209-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gannon BM, Rice KC, Collins GT. Reinforcing effects of abused “bath salts” constituents 3,4-methylenedioxypyrovalerone and α-pyrrolidinopentiophenone and their enantiomers. Behav Pharmacol. Behavioural Pharmacology; 2017. October;28(7):578–81. doi: 10.1097/FBP.0000000000000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Negreira N, Erratico C, Kosjek T, van Nuijs ALN, Heath E, Neels H, et al. In vitro Phase I and Phase II metabolism of α-pyrrolidinovalerophenone (α-PVP), methylenedioxypyrovalerone (MDPV) and methedrone by human liver microsomes and human liver cytosol. Anal Bioanal Chem. Springer Berlin Heidelberg; 2015. May 27;407(19):5803–16. doi: 10.1007/s00216-015-8763-6 [DOI] [PubMed] [Google Scholar]
- 10.Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy‐pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC–MS and LC–high‐resolution MS and its detectability in urine by GC–MS. J Mass Spectrom. John Wiley & Sons, Ltd; 2010. December 1;45(12):1426–42. doi: 10.1002/jms.1859 [DOI] [PubMed] [Google Scholar]
- 11.Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD. Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angewandte Chemie. 2016. March 7;128(11):3836–9. doi: 10.1002/ange.201511654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bremer PT, Schlosburg JE, Banks ML, Steele FF, Zhou B, Poklis JL, et al. Development of a Clinically Viable Heroin Vaccine. J Am Chem Soc. American Chemical Society; 2017. June 28;139(25):8601–11. doi: 10.1021/jacs.7b03334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med 1996. 2:10. Nature Publishing Group; 1996 Oct 1;2(10):1129. doi: 10.1038/nm1096-1129 [DOI] [PubMed] [Google Scholar]
- 14.McCluskie MJ, Thorn J, Mehelic PR, Kolhe P, Bhattacharya K, Finneman JI, et al. Molecular attributes of conjugate antigen influence function of antibodies induced by anti-nicotine vaccine in mice and non-human primates. Int J Immunopharmacol. 2015. April;25(2):518–27. doi: 10.1016/j.intimp.2015.02.030 [DOI] [PubMed] [Google Scholar]
- 15.Rüedi-Bettschen D, Wood SL, Gunnell MG, West CM, Pidaparthi RR, Carroll FI, et al. Vaccination protects rats from methamphetamine-induced impairment of behavioral responding for food. Vaccine. 2013. September;31(41):4596–602. doi: 10.1016/j.vaccine.2013.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janda KD, Treweek JB. Vaccines targeting drugs of abuse: is the glass half-empty or half-full? Nat Rev Immunol 2011. 12:1. Nature Publishing Group; 2012 Jan 1;12(1):67–72. doi: 10.1038/nri3130 [DOI] [PubMed] [Google Scholar]
- 17.Kosten TR, Domingo CB. Can you vaccinate against substance abuse? Expert Opin Biol Ther. 4 ed. Taylor & Francis; 2013. July 9;13(8):1093–7. doi: 10.1517/14712598.2013.791278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantak KM. Vaccines Against Drugs of Abuse. Drugs. Springer International Publishing; 2003;63(4):341–52. doi: 10.2165/00003495-200363040-00001 [DOI] [PubMed] [Google Scholar]
- 19.Shen XY, Orson FM, Kosten TR. Vaccines Against Drug Abuse. Clin Pharmacol Ther. John Wiley & Sons, Ltd; 2012. January 1;91(1):60–70. doi: 10.1038/clpt.2011.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen JD, Bremer PT, Ducime A, Creehan KM, Kisby BR, Taffe MA, et al. Active vaccination attenuates the psychostimulant effects of α-PVP and MDPV in rats. Neuropharmacology. 2017. April;116:1–8. doi: 10.1016/j.neuropharm.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClenahan SJ, Kormos CM, Gunnell MG, Hambuchen MD, Lamb P, Carroll FI, et al. Design, synthesis and biological evaluation of a bi-specific vaccine against α-pyrrolidinovalerophenone (α-PVP) and 3,4-methylenedioxypyrovalerone (MDPV) in rats. Vaccine. 2020. January 10;38(2):336–344. doi: 10.1016/j.vaccine.2019.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens MW, Gunnell MG, Tawney R, Owens SM. Optimization of a methamphetamine conjugate vaccine for antibody production in mice. Int J Immunopharmacol. 2016. June;35:137–41. doi. 10.1016/j.intimp.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam FC, Hung CT, Perrier DG. Estimation of Variance for Harmonic Mean Half-Lives. J Pharm Sci. 1985. February;74(2):229–31. doi: 10.1002/jps.2600740229 [DOI] [PubMed] [Google Scholar]
- 24.Karila L, Lafaye G, Scocard A, Cottencin O, Benyamina A. MDPV and α-PVP use in humans: The twisted sisters. Neuropharmacol. 2018. May 15;134(Pt A):65–72. doi: 10.1016/j.neuropharm.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 25.Knoy JL, Peterson BL, Couper FJ. Suspected impaired driving case involving α-pyrrolidinovalerophenone, methylone and ethylone. J Anal Toxicol. 2014. October;38(8):615–7. doi: 10.1007/s11419-017-0385-6 [DOI] [PubMed] [Google Scholar]
- 26.Wright TH, Harris C. Twenty-One Cases Involving Alpha-Pyrrolidinovalerophenone (α-PVP). J Anal Toxicol. 2016. June;40(5):396–402. doi: 10.1093/jat/bkw029 [DOI] [PubMed] [Google Scholar]
- 27.Banaś BP, Janus T, Majdanik S, Banaś T, Dembińska T, Borowiak K. Fatal Intoxication with α‐PVP, a Synthetic Cathinone Derivative. J Forensic Sci. John Wiley & Sons, Ltd (10.1111); 2017. March 1;62(2):553–6. doi: 10.1093/jat/bkw029 [DOI] [PubMed] [Google Scholar]
- 28.Sykutera M, Cychowska M, Bloch-Boguslawska E. A Fatal Case of Pentedrone and α-Pyrrolidinovalerophenone Poisoning. J Anal Toxicol. Narnia; 2015. May 1;39(4):324–9. doi: 10.1093/jat/bkv011 [DOI] [PubMed] [Google Scholar]
- 29.Nagai H, Saka K, International MN, 2014. Sudden death after sustained restraint following self-administration of the designer drug α-pyrrolidinovalerophenone. Int J Cardiol 172, 263–265. doi: 10.1016/j.ijcard.2013.12.262 [DOI] [PubMed] [Google Scholar]
- 30.Anizan S, Concheiro M, Lehner KR, Bukhari MO, Suzuki M, Rice KC, et al. Linear pharmacokinetics of 3,4‐methylenedioxypyrovalerone (MDPV) and its metabolites in the rat: relationship to pharmacodynamic effects. Addict Biol. 2016. March 1;21(2):339–47. doi: 10.1111/adb.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horsley RR, Lhotkova E, Hajkova K, Feriancikova B, Himl M, Kuchar M, et al. Behavioural, Pharmacokinetic, Metabolic, and Hyperthermic Profile of 3,4-Methylenedioxypyrovalerone (MDPV) in the Wistar Rat. Front Psychiatry. Frontiers; 2018. April 24;9. doi: 10.3389/fpsyt.2018.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valentine JL, Arnold LW, Owens SM. Anti-phencyclidine monoclonal Fab fragments markedly alter phencyclidine pharmacokinetics in rats. J Pharmacol Exp Ther. American Society for Pharmacology and Experimental Therapeutics; 1994. June 1;269(3):1079–85. doi: 10.1002/(ISSN)2052-1707 [DOI] [PubMed] [Google Scholar]
- 33.Owens SM, Mayersohn M. Phencyclidine-specific Fab fragments alter phencyclidine disposition in dogs. Drug Metab Dispos. American Society for Pharmacology and Experimental Therapeutics; 1986. January 1;14(1):52–8. doi: 10.1002/(ISSN)2052-1707 [DOI] [PubMed] [Google Scholar]
- 34.Stevens MW, Tawney RL, West CM, Kight AD, Henry RL, Owens SM, et al. Preclinical characterization of an anti-methamphetamine monoclonal antibody for human use. MAbs. 2014. March;6(2):547–55. doi: 10.4161/mabs.27620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novellas J, López-Arnau R, Carbó ML, Pubill D, Camarasa J, Escubedo E. Concentrations of MDPV in rat striatum correlate with the psychostimulant effect. J Psychopharmacol (Oxford). SAGE PublicationsSage UK: London, England; 2015. November;29(11):1209–18. Oxford). 29, 1209–1218. doi: 10.1177/0269881115598415 [DOI] [PubMed] [Google Scholar]
- 36.Hambuchen MD, Hendrickson HP, Gunnell MG, McClenahan SJ, Ewing LE, Gibson DM, et al. The pharmacokinetics of racemic MDPV and its (R) and (S) enantiomers in female and male rats. Drug Alcohol Depend. Elsevier; 2017. October 1;179:347–54. doi: 10.1016/j.drugalcdep.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]