Abstract
Crimean–Congo haemorrhagic fever (CCHF) is an emerging tick‐borne disease causing severe and fatal haemorrhagic syndrome in humans. Hyalomma spp. ticks are the primary vectors and sheep are important CCHF virus (CCHFV)‐amplifying hosts. In this study, blood samples and ticks collected in October 2019 from 270 sheep from 15 farms across Tunisia constituted the main research material. Moreover, the sera of the same animals taken at different periods between 2018 and 2019 were also used to obtain comparative results. To investigate the presence of anti‐CCHFV antibodies in sheep, all sera were tested using ELISA. Reactive sera were further characterised by a virus neutralisation test (VNT). Overall, one out of the 270 tested sheep was both ELISA‐ and strongly VNT‐positive to CCHFV. Another two sheep were borderline ELISA‐positive but did not exhibit neutralising antibodies. Ninety‐one ticks were collected from all sampled sheep, of which 34 (37.4%) belonged to Hyalomma spp. This is the first report of anti‐CCHFV antibodies in sheep from Tunisia. Both the results of this study and the recent CCHFV detection in ticks collected from camels in southern Tunisia indicate that further studies are needed to determine the competent tick vector in the country and to characterise the epidemiological cycle of CCHFV.
Keywords: Crimean–Congo haemorrhagic fever virus, ELISA, sheep, Tunisia, virus neutralisation test
This is the first report of Crimean Congo haemorrhagic fever virus seroprevalence in sheep in Tunisia. Despite the low seroprevalence, there is evidence of CCHFV introduction in Tunisia, may be through migratory birds carrying infected ticks from Europe. Further study on CCHFV epidemiology among ticks, hosts and humans should be urgently conducted.
1. INTRODUCTION
Crimean–Congo haemorrhagic fever virus (CCHFV) is an Orthonairovirus (Nairoviridae family) that causes severe haemorrhagic disease in humans (Wang et al., 2012). This emerging tick‐borne virus is endemic in several countries in Europe, Africa and Asia (Papa et al., 2017). In humans, after the occurrence of a severe haemorrhagic phase, the lethality rate can reach up to 50% (Centers for Disease Control and Prevention, 2013). Transmission occurs primarily through tick bites (mainly Hyalomma spp.) or through direct contact with blood or body tissues/fluids from viremic humans and animals (Appannanavar & Mishra, 2011). Farmers, physicians, veterinary surgeons and slaughterhouse workers are therefore considered particularly endangered occupational groups (Mostafavi et al., 2017).
In contrast to humans, infected animals do not develop any clinical symptoms despite being viraemic for a mean period of one week (Bente et al., 2010; Spengler et al., 2016a). During this period, they are infectious for humans, other animals and ticks. Most domestic animals (cattle, camels, sheep, goats, horses, pigs, dogs and chicken) seroconvert after being infected by CCHFV (Spengler et al., 2016b). Apart from having a role acting as a reservoir of CCHFV (Akuffo et al., 2016), livestock animals have an important role in spreading the infection via their movements and the ticks they harbour (Mertens et al., 2015).
Sheep are considered to be CCHFV‐amplification hosts (Schuster et al., 2016a; Wilson et al., 1991). In Senegal and Bulgaria, the reported seroprevalence using IgG ELISA ranged between 10.4% (n = 942) (Wilson et al., 1990) and 74% (n = 242) (Barthel et al., 2014), respectively. To our knowledge, there are no reports of CCHFV infections in sheep from North African countries.
Detection of emerging viruses such as CCHFV in Tunisia, located on one of the most important wild bird migration routes between Africa and Europe, could be of intercontinental significance. It is well documented that migratory birds can carry infected ticks and may participate in dissemination of emerging viruses under some circumstances (Gharbi, 2020). In this study, the presence of antibodies to CCHFV was investigated in sheep flocks in 15 farms distributed in three Tunisian regions (north, centre and southeast). Sheep were selected as sentinel species because these animals are considered good indicators of presence of CCHFV in some regions (Schuster et al., 2016a). Furthermore, the recent increase in case numbers of CCHF in the Middle East–North Africa region has been attributed to careless slaughtering of livestock, inadequate knowledge on the disease and the dissemination of CCHFV through uncontrolled animal movements (Al‐Abri et al., 2017).
2. MATERIALS AND METHODS
2.1. Sampling design
In this study, 270 sheep were screened for ticks, and blood samples were taken to test for the presence of anti‐CCHFV antibodies in October 2019, approximately 2 months after the natural peak activity of Hyalomma ticks in spring and summer in Tunisia (Bouattour et al., 1999). Sera of the same animals taken at different periods between April 2018 and July 2019 were also used to obtain comparative results. The sera belonged to ewes from 15 farms distributed in six locations representing the different agro‐bioclimatic systems in Tunisia (Table 1, Figure 1). The sampled animals were 5.4 (± standard deviation 2.1) years old on average and consisted of 66% (178/270) of Barbarine breed, 30.3% (82/270) of Queue Fine de l'Ouest breed as well as 3.7% (10/270) of cross‐bred sheep.
TABLE 1.
Characteristics of the selected localities and sheep farms
| Location (District) | Region | Number of farm(s) (total number of animals) | Bioclimatic status | Latitude (N) | Longitude (E) | Mean altitude (m) |
|---|---|---|---|---|---|---|
| Fernana (Jendouba) | Northwest | 1 (48) | Humid | 36°42′20.54″ | 8°48′46.5″ | 500 |
| Mornaguia (Manouba) | Northeast | 3 (58) | Sub‐humid |
36°37′20.84″ 36°39′38.58″ 36°46′30.86″ |
9°56′55.63″ 9°57′33.53″ 9°57′27.66″ |
122 121 57 |
| Saouef (Zaghouan) | Northeast | 3 (48) | Semi‐arid |
36°14′52″ 36°15′27″ 36°13′41.54″ |
10°9′7″ 10°8′49″ 10°10′47.17″ |
205 184 151 |
| Sbeitla (Kasserine) | Centre | 3 (62) | Arid high steppes |
35°17′44″ 35°17′44″ 35°14′28.36″ |
9°14′35.93″ 9°14′35.93″ 9°5′28.66″ |
470 470 561 |
| Bir Ali (Sfax) | Centre | 2 (29) | Arid low steppes |
34°38′75.9″ 34°46′19.4″ |
10°4′46.4″ 10°2′30.3″ |
117 203 |
| Bir Lahmar (Tataouine) | Southeast | 3 (25) | Saharan |
33°7′4.22″ 33°7′31″ 10°35′14.39″ |
10°33′42.13″ 10°33′54″ 10°35′14.39″ |
142 149 131 |
FIGURE 1.
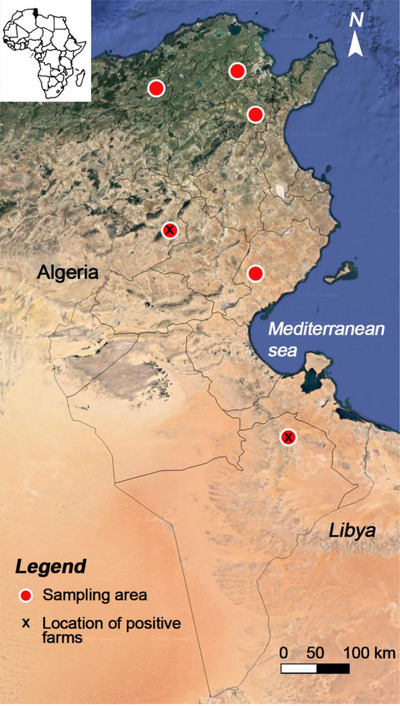
Map of Tunisia showing the location of sampled and positive farms where ELISA seropositive ewes to Crimean–Congo haemorrhagic fever virus were detected
2.2. Samples
The ewes were examined, and ticks were collected and preserved in identified tubes containing 70% ethanol. All ticks were morphologically identified at the species level under a stereomicroscope according to the identification keys described by Walker et al. (2003). For each sheep, 5 ml of blood was collected in sterile dry tubes from the jugular vein using a vacutainer. Sera were collected in Eppendorf tubes and stored at −20°C until used. Because sera were available for the previous six sampling rounds for all animals, those corresponding to ewes found positive in October 2019 were also later included in serological testing.
2.3. Serological methods
Sera were tested using the commercial CCHF Double Antigen Multi‐species ELISA (IDvet Screen®, Montpellier, France) as recommended by the manufacturer for the detection of specific antibodies against the CCHFV nucleoprotein. Optical density (OD) was read with an ELISA plate reader set at 450 nm wavelength. After plate validation, the serum titre was estimated by the ratio of Sample OD/Positive control OD and was considered positive if it exceeded 30%. In addition, to verify the ELISA result, positive sera were tested using a virus neutralisation test (VNT) as described by Hinkula et al. (2017).
Standard errors for proportions and standard deviations for means were estimated according to Schwartz (1993).
3. RESULTS
Three out of 270 (1.1% ± 0.6%) tested sera were seropositive to CCHFV by ELISA, one from the centre and two from southeast Tunisia (Table 2). The seropositive sheep (ewe no. 1) from Bir Ali locality (centre of Tunisia) was a 6‐year‐old ewe of Queue Fine de l'Ouest breed and showed a high ELISA titre (115%). The seropositive ewes no. 2 and no. 3 (Barbarine breed) were 4 and 7 years old, respectively, belonged to the same farm in Bir Lahmar region (southeast) and showed ELISA titres of 33.60% and 34.15%, respectively (Figure 2).
TABLE 2.
Distribution of ELISA seropositive animals according to age, breed, tick infestation and location
| Risk factors | Number of seropositive/number of tested (% ± SE) |
|---|---|
| Age group (years) a | |
| 1–2 | 0/20 (0) |
| >2 | 3/242 (1.2 ± 0.7) |
| Breed | |
| Barbarine | 2/178 (1.1 ± 0.8) |
| Queue Fine de l'Ouest | 1/82 (1.2 ± 1.2) |
| Cross‐bred | 0/10 (0) |
| Tick infestation | |
| Yes | 2/23 (8.6 ± 6) |
| No | 1/247 (0.4 ± 0.4) |
| Location | |
| North | 0/154 (0) |
| Centre | 1/91 (1 ± 1) |
| Southeast | 2/25 (8 ± 5.4) |
| Overall b | 3/270 (1.1 ± 0.6) |
Eight animals had no records for age.
One out of three seropositive ewes confirmed using a CCHFV serum neutralisation assay.
FIGURE 2.
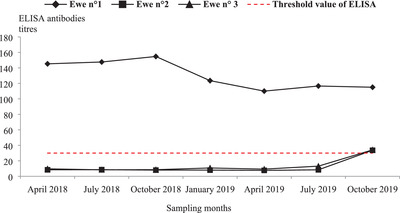
ELISA‐CCHFV antibody titre kinetics of three seropositive ewes during seven sampling rounds from April 2018 to July 2019
When testing sera collected prior to October 2019, only ewe no. 1 displayed high ELISA titres (> 110%) during the six successive sampling rounds, reaching 154.74% in October 2018 (Figure 2).
The VNT demonstrated that the serum of ewe no. 1 had a distinct titre as ND50 (50% neutralising dose) of 1:64. In contrast, the sera of ewes no. 2 and no. 3 showed no such a neutralising effect.
A total of 91 ticks were collected from 23 sheep in October 2019. Rhipicephalus sanguineus s.l. represented 63% (57/91) of the total tick species, followed by Hyalomma spp. (34/91; 37%). The Hyalomma ticks consisted of 26 H. impeltatum, 7 H. excavatum and 1 H. marginatum. The majority of ticks (81/91; 89%) were collected from sheep in the southern farm where the two seropositive animals were detected.
4. DISCUSSION
In this study, the presence of specific anti‐CCHFV antibodies was demonstrated in an animal (ewe no. 1) using ELISA followed by the VNT, which is considered one of the most specific serological tests (Canakoglu et al., 2013). Sera from ewes no. 2 and no. 3, with considerably weaker antibody levels compared to ewe no. 1, showed no neutralising effect. These two weak, positive ELISA results might be due to recent CCHFV infection in the sheep, since both ewes belonged to a farm in the same district (Tataouine) where CCHFV was recently detected in one tick collected on camels (Bouaicha et al., 2021).
The positive ewe (no. 1) for both ELISA and VNT, showed high ELISA titres during the six sampling rounds preceding the one of October 2019, hence suggesting long‐lasting IgG antibodies. In fact, after the experimental inoculation of CCHFV, naïve sheep developed persistent IgG high titres from day 8 and for more than 3 years (Gonzalez et al., 1998). Thus, persistence of IgG following the CCHFV infection makes sheep good sentinels for the presence of CCHFV in a given area (Schuster et al., 2016a). However, considering an animal species as sentinel depends mainly on the tick demography. Therefore, we suggest considering sheep as sentinel candidates for CCHFV in different parts of Tunisia taking into account their tick burden.
As far as we know, this is the first study reporting anti‐CCHFV antibody detection in sheep in Tunisia. This is in agreement with the previous detection report of anti‐CCHFV antibodies among Tunisian slaughterhouse workers reported by Wasfi et al. (2016), who suggested that CCHFV circulates in Tunisia through a cryptic cycle. Indeed, slaughterhouses pose higher‐risk areas for contracting CCHF in humans (Chiuya et al., 2020) and, to improve implementation of preventive measures, special awareness programs are recommended that aim at employees in the field.
However, the low seroprevalence that we found was not concordant with results reported elsewhere. Indeed, the seroprevalence in sheep using IgG ELISA ranged between 3.7% in Iran (Al‐Abri et al., 2017) and 75% in Afghanistan (Mustafa et al., 2011). In addition, differences in the demographic and phenological characteristics of the tick community in different territories and the vector competence and sheep affinity of these ticks are other possible factors to be considered. The VNT and the reverse transcription (RT)‐PCR require Biosafety Level 4 facilities, but this is not a strict rule for countries where the disease is mildly or highly endemic (Weidmann et al., 2016). Moreover, because viremia is short and of low intensity in livestock (OIE, 2018), ELISA offers the best alternative to detect CCHFV antibodies in sheep and is easy to implement in laboratories with limited resources (Vanhomwegen et al., 2012). Furthermore, the commercial ELISA showed better sensitivity (98%) and specificity (100%) compared to in‐house ELISA and was considered a good test for screening of CCHFV antibodies in sheep and goats (Schuster et al., 2016b).
Although it is not possible to link tick presence with the occurrence of antibodies, the geographical distribution of seropositive sheep across the centre and southeast of Tunisia is consistent with the distribution of Hyalomma spp. ticks, which are the most competent vectors for CCHFV (Spengler et al., 2016b). Indeed, H. impeltatum is found particularly in the arid bioclimatic zone (Bouattour et al., 1999), and this species was encountered in the southeast flock where we found weak ELISA‐CCHFV antibody titres in sheep. Recently, a serological survey performed in south Tunisia reported a high seropositivity rate of 89.7% (245/273) in camels (Camelus dromedarius), and 1 (H. impeltatum) out of 165 collected ticks was RT‐PCR positive (Bouaicha et al., 2021). This high seroprevalence in camels could be explained by the intensive and continuous movements of these animals in the Tunisian Sahara, between and across the Libyan and Algerian borders. This could expose them to viremic small ruminants, wild desert small mammals (rodents and hedgehogs), reptiles, birds and ticks. The presence of viral RNA in one tick collected from a host does not mean that the tick is the vector, but confirms the introduction of CCHFV in Tunisia. However, the vector role of H. impeltatum in CCHFV transmission should be confirmed.
Despite no CCHFV RNA being previously detected in Tunisia by Wasfi et al. (2016, 2019), the virus has possibly been introduced in different ways including bird migration. Tunisia is on one of the main bird migration flyways, and it is known that migratory birds play a key role in CCHFV spread, by introducing infected ticks to free areas (Leblebicioglu et al., 2014). Furthermore, there is an intense informal animal movement and trade with neighbouring countries where the presence of the virus is confirmed. For example, the virus was detected by nested RT‐PCR in Algeria in 28.6% (16/56) of H. aegyptium ticks collected from spur‐thighed tortoises (Testudo graeca) (Kautman, 2016).
It was shown that infected sheep were a virus source to 60% of H. truncatum ticks feeding on them (Wilson et al., 1991). Together with efficient transstadial and transovarian CCHFV transmission in ticks, sheep are considered as CCHFV amplifiers (Gonzalez et al., 1998); thus, active surveillance should target this animal species.
Although the seroprevalence was very low and the significance of one positive animal is difficult to interpret, our study provides evidence that sheep were exposed to CCHFV in the centre of Tunisia. Moreover, the fact that the positive sample was confirmed by the VNT underlines the validity of the study.
Because CCHFV was recently detected in the south of Tunisia, efforts should be done to deepen molecular studies in this direction. Overall, further epidemiological investigations are urgently needed, among humans, ticks, and domestic and wild animals to better characterise the epidemiology of CCHF in Tunisia.
ETHICAL STATEMENT
The sampled ewes were owned by private sheep farmers that were informed about the aim of the study and they gave their oral consent. The animals were sampled in their presence and under the supervision of a qualified veterinarians. The sampling procedures were performed according to the guidelines for the care and use of animals of the National School of Veterinary Medicine of Sidi Thabet (Tunisia).
CONFLICT OF INTEREST
Authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Khamassi Khbou, Médiha (Corresponding Author): Data curation, investigation, writing‐original draft, writing‐review and editing.
Romdhane, Rihab: Investigation
Bouaicha Zaafouri, Faten: Investigation
Bouajila, Mohsen: Investigation
Sassi, Limam: Investigation
Appelberg, Sofia K.: Investigation, writing‐review and editing
Schulz, Ansgar: Writing‐review and editing
Mirazimi, Ali: Investigation, resources, writing‐review and editing
Groschup, Martin H.: Funding acquisition, resources, writing‐review and editing
Rekik, Mourad: Funding acquisition, project administration, resources, writing‐review and editing
Benzarti, M'hammed: Conceptualisation, methodology, writing‐review and editing
Gharbi, Mohamed: Conceptualisation, methodology, project administration, supervision, visualisation, writing‐review and editing
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.597
ACKNOWLEDGEMENTS
The authors thank all the veterinarians for their valuable support and all the farmers that participated to the survey. We also acknowledge with thanks the valuable comments of the anonymous reviewers that commented and improved considerably our manuscript prior to publication. Authors acknowledge the financial support of the Ph.D. grant awarded by the ‘Arab Fund for Social and Economic Development (AFESD)’ and received through the International Center for Agricultural Research in the Dry Areas (ICARDA) under the agreement n°131001. This paper was also partly supported by the CGIAR research program on Livestock and the Tunisian Ministry of Higher Education and Scientific Research through the ‘Laboratoire d’épidémiologie d'infections enzootiques des herbivores, application à la Lutte’ LR16AGR01. This work was also supported from the European Union's Horizon 2020 research and innovation program under grant agreement n° 732732 (A.M. and M.G.).
Khamassi Khbou, M. , Romdhane, R. , Bouaicha Zaafouri, F. , Bouajila, M. , Sassi, L. , Appelberg, S. K. , Schulz, A. , Mirazimi, A. , Groschup, M. H. , Rekik, M. , Benzarti, M. , & Gharbi, M. (2021). Presence of antibodies to Crimean Congo haemorrhagic fever virus in sheep in Tunisia, North Africa. Veterinary Medicine and Science, 7, 2323–2329. 10.1002/vms3.597
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Akuffo, R. , Brandful, J. A. M. , Zayed, A. , Adjei, A. , Watany, N. , Fahmy, N. T. , Hughes, R. , Doman, B. , Voegborlo, S. V. , Aziati, D. , Pratt, D. , Awuni, J. A. , Adams, N. , & Dueger, E. (2016). Crimean‐Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infectious Diseases, 16(324), 1–5. 10.1186/s12879-016-1660-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Abri, S. S. , Al, I. , Fazlalipour, M. , Mostafavi, E. , Leblebicioglu, H. , Pshenichnaya, N. , Memish, Z. A. , Hewson, R. , Petersen, E. , Mala, P. , Nguyen, T. M. N. , Malik, M. R. , Formenty, P. , & Jeffries, R. (2017). Current status of Crimean‐Congo haemorrhagic fever in the World Health Organization Eastern Mediterranean Region: Issues, challenges, and future directions. International Journal of Infectious Diseases, 58, 82–89. 10.1016/j.ijid.2017.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appannanavar, S. B. , & Mishra, B. (2011). An update on Crimean Congo hemorrhagic fever. Journal of Global Infectious Diseases, 3(3), 285–292. 10.4103/0974-777X.83537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel, R. , Mohareb, E. , Younan, R. , Gladnishka, T. , Kalvatchev, N. , Moemen, A. , Mansour, S. S. , Rossi, C. , Schoepp, R. , & Christova, I. (2014). Seroprevalance of Crimean–Congo haemorrhagic fever in Bulgarian livestock. Biotechnology & Biotechnological Equipment, 28, (3), 540–542. 10.1080/13102818.2014.931685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente, D. A. , Alimonti, J. B. , Shieh, W.‐J. , Camus, G. , Ströher, U. , Zaki, S. , & Jones, S. M. (2010). Pathogenesis and immune response of Crimean‐Congo hemorrhagic fever virus in a STAT‐1 knockout mouse model. Journal of Virology, 84(21), 11089–11100. 10.1128/JVI.01383-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaicha, F. , Eisenbarth, A. , Elati, K. , Schulz, A. , Ben Smida, B. , Bouajila, M. , Sassi, L. , Rekik, M. , Groschup, M. H. , & Khamassi Khbou, M. (2021). Epidemiological investigation of Crimean‐Congo haemorrhagic fever virus infection among the one‐humped camels (Camelus dromedarius) in southern Tunisia. Ticks and Tick‐Borne Diseases, 12(1), 101601. 1–6. 10.1016/j.ttbdis.2020.101601 [DOI] [PubMed] [Google Scholar]
- Bouattour, A. , Darghouth, M. A. , & Daoud, A. (1999). Distribution and ecology of ticks (Acari: Ixodidae) infesting livestock in Tunisia: An overview of eighth years field collections. Parassitologia, 41(Suppl 1), 5–10. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11071534 [PubMed] [Google Scholar]
- Canakoglu, N. , Berber, E. , Ertek, M. , Yoruk, M. D. , Tonbak, S. , Bolat, Y. , Aktas, M. , Kalkan, A. , & Ozdarendeli, A. (2013). Pseudo‐plaque reduction neutralization test (PPRNT) for the measurement of neutralizing antibodies to Crimean‐Congo hemorrhagic fever virus. Virology Journal, 10(1), 6. 10.1186/1743-422X-10-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2013). Crimean‐Congo hemorrhagic fever (CCHF). Retrieved from https://www.cdc.gov/vhf/crimean‐congo/index.html
- Chiuya, T. , Masiga, D. K. , Falzon, L. C. , Bastos, A. D. S. , Fèvre, E. M. , & Villinger, J. (2020). Tick‐borne pathogens, including Crimean‐Congo haemorrhagic fever virus, at livestock markets and slaughterhouses in western Kenya. Transboundary and Emerging Diseases, 68(4), 2429–2445. 10.1111/tbed.13911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbi, M. (2020). Ticks crossing the Mare Nostrum, what risks? Bulletin de l'Académie Vétérinaire de France, 173(1), 168–179. 10.4267/2042/70868 [DOI] [Google Scholar]
- Gonzalez, J. P. , Camicas, J. L. , Cornet, J. P. , & Wilson, M. L. (1998). Biological and clinical responses of West African sheep to Crimean‐Congo haemorrhagic fever virus experimental infection. Research in Virology, 149(6), 445–455. 10.1016/s0923-2516(99)80013-2 [DOI] [PubMed] [Google Scholar]
- Hinkula, J. , Devignot, S. , Åkerström, S. , Karlberg, H. , Wattrang, E. , Bereczky, S. , Mousavi‐Jazi, M. , Risinger, C. , Lindegren, G. , Vernersson, C. , Paweska, J. , van Vuren, P. J. , Blixt, O. , Brun, A. , Weber, F. , & Mirazimi, A. (2017). Immunization with DNA plasmids coding for Crimean‐Congo hemorrhagic fever virus capsid and envelope proteins and/or virus‐like particles induces protection and survival in challenged mice. Journal of Virology, 91(10). 10.1128/JVI.02076-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautman, M. , Tiar, G. , Papa, A. , & Široký, P. (2016). AP92‐like Crimean‐Congo hemorrhagic fever virus in Hyalomma aegyptium ticks, Algeria. Emerging Infectious Diseases, 22(2), 354–356. 10.3201/eid2202.151528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblebicioglu, H. , Eroglu, C. , Erciyas‐Yavuz, K. , Hokelek, M. , Acici, M. , & Yilmaz, H. (2014). Role of migratory birds in spreading Crimean‐Congo hemorrhagic fever, Turkey. Emerging Infectious Diseases, 20(8), 1331–1334. 10.3201/eid2008.131547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens, M. , Vatansever, Z. , Mrenoshki, S. , Krstevski, K. , Stefanovska, J. , Djadjovski, I. , Cvetkovikj, I. , Farkas, R. , Schuster, I. , Donnet, F. , Comtet, L. , Tordo, N. , Mechlia, M. B. , Balkema‐Buschmann, A. , Mitrov, D. , & Groschup, M. H. (2015). Circulation of Crimean‐Congo hemorrhagic fever virus in the former Yugoslav Republic of Macedonia revealed by screening of cattle sera using a novel enzyme‐linked immunosorbent assay. PLOS Neglected Tropical Diseases, 9(3), e0003519. 10.1371/journal.pntd.0003519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi, E. , Pourhossein, B. , Esmaeili, S. , Bagheri Amiri, F. , Khakifirouz, S. , Shah‐Hosseini, N. , & Tabatabaei, S. M. (2017). Seroepidemiology and risk factors of Crimean‐Congo hemorrhagic fever among butchers and slaughterhouse workers in southeastern Iran. International Journal of Infectious Diseases, 64, 85–89. 10.1016/j.ijid.2017.09.008 [DOI] [PubMed] [Google Scholar]
- Mustafa, M. L. , Ayazi, E. , Mohareb, E. , Yingst, S. , Zayed, A. , Rossi, C. A. , Schoepp, R. J. , Mofleh, J. , Fiekert, K. , Akhbarian, Z. , Sadat, H. , & Leslie, T. (2011). Crimean‐Congo hemorrhagic fever, Afghanistan, 2009. Emerging Infectious Diseases, 17(10), 1940–1941. 10.3201/eid1710.110061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . (2018). Crimean Congo haemorrhagic fever. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 3.1.5., 399–406. Paris (France): OIE. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.05_CCHF.pdf [Google Scholar]
- Papa, A. , Tsergouli, K. , Tsioka, K. , & Mirazimi, A. (2017). Crimean‐Congo hemorrhagic fever: Tick‐host‐virus interactions. Frontiers in Cellular and Infection Microbiology, 7(213), 1–7. 10.3389/fcimb.2017.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster, I. , Mertens, M. , Köllner, B. , Korytář, T. , Keller, M. , Hammerschmidt, B. , Müller, T. , Tordo, N. , Marianneau, P. , Mroz, C. , Rissmann, M. , Stroh, E. , Dähnert, L. , Hammerschmidt, F. , Ulrich, R. G. , & Groschup, M. H. (2016b). A competitive ELISA for species‐independent detection of Crimean‐Congo hemorrhagic fever virus specific antibodies. Antiviral Research, 134, 161–166. 10.1016/j.antiviral.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Schuster, I. , Mertens, M. , Mrenoshki, S. , Staubach, C. , Mertens, C. , Brüning, F. , Wernike, K. , Hechinger, S. , Berxholi, K. , Mitrov, D. , & Groschup, M. H. (2016a). Sheep and goats as indicator animals for the circulation of CCHFV in the environment. Experimental and Applied Acarology, 68(3), 337–346. 10.1007/s10493-015-9996-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, D. (1993). Méthodes statistiques à l'usage des médecins et des biologistes 4th ed. (1–314). France: Médecine Sciences Publications. [Google Scholar]
- Spengler, J. R. , Bergeron, É. , & Rollin, P. E. (2016a). Seroepidemiological studies of Crimean‐Congo hemorrhagic fever virus in domestic and wild animals. PLoS Neglected Tropical Diseases, 10(1), 1–28. 10.1371/journal.pntd.0004210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler, J. R. , Estrada‐Peña, A. , Garrison, A. R. , Schmaljohn, C. , Spiropoulou, C. F. , Bergeron, É. , & Bente, D. A. (2016b). A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean‐Congo hemorrhagic fever virus. Antiviral Research, 135, 31–47. 10.1016/j.antiviral.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhomwegen, J. , Alves, M. J. , Županc, T. A. , Bino, S. , Chinikar, S. , Karlberg, H. , Korukluoglu, G. , Korva, M. , Mardani, M. , Mirazimi, A. , Mousavi, M. , Papa, A. , Saksida, A. , Sharifi‐Mood, B. , Sidira, P. , Tsergouli, K. , Wölfel, R. , Zeller, H. , & Dubois, P. (2012). Diagnostic assays for Crimean‐Congo hemorrhagic fever. Emerging Infectious Diseases, 18(12), 1958–1965. 10.3201/eid1812.120710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, A. R. , Bouattour, A. , Camicas, J. L. , Estrada‐Peña, A , Horac, I. G. , Latif, A. A. , Pegram, R. G. , & Preston, P. M. (2003). Ticks of domestic animals in Africa: a guide to identification of species. (1–228). Scotland, United Kingdom: Bioscience reports Edinburgh. https://www.researchgate.net/publication/265412942_Ticks_of_Domestic_Animals_in_Africa_a_guide_to_identification_of_species [Google Scholar]
- Wang, Y. , Dutta, S. , Karlberg, H. , Devignot, S. , Weber, F. , Hao, Q. , Tan, Y. J. , Mirazimi, A. , & Mirazimi, A. (2012). Structure of Crimean‐Congo hemorrhagic fever virus nucleoprotein: Superhelical homo‐oligomers and the role of caspase‐3 cleavage. Journal of Virology, 86(22), 12294–12303. 10.1128/JVI.01627-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasfi, F. , Dachraoui, K. , Najjar, C. , Younsi, H. , Findlay‐Wilson, S. , Petretto, M. , Dowall, S. , Hewson, R. , & Zhioua, E. (2019). Absence of Crimean‐Congo haemorrhagic fever virus in the tick Hyalomma aegyptium parasitizing the spur‐thighed tortoise (Testudo graeca) in Tunisia. Parasite, 26, 35. 10.1051/parasite/2019036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasfi, F. , Dowall, S. , Ghabbari, T. , Bosworth, A. , Chakroun, M. , Varghese, A. , Tiouiri, H. , Ben Jemaa, M. , Znazen, A. , Hewson, R. , Zhioua, E. , & Letaief, A. (2016). Sero‐epidemiological survey of Crimean‐Congo hemorrhagic fever virus in Tunisia. Parasite, 23(10), 1–5. 10.1051/parasite/2016010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann, M. , Avsic‐Zupanc, T. , Bino, S. , Bouloy, M. , Burt, F. , Chinikar, S. , Christova, I. , Dedushaj, I. , El‐Sanousi, A. , Elaldi, N. , Hewson, R. , Hufert, F. T. , Humolli, I. , van Vuren, P. J. , Tufan, Z. K. , Korukluoglu, G. , Lyssen, P. , Mirazimi, A. , Neyts, J. , … Zeller, H. (2016). Biosafety standards for working with Crimean‐Congo hemorrhagic fever virus. Journal of General Virology, 97(11), 2799–2808. 10.1099/jgv.0.000610 [DOI] [PubMed] [Google Scholar]
- Wilson, M. L , Gonzalez, J. P. , Cornet, J. P. , & Camicas, J. ‐L. (1991). Transmission of Crimean‐Congo haemorrhagic fever virus from experimentally infected sheep to Hyalomma truncatum ticks. Research in Virology, 142(5), 395–404. 10.1016/0923-2516(91)90007-p [DOI] [PubMed] [Google Scholar]
- Wilson, M. L. , LeGuenno, B. , Guillaud, M. , Desoutter, D. , Gonzalez, J.‐P. , & Camicas, J.‐L. (1990). Distribution of Crimean‐Congo hemorrhagic fever viral antibody in Senegal: Environmental and vectorial correlates. The American Journal of Tropical Medicine and Hygiene, 43(5), 557–566. 10.4269/ajtmh.1990.43.557 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


