Abstract
African swine fever (ASF) continues to cause outbreaks throughout regions of Africa, Europe and Asia. The disease can cause severe morbidity and mortality resulting in serious economic losses. Since there is no vaccine available to control ASF, early detection is critical to contain and control the disease. The aim of this study was to develop a novel real‐time PCR assay based on highly conserved ASFV gene E183L (p54). The limit of detection of the assay, VNUA‐p54 real‐time PCR, was 2.63 copies/reaction and 2 Log10 HAD50/ml. The VNUA‐p54 real‐time PCR was able to detect fifteen different ASFV reference strains representing p72 genotypes I, II and V. The assay was specific and did not amplify other swine viruses including CSFV, FMDV, PRRSV and PEDV. The diagnostic sensitivity of the real‐time PCR assay was evaluated using 200 field clinical specimens collected from swine farms located in different provinces in Vietnam. The VNUA‐p54 real‐time PCR assay is an additional tool for ASF diagnostics and can be used in combination with other p72 based ASFV real‐time PCR assays as a rapid confirmatory assay.
Keywords: African swine fever, real‐time PCR, VNUA‐p54
The aim of our study is to develop a novel real‐time PCR assay based on highly conserved ASFV gene E183L (p54) to detect ASFV strains circulating in Vietnam. The obtained results show that our novel real‐time PCR assay is highly specific and sensitive for ASFV detection
1. INTRODUCTION
African swine fever (ASF) was first described in 1921 in Kenya (Montgomery, 1921). It is a highly lethal swine disease caused by a large double‐stranded DNA virus belonging to the Asfarviridae family (Dixon et al., 2005). African swine fever virus (ASFV) infects exclusively suids including domestic swine, wild boars and warthogs. The disease can cause high mortality rates up to 100% resulting in large economic losses to the swine industry in affected countries due to loss of production and trade restrictions (Costard et al., 2013). ASF transmission can occur between a healthy and an infected pig through direct contact (Costard et al., 2013) or by a bite of an ASFV infected Ornithodoros soft tick (Plowright, 1977) which is responsible for maintenance of the sylvatic cycle in Africa.
Since its first discovery in Kenya, ASF has spread outside Africa twice. The first ASF outbreak in Europe was reported in Portugal in 1957 which later spread to Caribbean and South America between the 1970s and 1980s. This outbreak was completely eradicated from Europe and Americas at a great cost with the exception of Sardinia (Sánchez‐Vizcaíno et al., 2015). In 2007, ASF entered Europe for the second time through Georgia (Costard et al., 2013; Rowlands et al., 2008) from where it spread to the Caucasus region and Russia (Gogin et al., 2013; Khomenko et al., 2013). Then it continued its spread into Eastern Europe and entered China in 2018 where the virus became endemic (Zhao et al., 2019). Subsequently it spread to Mongolia (Heilmann et al., 2020), Vietnam (Le et al., 2019), Cambodia, Republic of Korea (Kim et al., 2020), Laos, Philippines, Myanmar and Timor‐Lester, Papua New Guinea and most recently to India (OIE). Since there is no vaccine for ASF, control of this disease relies on rapid detection and elimination of the infected animals (Oura et al., 2013). Real‐time PCR is the preferred first‐line diagnostic for ASF. It is highly sensitive and specific, rapid and highly scalable (Fernández‐Pinero et al., 2013; King et al., 2003). Several real‐time PCR assays have been developed and validated for ASF detection and most of them target ASF p72 gene (Fernández‐Pinero et al., 2013; Tignon et al., 2011; Wang, Jia et al., 2020; Wang, Xu et al., 2020; Zsak et al., 2005). Here we describe development of a novel real‐time PCR assay (VNUA‐p54) targeting a highly conserved region of the ASFV E183L gene that encodes an essential structural protein p54 (Brookes et al., 1998; Rodriguez et al., 1996; Mai et al., 2021).
2. MATERIALS AND METHODS
2.1. Clinical samples
Two hundred samples including whole blood (n = 127), serum (n = 17), spleen (n = 40) and kidney (n = 16) were collected from pigs displaying clinical signs of ASF from farms located in the different provinces in Vietnam during 2019–2020 outbreaks (Table S4). A sample volume of 200 μl of whole blood, serum or 10% tissue homogenate were used for nucleic acid extraction using DNeasy Blood & Tissue Kit (QIAGEN, Germany) according to the manufacturer's instructions.
2.2. Determination of analytical sensitivity and specificity
The limit of detection is defined as the highest dilution factor where 95% of the positive samples can be detected. To test the limit of detection of the VNUA‐p54 real‐time PCR assay, 10‐fold dilutions of 107, 106, 105, 104, 103, 102 and 10 HAD50/ml of VNUA/HY/ASF‐1/Vietnam/2019 ASFV strain (Le et al., 2019) were generated and the real‐time PCR was performed on a LightCycler® 96 Instrument (Roche, Switzerland). Additionally, the detection limit of the real‐time PCR was also conducted using the known amount of DNA templates of 0.1, 1, 2, 5 and 10 copies for each reaction. Each DNA concentration was run 12 times by VNUA‐p54 real‐time PCR assay as in the previous recommendation (Uhlig et al., 2015). To determine the diagnostic specificity of the real‐time PCR assays, fifteen different ASFV reference strains representing p72 genotypes I, II and V (Table S1) and other swine viruses including classical swine fever (Strain Vietnam/ND20/2014, GenBank accession no. MH979232), porcine reproductive and respiratory syndrome (Strain HUA/HP1963; GenBank accession no. KF699844), porcine epidemic diarrhea (Strain HUA‐14PED96, GenBank accession no. KT941120) and foot and mouth disease (Strain O/VN/PT555/2018, GenBank accession no. MN379784) were used in this study.
2.3. Development and optimisation of the VNUA‐54 real‐time PCR assay
In order to design an ASFV p54‐based real‐time PCR, the E183L (p54) gene sequences representing all 24 ASF p72 genotypes of ASFV were aligned by the Geneious software, and a highly conserved 100 bp region between nucleotide positions 287 and 386 was selected, and primers (forward: 5′‐CAAGTGTAGGCAAGCCAGTC‐3′ and reverse: 5′‐GCCATGACTAGTCTGTCCGT‐3′) and a TaqMan® probe (5′‐FAM ACGGGCAGACCGGCAACAAA‐3′TAM) were designed. The primer and probe concentrations and cycling conditions were extensively optimised and the optimised reaction mixture contained 5 μl of 4X TaqMan Fast Virus 1‐Step Master Mix (Applied Biosystems™); 10 μM of forward and reverse primers, and 10 μM of probe; 5 μl of extracted DNA and DNase & RNase‐free water in a 20 μl reaction. The optimal thermal profile for the VNUA‐p54 real‐time PCR assay is 50°C for 5 min; 95°C for 20 s; followed by 40 cycles of amplification (3 s at 95°C and 30 s at 58°C). All real‐time PCR reactions were performed on a LightCycle™ 96 (Rocher, Switzerland) and data was analysed by the manufacturer's software. For comparison, an ASF real‐time PCR developed by Tignon et al. (2011) was used as described.
3. RESULTS
3.1. Analytical sensitivity of the VNUA‐p54 real‐time PCR assay
The limit of detection of the VNUA‐p54 assay was analysed and compared with the validated p72‐based Tignon real‐time PCR assay (Tignon et al., 2011) using ASFV DNA ranging from 0.1 to 10 genome copies. Both assays were able to detect 10 and 5 ASF genome copies, while the VNUA‐p54 assay detected 10/12 times and the Tignon assay 9/12 times 1 and 2 genome copies. Both assays failed to detect 0.1 ASFV genome copies (Tables S2 and S3). Based on these findings LOD was calculated for both assays using https://quodata.de/content/validation‐qualitative‐pcr‐methods‐single‐laboratory. The LOD was 2.63 copies for the VNUA‐p54 real‐time PCR assay and 3.29 copies for the Tignon real‐time PCR assay (Table 1).
TABLE 1.
LOD95 value of real‐time PCR in detecting ASFV p54 gene with VNUA‐p54 primers and p72 gene with Tignon primers
| VNUA‐p54 | Tignon | |||
|---|---|---|---|---|
| Copies/reaction | Positive samples | LOD95 | Positive samples | LOD95 |
| 0.1 | 0/12 | 2.63 | 1/12 | 3.29 |
| 1 | 10/12 | 9/12 | ||
| 2 | 10/12 | 9/12 | ||
| 5 | 12/12 | 12/12 | ||
| 10 | 12/12 | 12/12 | ||
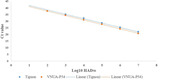
The sensitivity of the VNUA‐p54 assay compared with Tignon real‐time PCR assay was further evaluated using a 10‐fold dilution series of the ASFV strain VNUA/HY/ASF‐1/Vietnam/2019. The results showed that both assays were able to detect ASFV at viral titres of 107, 106, 105, 104 and 103 HAD50/ml. The Ct values obtained for VNUA‐p54 assay were 20.78, 24.23, 27.69, 30.92 and 34.39 and Tignon assay were 22.03, 25.29, 28.58, 32.2 and 35.16, respectively. In addition, at viral titre 102 HAD50/ml, VNUA‐p54 assay could result in Ct value of 37.66 while Tignon assay was negative. Both assays failed to detect ASFV at viral titre of 10 HAD50/ml. Basing on the Ct values generated from each known virus titres, a linear relationship was observed between VNUA‐p54 and Tignon real‐time PCR assays (Figure 1). Pearson's correlation test was used to determine the correlation of HAD50 values between VNUA‐p54 and Tignon assay and the result showed that the Pearson's correlation coefficient was equal to 0.89 (p < 0.05) (Figure 2).
FIGURE 1.

Comparison of the detection limit between the VNUA‐p54 and Tignon assays using VNUA/HY/ASF‐1/Vietnam/2019 ASFV strain
FIGURE 2.

Correlation test of HAD50 values between VNUA‐p54 and Tignon assay. Figure was created by R version 4.0.5. Pearson's correlation coefficient and p‐value are showed in the upper‐left corner
3.2. Analytical specificity of the VNUA‐p54 real‐time PCR assay
To determine the analytical specificity of the VNUA‐p54 real‐time PCR assay, a total of 15 different ASFV reference strains of p72 genotypes I, II and V were used and the result showed that the VNUA‐p54 real‐time PCR assay was able to detect all of them (Table S1). The specificity of the VNUA‐p54 real‐time PCR was also tested with other swine virus strains of CSFV, FMDV, PRRSV and PEDV and the assay did not have non‐specific reaction with any of those pathogens (Data not shown).
3.3. Field sample evaluation of the VNUA‐p54 real‐time PCR assay
A total of 200 field clinical samples including whole blood (n = 127), serum (n = 17), spleen (n = 40) and kidney (n = 16) collected from pigs displaying ASF‐ clinical signs were tested by both VNUA‐p54 and Tignon real‐time PCR assays. The results showed that both assays were able to detect ASFV in all sample types. The mean Log10 HAD50 value of VNUA‐p54 assay was 7.34 and Tignon assay was 7.00. Diagnosis results according to the type of samples showed that the mean Ct values obtained from spleen, kidney, serum and whole blood samples were 7.54, 7.31, 5.94 and 7.42 for VNUA‐p54 assay and 7.18, 6.98, 5.87 and 7.06 for Tignon assay, respectively (Table 2).
TABLE 2.
Diagnostic results of real‐time PCR for detection of ASFV in field samples collected from different provinces in Vietnam
| VNUA‐p54 (Log10 HAD50 values) | Tignon (Log10 HAD50 values) | ||||||
|---|---|---|---|---|---|---|---|
| Type of sample | Total samples | Positive samples | Range | Mean | Positive samples | Range | Mean |
| Spleen | 40 | 37 | 5.67–9.18 | 7.54 | 37 | 5.20–8.65 | 7.18 |
| Kidney | 16 | 16 | 5.07–9.31 | 7.31 | 16 | 4.49–9.22 | 6.98 |
| Serum | 17 | 12 | 3.30–7.76 | 5.94 | 12 | 3.55–7.89 | 5.87 |
| Whole blood | 127 | 122 | 3.17–9.10 | 7.42 | 122 | 3.04–9.38 | 7.06 |
| Total | 200 | 187 | 3.17–9.31 | 7.34 | 187 | 3.04–9.38 | 7.00 |
4. DISCUSSION
It has been almost a hundred years since ASF was first described in Kenya and today ASF is a greater threat to pig populations throughout the world. Despite numerous efforts by research groups across the globe, there is no vaccine for ASF and the disease is difficult to eradicate once established and has increased the geographic prevalence. Control of this disease relies on detection and containment and therefore rapid and sensitive diagnostics for ASF are really critical and important. Currently, there are numerous molecular and serological methods available to identify ASFV‐infected animals (Abad et al., 1998; Fernández‐Pinero et al., 2013; King et al., 2003; Tignon et al., 2011). Serological assays are used to determine if an animal has been exposed to ASFV. Molecular tests can detect the presence of ASFV in pigs even before to the clinical signs appear. A number of molecular assays including laboratory based, portable conventional and real‐time loop‐mediated isothermal amplification (LAMP) assays have been developed and validated for ASFV genome detection. Real‐time PCR is the preferred assay used in many diagnostic laboratories since it is quantitative and faster compared to conventional PCR (Daigle et al., 2020; King et al., 2003; Tignon et al., 2011; Wang, Jia et al., 2020; Wang, Xu et al., 2020). Most of these assays are based on B646L (p72) gene. In this study, we have developed a new assay that targets ASF p54 gene. The results obtained in this study also showed that the VNUA‐p54 assay is highly specific and sensitive and performs comparable to the widely used Tignon assay. The VNUA‐p54 assay can be used to detect ASFV in different sample types including blood, serum, spleen and kidney on its own or as an ancillary tool. The VNUA‐p54 real‐time PCR assay developed in this study will be an additional tool in our effort for rapid detection of ASFV in order to control the ongoing global epidemic.
ETHICAL APPROVAL
Ethical Statement is not applicable because sample collection from animals has been gathered.
AUTHOR CONTRIBUTIONS
TBNT, TT and VPL conceived the idea and designed a novel real‐time PCR assay. TBNT, VTN and XDV performed the experiments. VTN, AA, SB and TLN helped in revising of the manuscript. JO, DS and LAD participated in analysing the results. TBNT and VPL wrote the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.605
Supporting information
TABLE S1. ASFV reference strains representing for p72 genotype I, II and V used in the study
TABLE S2. Ct values of real‐time PCR in detecting p54 gene of ASFV with VNUA‐p54 primers and probe set
TABLE S3. Ct values of real‐time PCR in detecting p72 gene of ASFV with Tignon primers and probe set
TABLE S4. Detail diagnostic results of real‐time PCR for detection of ASFV in field samples collected from different provinces in Vietnam
ACKNOWLEDGEMENTS
This work was supported by the Vietnam National Project under project code no. DTDL.CN‐53/19.
Trinh, T. B. N. , Truong, T. , Nguyen, V. T. , Vu, X. D. , Dao, L. A. , Nguyen, T. L. , Ambagala, A. , Babiuk, S. , Oh, J. , Song, D. , & Le, V. P. (2021). Development of a novel real‐time PCR assay targeting p54 gene for rapid detection of African swine fever virus (ASFV) strains circulating in Vietnam. Veterinary Medicine and Science, 7, 2268–2272. 10.1002/vms3.605
Thi Bich Ngoc Trinh and Thang Truong contributed equally to this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abad, J. M , Pariente, F. , Hernández, L. , & Lorenzo, E. (1998). A quartz crystal microbalance assay for detection of antibodies against the recombinant African swine fever virus attachment protein p12 in swine serum. Analytica Chimica Acta, 368(3), 183–189. [Google Scholar]
- Parkhouse, R. M. , Brookes, S. M. , Dixon, L. K. , & Sun, H. (1998). Characterization of African swine fever virion proteins j5R and j13L: Immuno‐localization in virus particles and assembly sites. Journal of General Virology, 79(5), 1179–1188. [DOI] [PubMed] [Google Scholar]
- Costard, S. , Mur, L. , Lubroth, J. , Sanchez‐Vizcaino, J. M. , & Pfeiffer, D. U. (2013). Epidemiology of African swine fever virus. Virus Research, 173(1), 191–197. [DOI] [PubMed] [Google Scholar]
- Daigle, J. , Onyilagha, C. , Truong, T. , Van Phan, L. , Nga, B. T. T. , Nguyen, T. L. , Clavijo, A. , & Ambagala, A. (2020). Rapid and highly sensitive portable detection of African swine fever virus. Transboundary and Emerging Diseases, 68, 952–959. [DOI] [PubMed] [Google Scholar]
- Dixon, L. K. , Escribano, J. , Martins, C. , Rock, D. L. , Salas, M. , & Wilkinson, P. J. (2005). Asfarviridae. In Fauquet, C. M. , Mayo, M. A. , Maniloff, J. , Desselberger, U. , Ball, L. A. (Eds.), Virus taxonomy (pp. 135–143). VIIIth Report of the ICTV. Elsevier/Academic Press, London. [Google Scholar]
- Fernández‐Pinero, J. , Gallardo, C. , Elizalde, M. , Robles, A. , Gómez, C. , Bishop, R. , Heath, L. , Couacy‐Hymann, E. , Fasina, F. O. , Pelayo, V. , Soler, A. , & Arias, M. (2013). Molecular diagnosis of African swine fever by a new real‐time PCR using universal probe library. Transboundary and Emerging Diseases, 60(1), 48–58. [DOI] [PubMed] [Google Scholar]
- Gogin, A. , Gerasimov, V. , Malogolovkin, A. , & Kolbasov, D. (2013). African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus Research, 173(1), 198–203. [DOI] [PubMed] [Google Scholar]
- Heilmann, M. , Lkhagvasuren, A. , Adyasuren, T. , Khishgee, B. , Bold, B. , Ankhanbaatar, U. , Fusheng, G. , Raizman, E. , & Dietze, K. (2020). African swine fever in Mongolia: Course of the epidemic and applied control measures. Veterinary Sciences, 7(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomenko, S. , Beltrán‐Alcrudo, D. , Rozstalnyy, A. , Gogin, A. , Kolbasov, D. , Pinto, J. , Lubroth, J. , & Martin, V. (2013). African swine fever in the Russian Federation: Risk factors. Empres Watch, 28, 1–14. [Google Scholar]
- Kim, H.‐J. , Cho, K.‐H. , Lee, S.‐K. , Kim, D.‐Y. , Nah, J.‐J. , Kim, H.‐J. , Kim, H.‐J. , Hwang, J.‐Y. , Sohn, H.‐J. , Choi, J.‐G. , Kang, H.‐E. , & Kim, Y.‐J. (2020). Outbreak of African swine fever in South Korea, 2019. Transboundary and Emerging Diseases, 67(2), 473–475. [DOI] [PubMed] [Google Scholar]
- King, D. P. , Reid, S. M. , Hutchings, G. H. , Grierson, S. S. , Wilkinson, P. J. , Dixon, L. K. , Bastos, A. D. S. , & Drew, T. W. (2003). Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. Journal of Virological Methods, 107(1), 53–61. [DOI] [PubMed] [Google Scholar]
- Le, V. P. , Jeong, D. G. , Yoon, S.‐W. , Kwon, H.‐M. , Trinh, T. B. N. , Nguyen, T. L. , Bui, T To N , Oh, J. , Kim, J. B. , Cheong, K. M. , Van Tuyen, N. , Bae, E. , Vu, T. T. H. , Yeom, M. , Na, W. , & Song, D. (2019). Outbreak of African swine fever, Vietnam, 2019. Emerging Infectious Diseases, 25(7), 1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai, N. T. A. , Vu, X. D. , Nguyen, T. T. H. , Nguyen, V. T. , Trinh, T. B. N. , Kim, Y. J. , Kim, H.‐J. , Cho, K.‐H. , Nguyen, T. L. , Bui, T. T. N. , Jeong, D. G. , Yoon, S.‐W. , Truong, T. , Ambagala, A. , Song, D. , & Le, V. P. (2021). Molecular profile of African swine fever virus (ASFV) circulating in Vietnam during 2019–2020 outbreaks. Archives of Virology, 166(3), 885–890. [DOI] [PubMed] [Google Scholar]
- Eustace Montgomery, R. (1921). On a form of swine fever occurring in British East Africa (Kenya Colony). Journal of Comparative Pathology and Therapeutics, 34, 159–191. [Google Scholar]
- Oura, C. A. L. , Edwards, L. , & Batten, C. A. (2013). Virological diagnosis of African swine fever – Comparative study of available tests. Virus Research, 173(1), 150–158. [DOI] [PubMed] [Google Scholar]
- Plowright, W. (1977). Vector transmission of African swine fever virus. Paper presented at the Hog cholera/classical swine fever and African swine fever. Hannover, Germany, FR, 6 Sep 1976. [Google Scholar]
- Rodriguez, F. , Ley, V. , Gómez‐Puertas, P. , García, R. , Rodriguez, J. , & Escribano, J. (1996). The structural protein p54 is essential for African swine fever virus viability. Virus Research, 40(2), 161–167. [DOI] [PubMed] [Google Scholar]
- Rowlands, R. J. , Michaud, V. , Heath, L. , Hutchings, G. , Oura, C. , Vosloo, W. , Dwarka, R. , Onashvili, T. , Albina, E. , & Dixon, L. K. (2008). African swine fever virus isolate, Georgia, 2007. Emerging Infectious Diseases, 14(12), 1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Vizcaíno, J. M. , Mur, L. , Gomez‐Villamandos, J. C. , & Carrasco, L. (2015). An update on the epidemiology and pathology of African swine fever. Journal of Comparative Pathology, 152(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Tignon, M. , Gallardo, C. , Iscaro, C. , Hutet, E. , Van Der Stede, Y. , Kolbasov, D. , De Mia, G. M. , Le Potier, M.‐F. , Bishop, R. P. , Arias, M. , & Koenen, F. (2011). Development and inter‐laboratory validation study of an improved new real‐time PCR assay with internal control for detection and laboratory diagnosis of African swine fever virus. Journal of Virological Methods, 178(1–2), 161–170. [DOI] [PubMed] [Google Scholar]
- Uhlig, S. , Frost, K. , Colson, B. , Simon, K. , Mäde, D. , Reiting, R. , Gowik, P. , & Grohmann, L. (2015). Validation of qualitative PCR methods on the basis of mathematical–statistical modelling of the probability of detection. Accreditation and Quality Assurance, 20(2), 75–83. [Google Scholar]
- Wang, A. , Jia, R. , Liu, Y. , Zhou, J. , Qi, Y. , Chen, Y. , Liu, D. , Zhao, J. , Shi, H. , Zhang, J. , & Zhang, G. (2020). Development of a novel quantitative real‐time PCR assay with lyophilized powder reagent to detect African swine fever virus in blood samples of domestic pigs in China. Transboundary and Emerging Diseases, 67(1), 284–297. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Xu, L. , Noll, L. , Stoy, C. , Porter, E. , Fu, J. , Feng, Y. , Peddireddi, L. , Liu, X. , Dodd, K. A. , Jia, W. , & Bai, J. (2020). Development of a real‐time PCR assay for detection of African swine fever virus with an endogenous internal control. Transboundary and Emerging Diseases, 67, 2446–2454. [DOI] [PubMed] [Google Scholar]
- Zhao, D. , Liu, R. , Zhang, X. , Li, F. , Wang, J. , Zhang, J. , Liu, X. , Wang, L. , Zhang, J. , Wu, X. , Guan, Y. , Chen, W. , Wang, X. , He, X. , & Bu, Z. (2019). Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerging Microbes & Infections, 8(1), 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak, L. , Borca, M. V. , Risatti, G. R. , Zsak, A. , French, R. A. , Lu, Z. , Kutish, G. F. , Neilan, J. G. , Callahan, J. D. , Nelson, W. M. , & Rock, D. L. (2005). Preclinical diagnosis of African swine fever in contact‐exposed swine by a real‐time PCR assay. Journal of Clinical Microbiology, 43(1), 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. ASFV reference strains representing for p72 genotype I, II and V used in the study
TABLE S2. Ct values of real‐time PCR in detecting p54 gene of ASFV with VNUA‐p54 primers and probe set
TABLE S3. Ct values of real‐time PCR in detecting p72 gene of ASFV with Tignon primers and probe set
TABLE S4. Detail diagnostic results of real‐time PCR for detection of ASFV in field samples collected from different provinces in Vietnam
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


