Abstract
Background
Convallotoxin (CNT), present in lily of the valley (Convallaria majalis), is a toxin that causes food poisoning among humans and companion animals. Although various symptoms of CNT poisoning have been well described, hypercoagulability owing to CNT is only empirically known among some veterinarians, and the underlying mechanism remains to be elucidated. CNT exerts cytotoxic effects on endothelial cells.
Objectives
This study aimed to determine whether CNT induces the expression of tissue factor (TF), a potent initiator of the extrinsic coagulation cascade, in endothelial cells and leads to a hypercoagulable state.
Methods
Human umbilical vein endothelial cells (HUVECs) were used for in vitro experiments. HUVECs were treated with or without CNT (50 and 100 nM) for 4 h. Phosphate‐buffered saline was used as a control. Cell viability was determined using the WST‐8 assay. Quantitative real‐time polymerase chain reaction was performed to determine TF mRNA expression. TF protein expression was observed using a laser scanning confocal microscope.
Results
The viability of HUVECs significantly reduced after CNT treatment compared with that of non‐treated cells (p < 0.05). Moreover, a significant increase in TF mRNA and protein expression was observed after 4 h of CNT treatment. CNT elicited these effects in a dose‐dependent manner.
Conclusions
TF expression induced by CNT in endothelial cells can contribute to the development of a hypercoagulable state. The present study partially revealed the mechanisms underlying the CNT‐induced hypercoagulable state. The findings can contribute to the development of a novel therapy for lily of the valley poisoning.
Keywords: convallatoxin, endothelial cells, hypercoagulability, lily of the valley, tissue factor
Convallatoxin, the primary glycoside of lily of the valley, induced tissue factor expression in endothelial cells. Convallatoxin would induce coagulopathy to companion animals
.
1. INTRODUCTION
Lily of the valley (Convallaria majalis) is a well‐known plant and has been preferred for its fragrance and attractive flowers for a long time. Contrary to its attractive appearance, lily of the valley contains many toxins, such as saponins, and various cardiac glycosides (around 30 glycosides). Cardiac glycosides cause digitalis‐like toxicity, whereas saponin is responsible for digestive disorders (Welsh et al., 2014). The primary glycoside of lily of the valley is called convallatoxin (CNT).
Lily of the valley is considered a notorious poisonous plant among veterinarians because drinking water from a vase containing this plant has resulted in companion animal deaths (Cortinovis & Caloni, 2013). Besides, in humans, because of its similarity in appearance to alpine leek (Allium victorialis), an edible wildflower, cases of food poisoning owing to the consumption of lily of the valley have been reported occasionally in Japan (Goto et al., 2015). Furthermore, wild garlic (Allium ursinum) leaves can be confused with leaves of lily of the valley in Europe (Davanzo et al., 2011; Vončina et al., 2014). Although serious damage owing to the consumption of lily of the valley is rare in adults (Alexandre et al., 2012), it has been reported to be the most frequent source of plant poisoning among children in Finland (Lamminpää et al., 1996).
The diverse symptoms of CNT poisoning have been well described in animals as well as in humans (Edgerton, 1989; Mahdy et al., 2017), including salivation, nausea, vomiting, abdominal pain, pupil dilation, slow and irregular heartbeat, and hypertension. However, little is known about the occurrence of a hypercoagulable state. Izawa (1998) reported that the toxic components of lily of the valley have cardiotonic and blood‐clotting actions and that large intake of lily of the valley results in heart failure. Ouabain, the clinically used cardiac glycoside for the treatment of heart failure, has been shown to activate platelets (Lees et al., 1989). However, the detailed mechanisms underlying CNT‐induced hypercoagulability have not yet been elucidated.
In this study, we address the issue of CNT‐induced hypercoagulability from the standpoint of coagulation factors, especially tissue factor (TF). TF is expressed on monocytes and endothelial cells in response to invasive triggers, such as infection or inflammation (Grover et al., 2018). It forms a complex with coagulation factor VII and initiates the extrinsic coagulation pathway. Overactivation of the extrinsic pathway leads to the disturbance of the coagulation system (Yamamoto et al., 1992). It has been revealed that CNT is cytotoxic and inhibits the growth of human umbilical vein endothelial cells (HUVECs) (Yang et al., 2014). We hypothesised that CNT damages endothelial cells and induces TF expression. We confirmed this hypothesis via simple in vitro experiments. The clarification of CNT‐induced hypercoagulability would support an autopsy diagnosis, and it can be used to develop a novel treatment for lily of the valley intoxication.
2. MATERIALS AND METHODS
All methods, except for immunostaining, were performed as described previously (Kasuda et al., 2017).
2.1. Cell culture
HUVECs were purchased from Lonza and grown in endothelial cell growth medium‐2 (EGM‐2; Clonetics). Before each assay, HUVECs were serum‐starved using 0.1% bovine serum albumin‐containing medium for 2 h. For stimulation, CNT (Sigma‐Aldrich) was used at a final concentration of 50 and 100 nM. Phosphate‐buffered saline (Wako Pure Chemical Industries) was used as the control. A phase‐contrast microscope (EVOS XL Core Imaging System; Thermo Fisher Scientific) was used for cell observation.
2.2. Cell viability assay
The viability of HUVECs after CNT treatment was evaluated using the WST‐8 assay (Cell Counting Kit‐8; Dojindo). HUVECs seeded in a 96‐well plate (5 × 104 cells/well) were treated with or without CNT in 100 μl of EGM‐2. After 4 h, 10 μl of WST‐8 reagent was added and incubated for 1 h. Cell viability was determined using a spectrometer (SpectraMax M2e; Molecular Devices) at an absorbance of 450 nm.
2.3. Quantitative real‐time polymerase chain reaction
Total RNA was extracted from HUVECs using the RNeasy mini kit (Qiagen). The total RNA (1 μg) was reverse‐transcribed into cDNA using the SuperScript RT kit (Life Technologies), and real‐time polymerase chain reaction analysis was performed on a StepOne system (Applied Biosystems) using TaqMan probes and primers for human TF (assay ID; Hs01076032_m1). Human hypoxanthine phosphoribosyltransferase 1 (assay ID; Hs02800695_m1) served as an endogenous control.
2.4. Immunostaining for TF expression in HUVECs
HUVECs were seeded in the Labtek chamber (Thermo Fisher Scientific) and treated with CNT for 4 h or not treated. After stimulation, the cells were fixed with 4% paraformaldehyde, permeabilised with 0.1% Triton X‐100, and blocked with 10% goat serum for 1 h at 22°C. The cell samples were incubated with TF‐specific antibody (1:300, ab48647; Abcam) for 16 h at 4°C. Alexa Fluor 488‐conjugated goat anti‐rabbit IgG (1:500, ab150077; Abcam) was used as the secondary antibody. Vectashield Antifade Mounting Medium with 4′, 6‐diamidino‐2‐phenylindole (DAPI; Vector Laboratories) was used for sealing and nuclear staining. A laser scanning confocal microscope (C2; Nikon) was used to visualise TF expression as previously described (Morita‐Takemura et al., 2019).
2.5. Statistical analysis
Data were analysed using SPSS software version 19 (IBM). Statistical comparisons were performed using the Tukey–Kramer test. Differences were assessed at a significance level of p < 0.05. Data are shown as mean ± standard error of the mean.
3. RESULTS
3.1. CNT exerts cytotoxic effects and reduces endothelial viability
After 4 h of treatment with CNT, HUVECs were detached from the culture dish (Figure 1a), indicating the cytotoxicity of CNT. For the quantification of cell viability, we performed the WST‐8 assay. The cell viability after CNT treatment for 4 h decreased significantly compared with that of non‐treated cells (Figure 1b; p < 0.05). CNT exerted these cytotoxic effects in a dose‐dependent manner.
FIGURE 1.
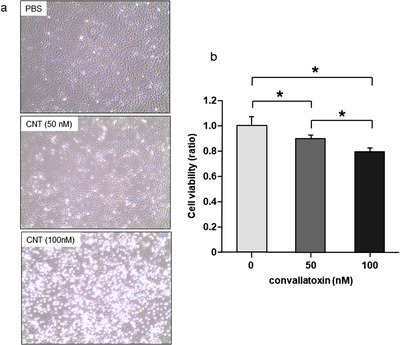
Cytotoxicity of convallatoxin (CNT) on human umbilical vein endothelial cells (HUVECs). (a) Phase‐contrast microscopy images of HUVECs after 4 h of treatment with PBS or CNT (50 and 100 nM). CNT detached the cells from the dish in a dose‐dependent manner (objective ×4). (b) CNT reduced cell viability in a dose‐dependent manner after 4 h of treatment. N = 6. *p < 0.05, determined using the Tukey–Kramer test
3.2. CNT increased TF mRNA and protein expression in HUVECs
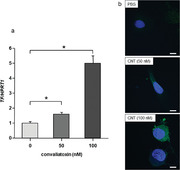
TF mRNA expression significantly increased after 4 h of treatment with CNT (Figure 2a; p < 0.05). TF protein expression also increased in HUVECs after CNT treatment (Figure 2b). Both TF mRNA and protein expressions were increased in a dose‐dependent manner in terms of CNT doses.
FIGURE 2.
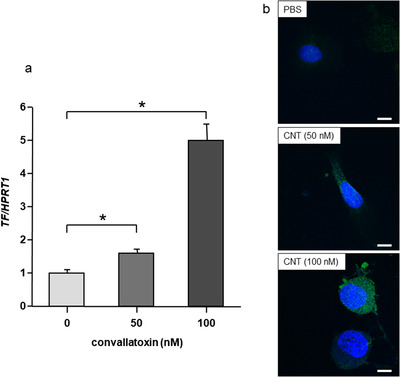
Convallatoxin‐induced tissue factor expression in HUVECs. (a) Convallatoxin (CNT; 50 and 100 nM) increased tissue factor (TF) mRNA expression in a dose‐dependent manner after 4 h of treatment. N = 4. *p < 0.05, determined using the Tukey–Kramer test. (b) Observation of human umbilical vein endothelial cells (HUVECs) using a laser‐scanning confocal microscope. CNT (50 and 100 nM) induced TF protein expression in HUVECs in a dose‐dependent manner after 4 h of treatment [scale bar, 10 μm; green, TF; blue, 4′,6‐diamidino‐2‐phenylindole (DAPI)]
4. DISCUSSION
Livestock or companion animals are at a high risk of contact with wild plants, while accidental consumption of wild vegetables due to similar appearance occurs only in humans as the collection of vegetables relies mainly on visual inspection. Plant poisoning is a major concern for veterinarians (Stegelmeier et al., 2020).
In the present study, we aimed to determine whether endothelial cells injured by CNT express TF that can lead to a hypercoagulable state. We found that CNT induced the synthesis of TF in HUVECs.
An appropriate level of TF expression leads to local thrombus formation. Local thrombi prevent the spread of microbes and inflammation, which is called immunothrombosis (Vazquez‐Garza et al., 2017). However, the overexpression of TF disturbs the coagulation system and causes systemic thrombus formation. CNT disrupts the delicate balance of TF expression and can lead to serious consequences.
Recently, CNT has garnered increasing attention for its anti‐proliferative effects in several cancer cells (Anderson & Barton, 2017; Schneider et al., 2016). Although these studies used CNT at nanomolar concentrations similar to that in this study, they treated cancer cells for 24–48 h. Nonetheless, in the present study, only 4 h of treatment with CNT induced cytotoxicity and reduced the viability of HUVECs. Our results suggest that CNT should be used with caution as an anti‐cancer drug because it kills both normal and cancer cells.
Notably, contrary to our observations, Stähli et al. (2007) reported that other types of cardiac glycosides, such as digoxin and ouabain, inhibit tumour necrosis factor‐α‐induced TF protein expression in human aortic endothelial cells. They also demonstrated that these inhibitory effects were mediated by Na+/K+‐ATPase. Another study also reported that the cytotoxic effects of CNT on cancer cells are regulated by Na+/K+‐ATPase; however, CNT cannot directly inhibit Na+/K+ ATPase in normal cells, such as pig kidney cells and human erythrocytes (Schneider et al., 2017). These findings suggest the possibility that CNT‐induced TF expression in endothelial cells is mediated by the Na+/K+‐ATPase‐independent pathway. Further studies are warranted to elucidate the detailed mechanisms of the signalling pathway.
Our study has limitations. First, we did not investigate the impact of CNT on monocytes. Although we used HUVECs in this study because of their availability, monocytes are the most potent cell type responsible for TF production in the blood. Monocytes have a greater role in the life‐threatening event, disseminated intravascular coagulation, than endothelial cells (Østerud & Bjørklid, 2001). Therefore, to obtain a more precise understanding of the influence of CNT on the coagulation system, further studies using monocytes are required. Second, the effects of CNT on whole blood coagulation were not elucidated. Additionally, animal experiments are required to demonstrate the alteration of haemostatic parameters in vivo after CNT treatment. These experiments are currently ongoing. However, our findings revealed a part of the toxic mechanisms of CNT and lead to a better understanding of it.
5. CONCLUSION
The results of the present study demonstrated that TF expression induced by CNT in endothelial cells would contribute to the development of a hypercoagulable state. In addition, a part of the mechanisms of CNT‐induced hypercoagulability was elucidated. Our results can contribute to the development of a novel treatment for lily of the valley intoxication, such as anticoagulation therapy.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this manuscript used only cultured cells.
AUTHOR CONTRIBUTIONS
Mami Morimoto contributed to the study design, data acquisition, analysis, interpretation, and drafting of the manuscript. Kohei Tatsumi contributed to conception, design, data acquisition, data analysis, interpretation, and drafting of the manuscript and critically revised the manuscript. Katsuya Yuui, Ikuko Terazawa, and Risa Kudo contributed to the design, data acquisition, analysis, and drafting of the manuscript. Shogo Kasuda contributed to the conception, design, data acquisition, analysis, and interpretation and drafted and edited the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work including its integrity and accuracy.
ACKNOWLEDGEMENT
We would like to thank Editage (www.editage.com) for English language editing. The authors received no financial support for the research, authorship, and publication of this article.
Morimoto, M. , Tatsumi, K. , Yuui, K. , Terazawa, I. , Kudo, R. , & Kasuda, S. (2021). Convallatoxin, the primary cardiac glycoside in lily of the valley (Convallaria majalis), induces tissue factor expression in endothelial cells. Veterinary Medicine and Science, 7, 2440–2444. 10.1002/vms3.614
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alexandre, J. , Foucault, A. , Coutance, G. , Scanu, P. , & Milliez, P. (2012). Digitalis intoxication induced by an acute accidental poisoning by lily of the valley. Circulation, 125, 1053–1055. [DOI] [PubMed] [Google Scholar]
- Anderson, S. E. & Barton, C. E. (2017). The cardiac glycoside convallatoxin inhibits the growth of colorectal cancer cells in a p53‐independent manner. Molecular Genetics and Metabolism Reports, 13, 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortinovis, C. & Caloni, F. (2013). Epidemiology of intoxication of domestic animals by plants in Europe. The Veterinary Journal, 197, 163–168. [DOI] [PubMed] [Google Scholar]
- Davanzo, F. , Miaglia, S. , Perego, S. , Assisi, F. , Bissoli, M. , Borghini, R. , Cassetti, F. , Puppa, T. D. , Dimasi, V. , Falciola, C. , Ferruzzi, M. , Moro, P. A. , Panzavolta, G. , Rebutti, I. , Sesana, F. , Severgnini, P. , Tomoiaga, A. , Travaglia, A. , Zanardi, S. , Georgatos, J. , & Colombo, M. L. (2011). Plant poisoning: Increasing relevance, a problem of public health and education. North‐western Italy, Piedmont region. Journal of Pharmaceutical Sciences and Research, 3, 1338–1343. [Google Scholar]
- Edgerton, P. H. (1989). Symptoms of digitalis‐like toxicity in a family after accidental ingestion of lily of the valley plant. Journal of Emergency Nursing, 15, 220–223. [PubMed] [Google Scholar]
- Goto, T. , Ozeki, F. , Aoyama, F. , Ito, Y. , Ueno, E. , & Ikai, Y. (2015). The simultaneous analysis of vegetable natural poison (lycorine and convallatoxin) that causes food poisoning. Report of Aichi Prefectural Institute of Public Health, 65, 31–38. [Google Scholar]
- Grover, S. P. & Mackman, N. (2018). Tissue factor: An essential mediator of hemostasis and trigger of thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 38, 709–725. [DOI] [PubMed] [Google Scholar]
- Izawa, K. (1998). Suzuran (Lily of the valley). Encyclopedia of herbs in color—All about Japanese medical plants (in Japanese). Shufunotomo Co., Ltd. [Google Scholar]
- Kasuda, S. , Kudo, K. , Yuui, K. , Sakurai, Y. , & Hatake, K. (2017). Acute ethanol intoxication suppresses pentraxin 3 expression in a mouse sepsis model involving cecal ligation and puncture. Alcohol, 64, 1–9. [DOI] [PubMed] [Google Scholar]
- Lamminpää, A. & Kinos, M. (1996). Plant poisonings in children. Human & Experimental Toxicology, 15, 245–249. [DOI] [PubMed] [Google Scholar]
- Lees, A. D. , Wilson, J. , Orchard, C. H. , & Orchard, M. A. (1989). Ouabain enhances basal and stimulus‐induced cytoplasmic calcium concentrations in platelets. Thrombosis and Haemostasis, 62, 1000–1005. [PubMed] [Google Scholar]
- Mahdy, C. E. L. , Popescu, S. , & Borda, C. (2017). Plants that can be poisonous for cows. A review. Bulletin UASVM Animal Science and Biotechnologies, 74, 69–83. [Google Scholar]
- Morita‐Takemura, S. , Nakahara, K. , Hasegawa‐Ishii, S. , Isonishi A., Tatsumi K., Okuda H., Tanaka T., Kitabatake T., Ito T., & Wanaka, A. (2019). Responses of perivascular macrophages to circulating lipopolysaccharides in the subfornical organ with special reference to endotoxin tolerance. Journal of Neuroinflammation, 16, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østerud, B. & Bjørklid, E. (2001). The tissue factor pathway in disseminated intravascular coagulation. Seminars in Thrombosis and Hemostasis, 27, 605–617. [DOI] [PubMed] [Google Scholar]
- Schneider, N. F. Z. , Geller, F. C. , Persich, L. , Marostica, L. L. , Pádua, R. M. , Kreis, W. , Braga, F. C. , & Simões, C. M. O. (2016). Inhibition of cell proliferation, invasion and migration by the cardenolides digitoxigenin monodigitoxoside and convallatoxin in human lung cancer cell line. Natural Product Research, 30, 1327–1331. [DOI] [PubMed] [Google Scholar]
- Schneider, N. F. Z. , Silva, I. T. , Persich, L. , de Carvalho, A. , Rocha, S. C. , Marostica, L. , Ramos, A. C. P. , Taranto, A. G. , Pádua, R. M. , Kreis, W. , Barbosa, L. A. , Braga, F. C. , & Simões, C. M. O. (2017). Cytotoxic effects of the cardenolide convallatoxin and its Na,K‐ATPase regulation. Molecular and Cellular Biochemistry, 428, 23–39. [DOI] [PubMed] [Google Scholar]
- Stähli, B. E. , Breitenstein, A. , Akhmedov, A. , Camici, G. G. , Shojaati, K. , Bogdanov, N. , Steffel, J. , Ringli, D. , Lüscher, T. F. , & Tanner, F. C. (2007). Cardiac glycosides regulate endothelial tissue factor expression in culture. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 2769–2776. [DOI] [PubMed] [Google Scholar]
- Stegelmeier, B. L. , Davis, T. Z. , Clayton, M. J. , & Gardner, D. R. (2020). Identifying plant poisoning in livestock in north America. The Veterinary Clinics of North America. Food and Practice, 36, 661–671. [DOI] [PubMed] [Google Scholar]
- Vazquez‐Garza, E. , Jerjes‐Sanchez, C. , Navarrete, A. , Joya‐Harrison, J. , & Rodriguez, D. (2017). Venous thromboembolism: thrombosis, inflammation, and immunothrombosis for clinicians. Journal of Thrombosis and Thrombolysis, 44, 377–385. [DOI] [PubMed] [Google Scholar]
- Vončina, M. , Baričevič, D. , & Brvar, M. (2014). Adverse effects and intoxications related to medicinal/harmful plants. Acta Agriculturae Slovenica, 103, 263–270. [Google Scholar]
- Welsh, K. J. , Huang, R. S. , Actor, J. K. , & Dasgupta, A. (2014). Rapid detection of the active cardiac glycoside convallatoxin of lily of the valley using LOCI digoxin assay. American Journal of Clinical Pathology, 142, 307–312. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M. , Nakagaki, T. , & Kisiel, W. (1992). Tissue factor‐dependent autoactivation of human blood coagulation factor VII. The Journal of Biological Chemistry, 267, 19089–19094. [PubMed] [Google Scholar]
- Yang, S. Y. , Kim, N. H. , Cho, Y. S. , Lee, H. , & Kwon, H. J. (2014). Convallatoxin, a dual inducer of autophagy and apoptosis, inhibits angiogenesis in vitro and in vivo. Public Library of Science One, 9, e91094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


